Abstract
AIM
To evaluate the effect of silymarin on the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma glutamyl transpeptidase (γGT) in patients with liver diseases.
METHODS
A systematic review with meta-analysis of ramdomized and controlled clinical trials was performed, evaluating the effects of sylimarin in patients with hepatic diseases, published by January 31, 2016. Clinical trials were sought on the basis of The Cochrane Central Register of Controlled Trials in the Cochrane Library, PubMed/Medline, Scopus, Web of Science, Lilacs and Clinical Trials. The trials with adult and elderly patients of both sexes, with Liver Diseases who took oral silymarin supplementation, as extract or isolated, as well as Silymarin combined with other nutrients, were included. The trials should provide information about the intervention, such as dosages and detailing of the product used, besides the mean and standard deviation of serum levels of ALT, AST and γGT of the baseline and at the end of the intervention.
RESULTS
An amount of 10904 publications were identified. From those, only 17 were included in the systematic review and 6 in the meta-analysis, according to the used selection criteria. In this meta-analysis, the results indicated a reduction of 0.26 IU/mL (95%CI: -0.46-0.07, P = 0.007) at the level of ALT and 0.53 IU/mL (95%CI: -0.74-0.32, P = 0.000) at the serum levels of AST after using the silymarin, both, statistically significant, but with no clinical relevance. There was no significant change in the γGT levels. Subgroup analyzes were also performed for the biochemical markers in relation to the type of intervention, whether silymarin isolated or associated with other nutrients and the time of intervention (whether ≥ 6 mo or < 6 mo). Significant differences were not found. The evaluated studies presented a high degree of heterogeneity and low methodological quality in the carried out analysis.
CONCLUSION
Silymarin minimally reduced, but without clinical relevance, the serum levels of ALT and AST. It is necessary to carry out studies with more appropriate methodological designs.
Keywords: Systematic review, Liver diseases, Milk thistle, Silymarin, Meta-analysis
Core tip: Silymarin is commonly prescribed in the practice of many professionals and ingested as self-medication for patients. Studies suggest benefits of its use in hepatic disorders, discussing its mechanisms of action and potential as a coadjutant in the treatment of those diseases. Favorable clinical outcomes as improvement of biochemical indicators and liver profile were observed in clinical trials. However, other studies are controversial or have not reported statistical significance in the improvement of these indicators. Facing the differences and methodological peculiarities of these studies, a systematic review with meta-analysis was performed to clarify the real benefits of silymarin in liver diseases.
INTRODUCTION
The most frequent liver diseases are of an inflammatory nature, which have different etiologies and characteristics. The most common causes of chronic inflammatory liver diseases are viral infections (hepatitis B and C viruses), autoimmune diseases, alcoholic liver disease and non-alcoholic fatty liver disease (NAFLD). Other diseases also occur with inflammation such as chronic biliary diseases, hereditary metabolic diseases and hepatic attacks by hepatotoxic substances[1]. Méndez-Sánches et al[2] have estimated approximately two million cases of chronic liver disease by the year 2050.
The nutritional treatment comprises a fundamental step in the clinical treatment of these patients, as well as in the minimization and/or postponement of the common symptomatology in these diseases[3] and, the prescription of herbal medicines can be a complementary tool to conventional dietary strategies[4].
Silymarin is part of the flavonoid group and is extracted from the plant Silybum marianum, an herbal remedy that has been extensively studied in various hepatic disorders. It is composed of approximately 50% silibinin, which is considered the biologically active component of silymarin[5,6]. Silybum marianum is one of the most commonly plants used in liver diseases treatments, because it is considered hepatoprotective and it has been widely used in patients with cirrhosis, chronic hepatitis and liver disease associated with alcohol consumption and exposure to environmental toxins[7-9]. Currently, it is one of the most studied medicinal herbs for the treatment of NAFLD and steatohepatitis (NASH) and its use has been shown to be safe, well tolerated, with limited adverse effects also for these patient groups[10-12].
Silymarin acts primarily as an antioxidant, reducing the production of reactive oxygen species and lipid peroxidation, increasing the endogenous concentrations of antioxidant enzymes such as glutathione peroxidase, glutathione reductase, superoxide dismutase and catalase[13-16]. It exerts a significant anti-inflammatory effect, mainly by inhibition of nuclear transcription factor NFκB and consequently reduction of inflammatory cytokines in the hepatic parenchyma, in addition to interaction with protein kinases and downregulation of cyclooxygenase 2[17,18].
It also acts as an immunomodulator and anti-fibrotic agent, due to the reduction of the activation or stimulation of apoptosis of the hepatic stellate cells, or increasing the degradation of the collagen deposits in the hepatic parenchyma[19-21]. In addition, it’s considered a hepatoprotective for the ability to stabilize the cell membranes of hepatocytes, preventing the entry of toxic chemicals into these cells. Silymarin binds to receptors present on these membranes, inhibiting the binding of toxins in these sites, reducing drug-induced hepatocellular damage[22,23]. It also stimulates the synthesis and activity of enzymes responsible for the hepatic biotransformation process, such as glutathione S-transferase[24,25].
Studies have shown that silymarin has an important effect on the reduction of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in liver diseases, being considered beneficial in the treatment of these patients[22,26-28].
However, it is important to point out that most of these studies present considerable methodological variations. In addition to having used different doses with different concentrations of silymarin and various formulations, which makes it difficult to perform a comparative analysis of the studies and a consensus about the clinical use of this herbal medicine and its effects on biochemical indicators such as liver enzymes. Thus, the objective of this article is to perform a systematic review with meta-analysis on the effect of silymarin on the ALT, AST and gamma glutamyl transpeptidase (γGT) levels in patients with liver diseases. The present systematic review can be considered a useful publication to evaluate the real benefit of silymarin as commonly prescribed and used as a coadjutant in the treatment of liver diseases.
MATERIALS AND METHODS
Identification and selection of articles
This is a systematic review with a meta-analysis of randomized controlled trials evaluating the effect of silymarin in patients with liver disease, published by January 31, 2016. This review was carried out taking into account the provisions of PRISMA (Peferred Reposting Items for Systematic Reviews and Meta-Analyzes)[29]. We searched for randomized controlled trials in the Cochrane Central Register of Controlled Trails databases in the Cochrane Library, PubMed/Medline, Scopus, Web of Science, Lilacs and Clinical Trials. The research was conducted with no restrictions regarding the year of publication.
The terms “silybum marianum”, “milk thistle”, “silymarin”, “silybin”, “silibinin”, “silydianin”, “silychristin”, “cardus marianus”, “liver disease”, “chronic liver disease”, “end-stage liver disease”, “drug-induced liver injury”, “Non-alcoholic fatty liver disease”, “fatty liver”, “alcoholic fatty liver”, “alcoholic liver disease”, “fibrosis”, “liver cirrhosis, “Hepatocellular carcinoma”, “viral liver disease”, “hepatitis B”, “hepatitis C”, “hemochromatosis”, “liver steatosis”, “alcoholic hepatitis” and “chronic hepatitis” were searched in English, Portuguese and Spanish. All these keywords were combined using the Boolean operators “OR” and “AND” in several databases. The construction of the search strategy took into account the research question structured by the acronym PICO. Only the terms for the components Population, Intervention and Control had been defined. The terms for outcome ”O” were not defined to avoid assigning undesirable specificity at this stage of data collection[30].
