Abstract
Helping women make choices to reduce cancer risk and to improve breast health behaviors is important, but the best ways to reach more people with intervention assistance is not known. To test the efficacy of a web-based intervention designed to help women make better breast health choices, we adapted our previously tested, successful breast health intervention package to be delivered on the Internet, and then we tested it in a randomized trial. We recruited women from the general public to be randomized to either an active intervention group or a delayed intervention control group. The intervention consisted of a specialized website providing tailored and personalized risk information to all participants, followed by offers of additional support if needed. Follow-up at one-year post randomization revealed significant improvements in mammography screening in intervention women compared with control women (improvement of 13 percentage points). The intervention effects were more powerful in women who increased breast health knowledge and decreased cancer worry during intervention. These data indicate that increases in mammography can be accomplished in population-based mostly insured samples by implementing this simple, low resource intensive intervention.
Keywords: Randomized trial, Mammography screening, Web/Internet intervention, Genetic testing
INTRODUCTION
Helping women make choices to reduce cancer risk and to improve breast health behaviors is important, but the best ways to reach more people with intervention assistance are not known. Counseling and intervention programs have been designed and evaluated to support women in making breast health choices [1–19]. There are limitations to these existing programs, however, the counseling programs can be labor intensive for the staff delivering the programs and for the participants, and the reminder system programs focus on only a limited number of medically oriented variables for tailoring [19–21]. Our previous reminder system intervention [22], built on a stepped care model of health behavior intervention [23], delivered very simple information and support to some women, while to others, because of their risk level, worry level, or other key variables, delivered more support and guidance in a counseling setting. Our reminder system intervention was successful in improving a variety of breast health behaviors, but it required personalized and tailored mailings to be created for each participant as well as in-person contact for counseling settings [24]. A recent review of dissemination and implementation research recently called for the study of dissemination in a variety of settings and populations [25]. Therefore, we decided to deliver breast health communication by using the Internet. Specifically, we selected the World Wide Web (Web) as an ideal interactive, electronic system for delivering a new version of our previous intervention content. The Web is an attractive interface because it provides information in a variety of media (images, video, sound, animation, different font sizes), instant access to global information, and the ability to network with other consumers and health care providers. The Web can facilitate two-way interactive dialogue between author and reader. Tailoring is relatively easy over the Web, and tailored interventions hold promise for increasing effects over non-tailored interventions, providing more powerful health behavior change [26, 27]. A Web site can provide many resources in a single, focused, and widely accessible location. Unlike conventionally published textual materials, which are two-dimensional and linear in form, web-based information can be cross-referenced over a limitless number of levels and layers, resulting in an almost three-dimensional experience and potentially more efficient navigation and information retrieval. The publication of printable and/or downloadable tailored materials on the Web obviates the need for costly phone, duplication, fax, publishing, printing, and mailing expenses. In addition, and of prime importance to medical information, authors can update materials on the Web almost instantly. The advantages of delivering information on genetic risk on the Internet are clear [28]. As systems are developed and put into place to communicate genetic risk, it seems obvious to make use of the Internet as an ideal vehicle for disseminating health and health risk information and support within the context of health care delivery [29, 30]. We used all of these properties and others during our design of the study Web site.
Evaluating Web delivery systems is critical, given the plethora of Web sites developed and available. Several articles have documented the growing number of Web-based support systems available as well as their mixed quality and focus [31, 32]. The number of patients who rely on the Web as their main source of detailed information and support about health and illness processes is also increasing [33]. According to a Pew Report published in 2009, 77 % of US adults were online—87 % of those users access from home and 48 % have access from work—and 66 % of them have looked for health information [34]. In addition, tens of thousands of Web sites currently disseminate health information.
Given the plethora of web material on health, we feel that it is critical to test the effects of health websites on measures of health behavior. The present paper reports the results of a randomized controlled behavioral trial of a web-based intervention to improve women’s breast health choices. Women from the general public were recruited and randomized to receive the intervention package or to serve as control participants. Intervention participants received access to the Web site and cues and support to use the intervention. We examined the following study outcomes: breast health behaviors (mammography screening and breast self-examination), genetic testing pursuit, and quality of life. We also examined variables from our theoretical model, described below, as mediators of the intervention effects.