Two reviewers independently carried out the active search of the scientific articles. The identified disagreements were evaluated and discussed by a third evaluator. The review team through the screening phase, reading the titles and abstracts, carried out a process of evaluation of the eligibility of the studies. Subsequently, reading the full text and identifying the duplicates in all databases described, the confirmation phase was performed. In this stage, the reason for the exclusion of each article was recorded in an article selection flow form. The third reviewer solved the disagreements between the former reviewers, regarding the eligibility of the articles.
Inclusion criteria
Randomized and controlled clinical trials with adult and elderly patients of both sexes with liver disease who took oral silymarin supplementation, as an extract or in its isolated form, as well as silymarin combined with other nutrients were included. We included studies in English, Portuguese and Spanish. The trials should provide information on the intervention such as doses and details of the product used, as well as mean and standard deviation of ALT, AST and γGT serum levels at baseline and at the end of the intervention.
Exclusion criteria
We excluded articles that reported the use of drugs associated with silymarin, did not provide descriptive data of the control or intervention group, used a crossover study design and also those who after contact, did not obtain answers from the authors to provide data not available in the articles. Studies using median and interquartile range with descriptive measures of outcome variables could not be included in the meta-analysis. Trials whose full accesses were not possible due to year of publication, or by online unavailability were also excluded.
Data extraction
Two reviewers independently reviewed eligible articles. For the data extraction process, the eligible articles were read in full and a standardized sheet was used for each article, with all the selection criteria established. The variables data in the baseline and at the end of the intervention were recorded in a spreadsheet in the Excel® program.
Evaluation of the methodological quality of articles
The methodological quality of the articles included in this review was evaluated according to the adapted Downs and Black[31] checklist. This checklist evaluates criteria such as description of the information in the studies, items to analyze external validity, items referring to participants, intervention and statistical tests, besides the internal validity, confounding factors and possible selection biases and the power of the studies.
The articles were evaluated based on the following criteria: (1) definition of the objectives/hypothesis; (2) description of results; (3) characterization of participants included in the study; (4) description of the exposure; (5) quality of the description of the main results; (6) reports of 95% confidence intervals and/or P value for the main outcomes; (7) representativeness of individuals invited to participate in the study; (8) representativeness of the individuals included in the research; (9) clarity if any of the studies were post hoc-based; (10) appropriate use of statistical tests to evaluate the main results; (11) validity and reliability of measures of the main outcomes; and (12) whether the statistical analysis includes adequate adjustment for the main confounding variables.
Clinical trials were also evaluated according to the following items: (1) information on the characteristics of the loss of follow-up; (2) analysis adjusted for different follow-up times; (3) whether participants in the intervention and control groups were recruited from the same population; (4) whether participants in the intervention and control groups were recruited within the same time period; (5) reporting blinding of the intervention to participants and evaluators; and (6) whether follow-up losses were considered.
In order to evaluate the quality, a dichotomous response was defined as “yes”, with a score of 1, or “no”, with a score of 0, for each item in the checklist. At the end, a sum of the scores and the percentage for each publication was calculated. The percentage of ideal methodological quality was equal to or greater than 80%, according to Downs and Black[31].
The risk of bias in the studies was assessed according to the criteria of the Cochrane Collaboration for the development of systematic reviews of intervention[32]. It was not possible to assess publication bias by the Funnel plot and test its asymmetry by the Egger’s test because of the small number of included studies.
Statistical analysis
For the data extraction process, the eligible articles were read in full and a standardized clinical record was used for each article, with all the selection criteria established. The data referring to the descriptive measures of the outcome variables at the baseline and at the end of the intervention were recorded in an Excel® worksheet.
The summary measure used in this meta-analysis was the difference of standardized means among the groups for each indicator evaluated (ALT, AST and γGT) and their respective confidence intervals, which were presented in Forest plot charts. The difference of global standard means was calculated using the random effects model, due to the high heterogeneity of the studies. The assumption of the homogeneity of the studies was tested by the extent of the heterogeneity interpreted by the total percentage of variation between the studies analyzed with the I2 statistic (Higgins inconsistency test). This test of inconsistency greater than 50% was used as an indicator of moderate heterogeneity[33]. The statistical methods of this study were reviewed by Priscila Costa from the School of Nutrition of the Federal University of Bahia, Brazil.
Subgroup analyzes were also performed according to the type of intervention (isolated silymarin or silymarin associated with nutrients) and the intervention time (≥ 6 mo or < 6 mo) to identify possible differences. The heterogeneity of the meta-analysis was evaluated by meta-regression and the influence of the variables: sample size, treatment time and type of intervention were tested. In all analyzes, a P-value less than 0.05 was considered significant.
Statistical analysis was performed using the STATA Program for MAC, version 12 (Stata Corp. College Sattion, TX, United States).
RESULTS
Selection of studies
The electronic search identified 10904 publications, excluding 207 duplicates and 10413 articles by reading the title and abstracts, totaling 284 eligible studies for in-depth analysis. A total of 267 articles were excluded due to issues such as: impossibility of access to the full article (n = 68), non-randomization (n = 104), uncontrolled clinical trials (n = 80), crossover design (n = 1), medication-associated intervention (n = 4), absence of biochemical markers (AST, ALT and γGT) after intervention (n = 2) and absence of Data from the control group (n = 3). Thus, the systematic review was performed with 17 publications and of these, only 6 were included in the meta-analysis (Figure 1), since 5 of them used the median as descriptive measure and 6 had no descriptive data necessary for the analysis.
Figure 1.
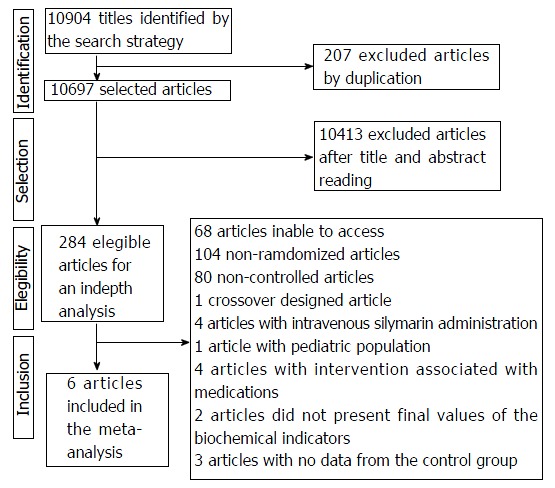
Flow of selection of articles included in the meta-analysis.
Characteristics of the studies
Table 1 presents the main characteristics of the studies and patients included in the systematic review. Seven studies[8,19,26,34-37] were performed in Europe, six[27,28,38-41] in Asia, three[42-44] in Africa and one in America[45]. The year of publication varied from 1994 to 2016. The sample size varied from 30 to 370 individuals, totalizing 1558 adults and elderly, of both sexes. The studies evaluated drug-induced hepatic injury[8,40,41], with C virus[43,44,45], individuals with acute hepatitis[42], NAFLD and of these studies, three articles[28,38,39] included patients diagnosed with NASH and one[35] evaluated individuals with DHGNA and metabolic syndrome , being a pilot article[19].
Table 1.
Summary of clinical trial characteristics
| Study | Year | Origin | Population | Silymarin dose | Intervention | Inclusion criteria | Follow-up | Outcomes |
| Loguercio et al[19] | 2007 | Italy | 59 adult patients with NAFLD | 4 × 94 mg silibin + 194 mg phosphatidylcholine + 90 mg vitamin E (Reasil®) daily | Silymarin | NAFLD with no chronic liver disease | 6 mo and 12 mo | ALT, gGT, insulin and HOMA |
| Control untreated (diet + physical activity) | ||||||||
| Hashemi et al[38] | 2009 | Iran | 100 adult patients with NAFLD (NASH) | 2 × 140 mg silymarin (Livergol®) daily | Silymarin | USG evidencing steatosis, ALT elevation in more than 1.2 of the normal value, exclusion of conical diseases of the liver, histological evidence of NASH or presence of risk factor such as MD or obesity | 6 mo | ALT, AST, gGT, FA, glycemia, triglycerides and cholesterol |
| Control | ||||||||
| Massodi et al[39] | 2013 | Iran | 100 adult patients with NAFLD (NASH) | 2 × 140 mg silymarin daily | Silymarin | NASH confirmada por USG e níveis elevados de AST e ALT | 3 mo | AST and ALT |
| Control | ||||||||
| Solhi et al[28] | 2014 | Iran | 64 adult patients with NAFLD (NASH) | 3 × 70 mg silymarin (Livergol®) daily | Silymarin | NASH confirmada por USG abdominal e elevação persistente de AST e ALT mais de 1,2 acima do valor normal nos últimos 6 meses | 8 wk | ALT and AST |
| Control | ||||||||
| Aller et al[34] | 2015 | Spain | 36 adult patients with NAFLD | 2 × Silybum marianum 540.3 mg + vitamin E - 36 mg (Eurosil 85®) daily | Silymarin | NAFLD confirmed by liver biopsy | 3 mo | Glycemia, triglycerides, AST, ALT, gGT and HOMA IR |
| Control untreated (diet + physical activity) | ||||||||
| Sorrentino et al[35] | 2015 | Italy | 78 adults with MS and NAFLD | 2 × silymarin 210 mg+ 30 IU vitamin E (Eurosil 85®) daily | Silymarin | MS and NAFD confirmed by USG | 3 mo | Hepatic steatosis, lipid accumulation index, ALT, AST, gGT, triglycerides, cholesterol, LDL, HDL, glycated Hb and Glycemia |
| Control untreated (diet) | ||||||||
| Abenavoli et al[36] | 2015 | Italy | 30 overweight Caucasian adults with NAFLD | 2 × Silibin 94 mg + phosphatidylcholine 194 mg + vitamin E 89.28 mg daily | Group A: Hypochloric diet | Overweight and NAFLD confirmed by USG | 6 mo | BMI, weight, waist circumference, blood pressure, AST, ALT, gGT, bilirubin, glycemia, HOMA-IR, insulin, triglycerides, total cholesterol, HDL, LDL, creatinine, azotemia, hepatic steatosis index |
| Group B: Diet + silymarin | ||||||||
| Group C: control | ||||||||
| Luangchosiri et al[40] | 2015 | Thailand | 55 adults and elderly with pulmonary tuberculosis | 3 × silymarin 140 mg daily | Silymarin | Diagnosis of pulmonary tuberculosis, > 18 yr, treatment with anti-tuberculosis drugs | 4 wk | ALT, AST, alkaline phosphatase, gGT, total proteins, albumin, bilirubin, SOD, glutathione, malonyldialdehyde, risk of hepatic injury by anti-tuberculosis drug, adverse events |
| Control | ||||||||
| El-Kamary et al[42] | 2009 | Egypt | 105 adults with acute hepatitis of varied etiologies | 3 × silymarin 140 mg daily (Legalon®) | Silymarin | ALT > 100 IU/L with jaundice and 3 or more symptoms of acute hepatitis | 8 wk | ALT, AST, bilirubin, acute hepatitis symptoms, adverse events |
| Control (multivitamin) | ||||||||
| Fried et al[45] | 2012 | United States | 154 adults with HCV | 5 × silymarin 140 mg daily (Legalon®) - 700 mg | Group 1: silymarin 420 mg | HCV and ALT > 65 U/L or unsuccessful patients on interferon therapy | 24 wk | ALT, RNA HCV |
| 3 × silymarin 140mg daily (Legalon®) - 420 mg | Group 2: silymarin 700 mg | |||||||
| Group 3: control | ||||||||
| Hajaghamohammadi et al[27] | 2008 | Iran | 50 adults with NAFLD | 1 × 140 mg silymarin (Livergol®) daily | NAFLD confirmed by USG and elevated levels of ALT and AST | 2 mo | Weight, BMI, AST, ALT | |
| Stiuso et al[37] | 2014 | Italy | 30 adults with NASH | 2 × 94 mg silibin + 194 mg phosphatidylcholine + 89.28 mg vitamin E (Reasil®) daily | Silymarin | NASH histologically confirmed | 12 mo | Levels of substances that react with thiobarbituric acid, nitric oxide, SOD, catalase, BMI, glycemia, insulin, HOMA, AST, ALT, gGT, score for NAFLD |
| Control | ||||||||
| Velussi et al[26] | 1997 | Italy | 60 diabetic adults and elderly with alcoholic cirrhosis | 600 mg siymarin daily | Silymarin | Diabetics treated with insulin with alcoholic cirrhosis (biopsy), aged between 45 and 70 years old | 12 mo | Glycemia, postprandial glycemia, glycated hemoglobin and malonildialdehyde |
| Control | ||||||||
| Yakoot et al[43] | 2012 | Egypt | 66 adult and elderly patients with HCV genotype 4 | 3 × silymarin 140mg daily | Group 1: spirulina 500 mg | HCV genotype 4, elevated liver enzymes, virgin antiviral therapy | 6 mo | Virological response, ALT, quality of life score, adverse events |
| Group 2: silymarin | ||||||||
| Group 3: control | ||||||||
| Zhang et al[41] | 2015 | China | 370 adult patients with tuberculosis on antituberculosis therapy | 2 × S. marianum 200 mg | Silymarin | > 12 yr with tuberculosis and in anti-tuberculosis therapy | 8 wk | ALT, AST, bilirubin, gGT, alkaline phosphatase |
| Control | ||||||||
| Tanamly et al[44] | 2004 | Egypt | 141 adults and elderly with HCV | 3 × silymarin 140 mg daily (Legalon®) | Silynarin | HCV | 12 mo | RNA HCV, ALT, fibrosis, adverse events |
| Control (multivitamin) | ||||||||
| Palasciano et al[8] | 1994 | Italy | 60 adult women using psychotic drugs | 2 × 400 mg sliymarin daily | Group 1A: drugs + silymarin | Women between 40 and 60 yr of age, treated with phenothiazines and/or butyrenes for at least 5 yr, AST or ALT with values 2 × higher than the regular range | 3 mo | AST, ALT, gGT, malonildialdehyde, bilirubin |
| Group 1B: drugs + control | ||||||||
| Group 2A: no drugs and with silymarin | ||||||||
| Group 2B: no drugs but control |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; γGT: Gamma glutamyl transpeptidase; NAFLD: Non-alcoholic fatty liver disease; HCV: Hepatitis C virus.
The work of Loguercio et al[19] presented a subgroup with patients with hepatitis C virus, but it was decided not to include this subgroup in the analyzes, since it was considered impracticable to analyze this small number of patients. The duration of follow-up ranged from 4 wk to 12 mo, the dose of oral silymarin used was 210 mg to 700 mg and the frequency of ingestion was two to five times a day. Four studies[8,39,40,45] reported blinding, describing methodological design as double-blind. Twelve studies[8,26-28,38-45] used only dry extract of Silybum marianum, which contains silymarin or silymarin alone, two[34,35] used silymarin associated with vitamin E and three[19,36,37] studies used silybin with vitamin E and phosphatidylcholine. All articles evaluated ALT, however the study by Velussi et al[26] only evaluated liver enzymes in the baseline, four[16,43-45] did not evaluate AST and eight[27-29,36,39,42-44] did not evaluate γGT. Six studies[26-28,36,37,39] did not report data on adverse effects, six[8,19,34,35,41,45] did not identify any of these effects, four[40-43] performed specific evaluation and only one[39] described that serious adverse events were not observed and that side effects were similar in frequency and uncommon in both groups.
Evaluation of the methodological quality of the studies included in the meta-analysis
Among the 6 studies included in this meta-analysis, only one[39] was double-blind and reported the randomization method used. No intention-to-treat analyzes were described in the studies evaluated. Only one study[39] presented methodological adequacy (92.5%), higher, therefore, to 80% according to the checklist score adapted from Downs and Black[31]. The main limitations observed in the studies were: (1) absence in the description of the characterization of participants with loss of follow-up[19,34,35]; (2) failure to report blinding for the intervention of the participants and evaluators[19,28,34,35,38]; (3) no adjusted analyzes were performed for different follow-up times[19,25,28,34,35,38]; (4) randomization was not concealed for patients and staff until complete recruitment[19,28,34,35,38]; and (5) absence of adequate adjustments for confounding factors in the analyzes of which the main findings were withdrawn[19,28,34,35,38,39].
Risk analysis of bias
Fungal plot analysis and the Egger test were not performed since these are recommended for meta-analyzes with at least 10 studies and are not indicated for this study[32].
A bias risk assessment of randomized controlled trials was performed according to the Cochrane Collaboration criteria[32] for the development of systematic reviews of intervention (Figure 1). There was a high risk of bias in relation to the blinding of the participants and the researcher, since only one study was double-blind[39] and reports on allocation, blinding of outcome evaluation and other potential biases were not well understood in the studies evaluated. There was a low risk of bias for selective information[28,38,39] and random sequence generation in half of the studies analyzed[28,34,39] (Figure 2). Only studies by Massodi et al[39] and Solhi et al[28] presented half of the items assessed as low risk for bias (Figure 2).
Figure 2.
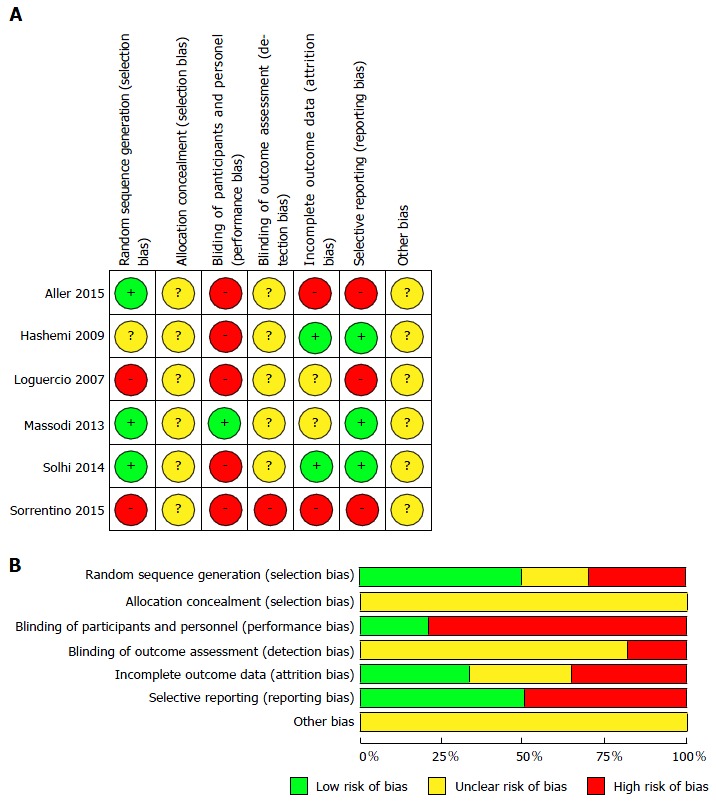
Risk of bias assessment. A: Risk of bias summary: review authors’ judgments about each risk of bias item for each included study; B: Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Meta-analysis results
The results of the meta-analysis are shown in Table 2, Figures 3, 4, 5, 6, 7 and 8. This meta-analysis included 437 individuals. All articles evaluated ALT levels. One study had no AST levels measurements and only three[19,34,35] had γGT dosages. The included studies evaluated only patients with NAFLD and publications evaluating other liver diseases were naturally excluded in the screening and confirmation stages of eligible articles. However, the work of Loguercio et al[19] presented a subgroup with patients with HCV, but it was decided not to include these patients in this meta-analysis. In the groups treated with silymarin, four studies[19,28,38,39] observed a significant reduction in serum ALT levels, three[28,38,39] showed a significant reduction in AST and only one[19] observed a significant decrease in γGT serum levels.
Table 2.
Results of selected studies for meta-analysis
| Ref. | Used Indicators | Results |
| Loguercio et al[19], 2007 | ALT, γGT | There were no adverse events in either group. The intervention group presented a significant reduction of hepatic steatosis in the ultrasonography score (change from 2-3 to 1-2) after 6 mo and 12 mo (P < 0.01). Significant reduction of ALT and γGT after 6 mo and 12 mo only in the intervention group (P < 0.01). Treatment affected the levels of ALT and γGT Range independent of changes in BMI of the participants. We did not evaluate data from the group with HCV patients |
| Hashemi et al[38], 2009 | ALT, AST | There was a significant reduction in the average of ALT levels only in the intervention group (113.54 IU/mL vs 73.14 IU/mL) (P < 0.001). The percentage of patients with normalization (ALT < 40) was 32% after 3 mo and 52% after 6 mo in the intervention group and the difference in these percentage between control and intervention group was significant (P = 0.001). There was also a significant reduction in AST averages only in the intervention group (71.42 IU/mL vs 49.66 IU/mL) (P = 0.006). The percentage of patients with normalization (AST < 40) was 46% after 3 mo and 62% after 6 mo in the intervention group and the difference in these percentages between control group and intervention was also significant (P = 0.0001) |
| Massodi et al[39], 2013 | ALT, AST | There were no serious adverse events and the side effects were similar in frequency and uncommon in both groups. There was a significant reduction in the average of ALT levels only in the intervention group (84.06 IU/mL vs 68.54 IU/mL) (P < 0.001) and in the average AST levels only in the intervention group (71.94 IU/mL vs 54.70 IU/mL) (P < 0.001) |
| Solhi et al[28], 2014 | ALT, AST | There was a significant difference in the mean values of ALT levels only in the intervention group (91.3 IU/mL vs 38.4 IU/mL) (P = 0.026) and in the AST levels only in the intervention group (62.8 IU/mL vs 30.5 IU/mL) (P = 0.038). |
| Aller et al[34], 2015 | ALT, AST, γGT | There were no adverse events in both groups. There was a significant improvement in the fibrosis score in both groups (P < 0.05). There was a significant difference in the reduction of the average γGT levels (81.5 IU/L vs 46.2 IU/L) (P < 0.05) in the intervention group and also in the control group (80.5 IU/L vs 50.3 IU/L) (P < 0.05). There was a significant reduction only in the average of ALT levels (70.8 IU/L vs 54.7 IU/L) (P < 0.05) and AST (41.6 IU/L vs 36 IU/L) (P < 0.05) in the control group. |
| Sorrentino et al[35], 2015 | ALT, AST, γGT | No adverse events were reported in both groups. Mean levels of ALT, AST and γGT were within normal limits at the baseline. There was a significant reduction only in the average values of right lobe size of the liver by the USG (17.24 cm vs -0.96 cm) (P = 0.044) |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; γGT: Gamma glutamyl transpeptidase.
Figure 3.
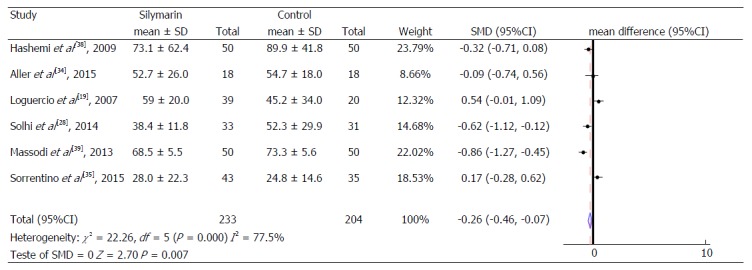
Alanine aminotransferase levels.
Figure 4.
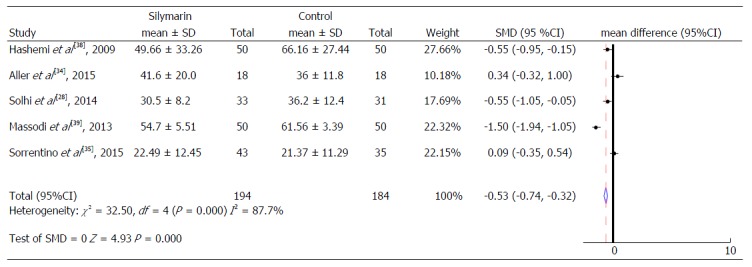
Aspartate aminotransferase levels.
Figure 5.
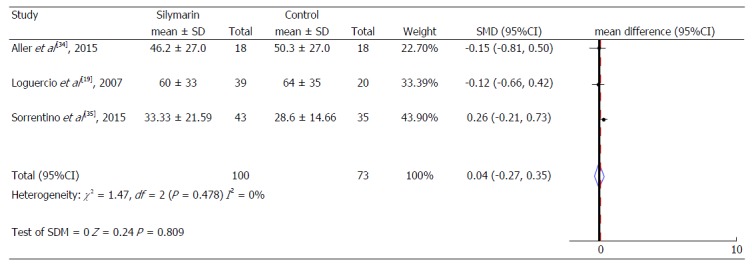
Gamma glutamyl transpeptidase levels.
Figure 6.
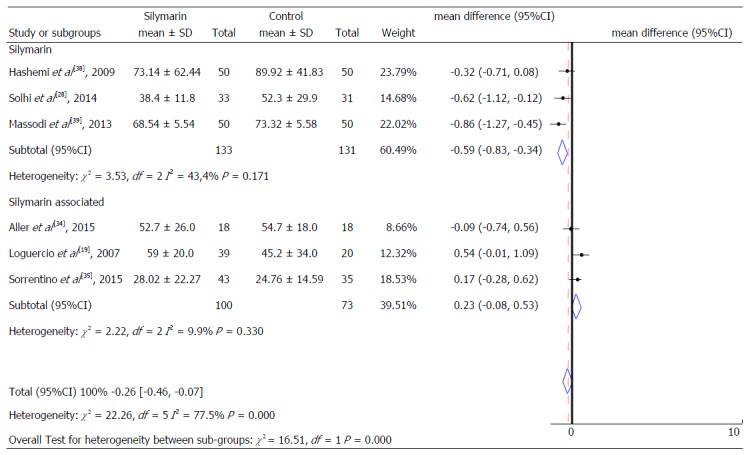
Alanine aminotransferase levels according to the type of product used.
Figure 7.
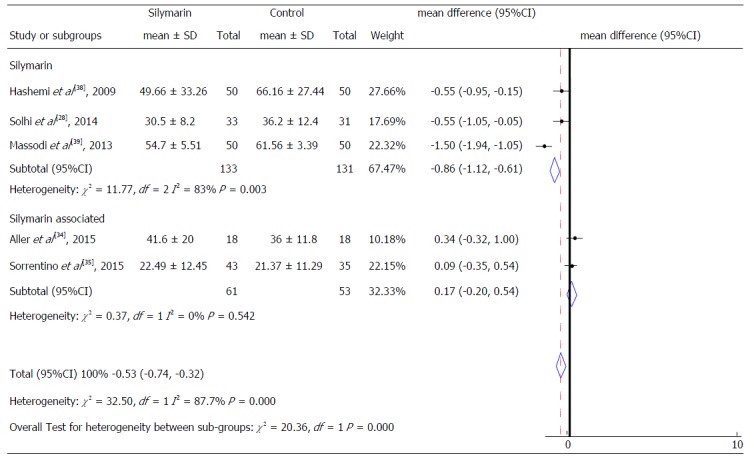
Aspartate aminotransferase levels according to the type of product used.
Figure 8.
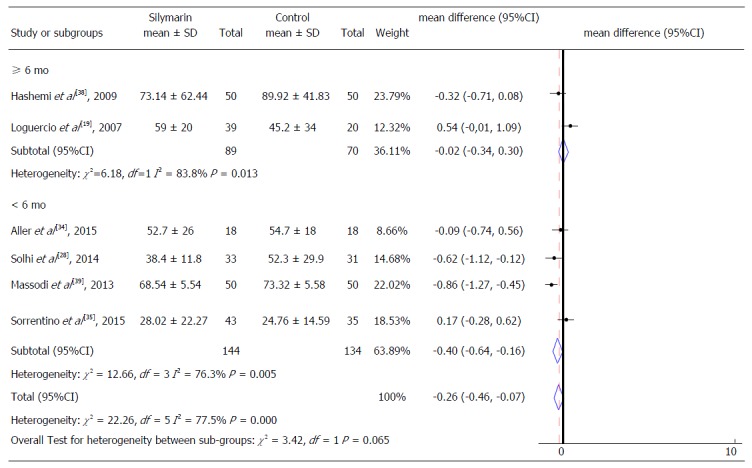
Alanine aminotransferase levels according to intervention time.
Thus, when the intervention groups were compared with the control groups of all studies included in the meta-analysis, a reduction of 0.26 IU/mL (95%CI: -0.46-0.07) was observed in the mean ALT serum values and a reduction of 0.53 IU/mL (95%CI: -0.74-0.32) in the mean AST serum values of the treated group, compared to the control group (Figure 4), both of which are statistically significant. No significant change in the Gamma γGT serum levels was identified (Figure 5).
A subgroup analyses was also performed to identify possible differences in relation to intervention characteristics. We considered as subgroups different studies that performed intervention with isolated silymarin[28,38,39] or silymarin associated with other nutrients[19,34,35], as well as the studies that presented different follow-up time (equal or superior to 6 mo and less than 6 mo) for both ALT and AST serum levels (Figures 6-8). It was not possible to consider these subgroups for the evaluation of γGT and for the AST levels regarding the intervention time due to the insufficient number of studies to enable these analyzes.
When comparing control and treatment groups, a reduction trend of 0.59 IU/mL (95%CI: -0.83-0.34) was found in the mean ALT serum values of subjects treated with isolated silymarin and 0.23 IU/mL in this same marker (95%CI: -0.08-0.53), in those treated with silymarin associated with other nutrients. However, there was no statistical significance (Figure 6). Therefore, no significant differences were observed in these forms of intervention (isolated or associated silymarin).
Analysis of the mean AST serum values showed a reduction of 0.86 IU/mL (95%CI: -1.12 to -0.61, P = 0.003) in subjects treated with isolated silymarin (Figure 7). These results also did not present significant differences between the types of intervention, similar to the analysis referring to ALT levels. Likewise, the assessment of intervention time subgroups and ALT levels did not show significant differences between them (Figure 8).
Heterogeneity and meta-regression
The present study observed that the studies evaluated presented a high degree of heterogeneity, with an inconsistency test (I2) greater than 50%. Two meta-regressions were performed, one having ALT as the outcome and another for AST. It was not possible to perform meta-regression for γGT, considering the small number of studies. In the first meta-regression, the sample size (P = 0.901), treatment time (P = 0.233) and type of intervention (isolated silymarin and associated silymarin) (P = 0.143) did not explain the heterogeneity between the studies. Likewise, in the second meta-regression, the sample size (P = 0.941), treatment time (P = 0.163) and type of intervention (P = 0.089) also failed to explain the heterogeneity of the studies (data not shown in tables).
DISCUSSION
In this review, some intervention studies have observed an improvement in the biochemical and clinical indicators evaluated in patients with NAFLD, including hepatic steatosis and NASH, after the use of silymarin. Although the results of the meta-analysis indicate that the use of silymarin is associated with a reduction in serum levels of ALT and AST, the values found are not clinically relevant. The studies[19,34,35,39] also showed limited adverse effects and good tolerance to the use of silymarin as reported in other studies[10-12].
Some studies report that silymarin is capable to improving biochemical indicators in patients with liver diseases of different etiologies[40,46-50], in addition to the reduction of ALT and AST levels are commonly described in other studies[48,49,51,52]. The hypothesis described by the researchers is that the antioxidant properties of silymarin are capable of reducing reactive oxygen species, thus inhibiting cellular damage[53]. In addition to the improvement in the antioxidant system, observed in experimental studies, due to the increase of enzymes such as glutathione reductase, glutathione peroxidase, superoxide dismutase and catalase, all with antioxidant function[13,16,54] and non-enzymatic antioxidants, through the modulation of associated transcription factors[55,56]. On the other hand, there are reports of similar studies that, despite showing differences in the values of these indicators, these were not statistically significant[45,57].
It is important to emphasize that there are a few trials with rigorous methodologies that consider important issues such as the use of well-characterized products, evaluation of specific liver diseases, adequate sample size, representativeness of the study population, adequate intervention time and appropriate statistical analysis. These factors are quite divergent among the studies, which may directly interfere both in the positive results and in the controversial findings, representing an important limitation for conclusions on this topic.
It was identified in this meta-analysis that a clinical trial[35] that found normal values of ALT, AST and γGT in the baseline, which is not surprising, since some patients may be carriers of NAFLD and do not present alterations in liver enzyme levels[58-60]. Thus, in the aforementioned study[35], there was no relevance in the results of these markers after intervention, since in the baseline; the patients no longer presented alteration in these markers. It was also found that another clinical trial[34] included in this analysis demonstrated a significant reduction of γGT in the control and intervention group, probably due to differences in the methodological design used. In this study, both groups had prescriptions for hypocaloric diet and physical activity, which probably influenced the clinical and biochemical parameters of patients with NAFLD.
Studies have associated high levels of ALT or the AST:ALT ratio > 1 in patients with NAFLD and with disease progression and the presence of hepatocellular fibrosis[61-63]. Several publications[64-66] have been considered to change lifestyle with dietary intervention and physical activity practice, as strategies with an impact on the improvement of markers and liver function for individuals with NAFLD. Despite this, it was observed that there are still more data available in the literature regarding the pattern of adherence of this profile of patients to lifestyle changes and nutritional guidelines provided by health professionals. It is also important to consider the growing increase in the prevalence of NAFLD in recent years and is even considered a global public health problem[1-3]. Considering this scenario, researchers have investigated adjuvant therapeutic strategies, such as phytotherapy and considered the use of silymarin as a possibility to improve biochemical indicators of these patients[28,38,39]. However, the available studies present low methodological quality and the positive results found are not of clinical relevance, as found in this meta-analysis. Therefore, there is still insufficient scientific evidence for the recommendation of silymarin as a possibility of adjunctive therapeutic alternatives for the reduction of biochemical indicators in patients with hepatic disease.
It is important to highlight that the inconsistency tests performed in this meta-analysis showed that the studies evaluated presented a high degree of heterogeneity, which is generally present in meta-analyzes involving clinical trials[30], especially when evaluating such specific topics and presenting few studies with well-designed methodological designs. The case of phytotherapy and specifically, the use of silymarin. In addition, details of intervention, blinding, selection and recruitment of the population and absence of adjustments in the statistical analyzes may be factors that interfere in the final results, as well as the high and medium risk of bias observed in the studies.
In this meta-analysis, trials with small samples and, therefore, little representativeness of the population were identified, which may have favored the high heterogeneity, since studies with larger samples provide greater precision in the association. Absence of intention-to-treat analyzes in the studies can also be considered factors that interfered in the final results and conclusions. Another relevant methodological factor refers to the blinding of the studies evaluated: only one is double-blind, representing another inconsistency of the studies evaluated. Although meta-regression did not identify interference with sample size, time of treatment and type of intervention in the results, it’s considered that these results might have been strongly influenced by the low methodological quality, observed in all studies, in general, according to the used methods.
In conclusion, the results of this meta-analysis demonstrate that the use of silymarin minimally reduced, but without clinical relevance, the ALT and AST serum levels in patients with non-alcoholic fatty liver disease. Although the reductions observed do not translate into clinical relevance, they may signal to a possible additional therapeutic strategy in the control of NAFLD. When discussing the data found, it is important to consider the great variability and methodological fragility of these studies, a finding very common in publications that evaluate herbal medicines. Therefore, it is necessary to carry out new studies with more adequate methodological designs, with special attention in the accomplishment of the planning stages and execution of clinical trials. This will provide more consolidated scientific evidence and may contribute to a greater safety in the indication or not of doses of silymarin to be prescribed by qualified health professionals.
COMMENTS
Background
The use of phytotherapic medicines is very common and, for many individuals, represents a simple and easily accessible therapeutic option. Despite the use of silymarin in liver diseases be described as millenarian and prescribed by many professionals, there is still controversy in the literature about its real effects on biochemical indicators in patients with liver diseases.
Research frontiers
Chronic liver disease is one of the main causes of morbidity and mortality in the world. High levels of indicators, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma glutamyl transpeptidase (γGT), have been associated with progression of these diseases. Researchers have investigated supporting therapeutic strategies such as phytotherapy and discussed the use of silymarin as a possibility to improve the biochemical indicators of these patients.
Innovations and breakthroughs
In the present study, the authors investigated the effect of the use of silymarin on ALT, AST and γGT levels in patients with liver diseases. This is the first meta-analysis which evaluates the effect of oral use of silymarin on biochemical indicators of patients with liver disease and the methodological quality of the included studies.
Applications
This study allows us to understand the real effects of silymarin on ALT, AST and γGT levels, from patients with liver diseases, in addition to signaling to the need of new clinical trials with more appropriate methodological designs.
Peer-review
This manuscript describes the results of a meta-analysis evaluating effect of silymarin on the serum levels of ALT, AST and GGT in patients with liver diseases. Silymarin has been used in several studies of liver diseases for its hepatoprotective effects. Consequently, this systematic review with meta-analysis evaluating large majority of the literature is crucial to be understood of its actual effectiveness.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors deny any conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: February 8, 2017
First decision: March 3, 2017
Article in press: July 19, 2017
P- Reviewer: Cengiz M, Dabak DO, Sharaf IA S- Editor: Gong ZM L- Editor: A E- Editor: Wang S
References
- 1.Freitas LAR, Freitas JR. Alterações Histológicas nas Doenças Crônicas do Fígado. (Eds) Nutrição e Hepatologia: abordagem terapêutica, clínica e cirúrgica. In: Jesus, RP, Oliveira, LPM, Lyra, et al., editors. Rio de Janeiro: Rubbio; 2014. pp. 1–17. [Google Scholar]
- 2.Méndez-Sánchez N, Villa AR, Chávez-Tapia NC, Ponciano-Rodriguez G, Almeda-Valdés P, González D, Uribe M. Trends in liver disease prevalence in Mexico from 2005 to 2050 through mortality data. Ann Hepatol. 2005;4:52–55. [PubMed] [Google Scholar]
- 3.Roriz AKC, Oliveira LPM, Boulhosa RSSB, Oliveira, TM, Araújo, ANM . Avaliação e Diagnóstico Nutricionais das Doenças Crônicas do Fígado. Nutrição e Hepatologia: abordagem terapêutica, clínica e cirúrgica. In: Jesus RP, Oliveira LPM, Lyra LGC, et al., editors. Rio de Janeiro: Rubbio; 2014. pp. 93–112. [Google Scholar]
- 4.Passos, RP, Rios CS, Casé NA, Avelar CR, Cardoso RM, Borges FM. Rio de Janeiro: Rubbio; 2014. Fitoterapia em Hepatologia. Jesus RP, Oliveira LPM, Lyra LGC Nutrição e Hepatologia: abordagem terapêutica, clínica e cirúrgica; pp. 383–406. [Google Scholar]
- 5.Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med. 2002;113:506–515. doi: 10.1016/s0002-9343(02)01244-5. [DOI] [PubMed] [Google Scholar]
- 6.Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos. 2008;36:1909–1916. doi: 10.1124/dmd.107.019604. [DOI] [PubMed] [Google Scholar]
- 7.Tănăsescu C, Petrea S, Băldescu R, Macarie E, Chiriloiu C, Purice S. Use of the Romanian product Silimarina in the treatment of chronic liver diseases. Med Interne. 1988;26:311–322. [PubMed] [Google Scholar]
- 8.Palasciano G, Portincasa P, Palmieri V, Ciani D, Gianluigi V, Altomare E. The effect of silymarin on plasma levels of malon-dialdehyde in patients receiving longterm treatment with psychotropic drugs. Curr Ther Res-Clin Exp. 1994;55:537–545. [Google Scholar]
- 9.Parés A, Planas R, Torres M, Caballería J, Viver JM, Acero D, Panés J, Rigau J, Santos J, Rodés J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 10.Rainone F. Milk thistle. Am Fam Physician. 2005;72:1285–1288. [PubMed] [Google Scholar]
- 11.Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15:9–20. doi: 10.1159/000113648. [DOI] [PubMed] [Google Scholar]
- 12.Milosević N, Milanović M, Abenavoli L, Milić N. Phytotherapy and NAFLD--from goals and challenges to clinical practice. Rev Recent Clin Trials. 2014;9:195–203. [PubMed] [Google Scholar]
- 13.Fehér J, Láng I, Nékám K, Müzes G, Deák G. Effect of free radical scavengers on superoxide dismutase (SOD) enzyme in patients with alcoholic cirrhosis. Acta Med Hung. 1988;45:265–276. [PubMed] [Google Scholar]
- 14.Muriel P, Mourelle M. Prevention by silymarin of membrane alterations in acute CCl4 liver damage. J Appl Toxicol. 1990;10:275–279. doi: 10.1002/jat.2550100408. [DOI] [PubMed] [Google Scholar]
- 15.Mourelle M, Muriel P, Favari L, Franco T. Prevention of CCL4-induced liver cirrhosis by silymarin. Fundam Clin Pharmacol. 1989;3:183–191. doi: 10.1111/j.1472-8206.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- 17.Köksal E, Gülçin I, Beyza S, Sarikaya O, Bursal E. In vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem. 2009;24:395–405. doi: 10.1080/14756360802188081. [DOI] [PubMed] [Google Scholar]
- 18.Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- 19.Loguercio C, Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Niosi M, D’Auria MV, Capasso R, Del Vecchio Blanco C. The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:2387–2395. doi: 10.1007/s10620-006-9703-2. [DOI] [PubMed] [Google Scholar]
- 20.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24:1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 21.Abenavoli L. Role of silymarin to treat fibrosis development in non-alcoholic fatty liver disease. Hepatol Res. 2011;41:668. doi: 10.1111/j.1872-034X.2011.00823.x. [DOI] [PubMed] [Google Scholar]
- 22.Salamone F, Galvano F, Cappello F, Mangiameli A, Barbagallo I, Li Volti G. Silibinin modulates lipid homeostasis and inhibits nuclear factor kappa B activation in experimental nonalcoholic steatohepatitis. Transl Res. 2012;159:477–486. doi: 10.1016/j.trsl.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Tuchweber B, Sieck R, Trost W. Prevention of silybin of phalloidin-induced acute hepatoxicity. Toxicol Appl Pharmacol. 1979;51:265–275. doi: 10.1016/0041-008x(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 24.Faulstich H, Jahn W, Wieland T. Silybin inhibition of amatoxin uptake in the perfused rat liver. Arzneimittelforschung. 1980;30:452–454. [PubMed] [Google Scholar]
- 25.Mateen S, Raina K, Agarwal R. Chemopreventive and anti-cancer efficacy of silibinin against growth and progression of lung cancer. Nutr Cancer. 2013;65(Suppl 1):3–11. doi: 10.1080/01635581.2013.785004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26:871–879. doi: 10.1016/s0168-8278(97)80255-3. [DOI] [PubMed] [Google Scholar]
- 27.Hajaghamohammadi AA, Ziaee A, Raflei R. The efficacy of silymarin in decreasing transaminase activities in nonalcoholic fatty liver disease. A randomized controlled clinical trail. Hepat Mon. 2008;8:191–195. [Google Scholar]
- 28.Solhi H, Ghahremani R, Kazemifar AM, Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: A randomized clinical trial. Caspian J Intern Med. 2014;5:9–12. [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 30.de Saúde M. Diretrizes metodológicas: elaboração de revisão sistêmica e metánalise de ensaios clínicos randomizado 2012. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes metodologicas elaboracao sistematica.pdf.
- 31.Serum oxidative stress markers and lipidomic profile to detect NASH patients responsive to an antioxidant treatment: a pilot study. Oxid Med Cell Longev. 2014;2014:169216. doi: 10.1155/2014/169216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0, The Cochrane Collaboration. Available from: http://handbook.cochrane,org/ [Google Scholar]
- 33.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 34.Aller R, Izaola O, Gómez S, Tafur C, González G, Berroa E, Mora N, González JM, de Luis DA. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015;19:3118–3124. [PubMed] [Google Scholar]
- 35.Sorrentino G, Crispino P, Coppola D, De Stefano G. Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs R D. 2015;15:21–25. doi: 10.1007/s40268-015-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abenavoli L, Greco M, Nazionale I, Peta V, Milic N, Accattato F, Foti D, Gulletta E, Luzza F. Effects of Mediterranean diet supplemented with silybin-vitamin E-phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:519–527. [Google Scholar]
- 37.Stiuso P, Scognamiglio I, Murolo M, Ferranti P, Simone C, Rizzo MR, Tuccillo C, Caraglia M, Loguercio C, Federico A. Serum oxidative stress Markers and Lipidomic profile to detect NASH Patients Responsive to an antioxidant Treatment: A Pilot Study. Oxidative Medicine and Cellular Longevity. 2014:1–8. doi: 10.1155/2014/169216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashemi SJ, Hajiani E, Sardabi EH. A placebo-controlled Trial of Silymarin in Patients with Nonalcoholic Fatty Liver Disease. Hepat Mon. 2009;9:265–270. [Google Scholar]
- 39.Massodi M, Rezadoost A, Panahian M, Vojdanian M. Effects of silymarin on reducing liver aminotransferases in patients with nonalcoholic fatty liver diseases. Govaresh. 2013;18:181–185. [Google Scholar]
- 40.Luangchosiri C, Thakkinstian A, Chitphuk S, Stitchantrakul W, Petraksa S, Sobhonslidsuk A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 2015;15:334. doi: 10.1186/s12906-015-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Pan H, Peng X, Lu H, Fan H, Zheng X, Xu G, Wang M, Wang J. Preventive use of a hepatoprotectant against anti-tuberculosis drug-induced liver injury: A randomized controlled trial. J Gastroenterol Hepatol. 2016;31:409–416. doi: 10.1111/jgh.13070. [DOI] [PubMed] [Google Scholar]
- 42.El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, Metwally M, Mikhail N, Hashem M, Mousa A, Aboul-Fotouh A, et al. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine. 2009;16:391–400. doi: 10.1016/j.phymed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yakoot M, Salem A. Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. A pilot randomized, comparative clinical trial. BMC Gastroenterol. 2012;12:32. doi: 10.1186/1471-230X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanamly MD, Tadros F, Labeeb S, Makld H, Shehata M, Mikhail N, Abdel-Hamid M, Shehata M, Abu-Baki L, Medhat A, et al. Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: study description and 12-month results. Dig Liver Dis. 2004;36:752–759. doi: 10.1016/j.dld.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, Meyers CM, Reddy KR. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA. 2012;308:274–282. doi: 10.1001/jama.2012.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bares JM, Berger J, Nelson JE, Messner DJ, Schildt S, Standish LJ, Kowdley KV. Silybin treatment is associated with reduction in serum ferritin in patients with chronic hepatitis C. J Clin Gastroenterol. 2008;42:937–944. doi: 10.1097/MCG.0b013e31815cff36. [DOI] [PubMed] [Google Scholar]
- 47.Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJ, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275–280. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- 48.Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic fatty disease: A randomized controlled pilot study. Hepat Mon. 2012;12:e6099. doi: 10.5812/hepatmon.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalantari H, Shahshahan Z, Hejazi SM, Ghafghazi T, Sebghatolahi V. Effects of silybum marianum on patients with chronic hepatitis C. J Res Med Sci. 2011;16:287–290. [PMC free article] [PubMed] [Google Scholar]
- 50.Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, de Sio I, Floreani A, et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med. 2012;52:1658–1665. doi: 10.1016/j.freeradbiomed.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Jelodar G, Rafiee B, Moosavi SH. Silymarine extract improved plasma homocysteine, lipids and liver enzymes in hyperhomocysteinemic non-alcoholic steatohepatitis. Physiol Pahrmacol. 2015;19:139–145. [Google Scholar]
- 52.Flisiak R, Prokopowicz D. One year follow-up of patients treated with misoprostol in acute phase of viral hepatitis B. Prostaglandins Other Lipid Mediat. 2000;60:161–165. doi: 10.1016/s0090-6980(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 53.Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439–442. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 55.Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu YT, Wu SB, Wei YH. Metabolic reprogramming of human cells in response to oxidative stress: implications in the pathophysiology and therapy of mitochondrial diseases. Curr Pharm Des. 2014;20:5510–5526. doi: 10.2174/1381612820666140306103401. [DOI] [PubMed] [Google Scholar]
- 57.Freedman ND, Curto TM, Morishima C, Seeff Lb, Goodman ZD, Wright EC, Sinha R, Everhart JE, the HALT-C Trial Group. Silymarin use and liver disease progression in the HALT-C trial. Aliment Pharmaol Ther. 2011;33:127–137. doi: 10.1111/j.1365-2036.2010.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 59.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 60.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 61.Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, Rosen CB, Batts KP. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 62.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 63.Ong JP, Elariny H, Collantes R, Younoszai A, Chandhoke V, Reines HD, Goodman Z, Younossi ZM. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15:310–315. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- 64.Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408–1413. doi: 10.1016/0016-5085(90)91169-7. [DOI] [PubMed] [Google Scholar]
- 65.Andersen T, Gluud C, Franzmann MB, Christoffersen P. Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol. 1991;12:224–229. doi: 10.1016/0168-8278(91)90942-5. [DOI] [PubMed] [Google Scholar]
- 66.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]