Experimental methods
Participant recruitment
The eligibility criteria for this project were simple and minimal, in keeping with the public health nature of the proposal. The study subjects were women, ages 18–74, living in the Seattle metropolitan area, not been previously diagnosed with breast cancer, who had a working telephone number and address, who spoke English, who planned to be in their present residence for at least 1 year, who had Internet access in their homes, and who were willing to complete the survey requirements for the baseline and follow-up assessments. Sample size was determined through prestudy power calculations. We purchased names from Mailing Lists Plus*, a company that brokers name lists, in this case from Polk, a source company. Polk gathered the contact information and addresses by using both public (voter registration and driver’s license rolls) and private sources (credit bureaus, insurance lists, and other lists of names). The lists were obtained in blocks of 1500 names approximately every 3–4 months in order to keep the phone numbers and addresses as current as possible and to reduce the occurrence of incorrect or out-of-service phone numbers during recruitment.
Each potential participant received a letter that introduced the project and provided the participants with a number they could call to indicate unwillingness to participate in the study. Individuals who called requesting not to participate were removed from the call list and were not contacted again. All other potential study subjects were called to obtain verbal consent to participate in the study. The consent process script included descriptions of the surveys, the randomization process, the personalized risk information sheet, and the potential for follow-up support. If a participant consented to participate in the study and then completed the baseline assessments, study staff randomized her to either intervention or control status in 50/50 ratio using a pre-assigned number on a list created by the study statistician.
Survey procedures
Telephone surveys were used to gather eligibility, baseline, and follow-up data. Potential participants were contacted on the phone by trained interviewers (unaware of study condition) to complete a 15-min eligibility screening survey. Respondents who were both eligible and interested in the study went on to complete the baseline survey, which measured perceived risk, cancer worry, demographics, estimated actual risk, quality of life, knowledge of breast cancer, screening intentions and behaviors, previous genetic testing, and interest in genetic testing. The follow-up survey was conducted 1 year after the baseline to measure study outcomes.
The intervention
We based the content of this intervention on the self-regulation model developed by Leventhal and colleagues [35] applied to women at risk for breast cancer [4, 5, 36]. The self-regulation model of health behavior addresses health-risk communications and the use and effects of health screening. This model emphasizes the ways in which people actively cope with information about their health and make decisions regarding medical procedures. The model also included variables on the importance of understanding both a woman’s understanding of her health risk and her emotional reaction to that risk. According to this model, a woman begins coping with her risk for breast cancer when she learns that she is at risk for the disease. This realization may occur to her on her own, when she learns of a friend or relative’s illness, when she learns that breast cancer may be partially hereditary, or when she is invited to participate in a screening program. The woman reacts emotionally to the realization and develops an internal understanding or mental representation of what her elevated risk for breast cancer means to her. This representation will include her knowledge and beliefs about breast cancer and her beliefs about her own risk for breast cancer. The representations will then influence her emotional reactions, including her development and execution of plans for action regarding her risk and emotional reactions to that risk.
Each woman in this study was placed into an average risk group, a mixed risk group, or a genetic risk group, based on her baseline data. These criteria are described in Table 1.
Table 1.
Risk categories used in intervention delivery
| Average risk category | Mixed risk category | Genetic risk category |
|---|---|---|
| 1) Does not meet requirements of mixed or genetic risk categories and
2) Cancer worry scale score below 8a |
1) Does not meet requirements of genetic risk and
2) Has had one or more breast biopsies, or 3) Breast biopsy with atypical hyperplasia, or 4) Cancer worry of 8 and above (range 4–16), or 5) Ppt requests counseling |
1) One first-degree relative w/BC before 60, or
2) Two first-degree relatives w/breast or ovarian cancer at any age, or 3) Two second-degree relatives w/breast or ovarian cancer on same side, or b 4) One first-degree relative w/breast or ovarian cancer and one second-degree relative w/breast or ovarian cancer on same side, or b 5) One first-degree male relative w/breast cancer |
aDetermined in our previous research
bFor siblings, offspring, and sibling’s offspring (where maternal or paternal side cannot be determined) relatives were included in both maternal and paternal criteria
Average risk women had no major risk factors for breast cancer and a Gail risk score (described below) under 15. Mixed-risk women either had reported a specific mixed risk factor for breast cancer or reported a cancer worry score of 8 or over. We used the data in our previous research to determine a “high” worry score, indicating women who might need more intensive assistance [4, 5]. Genetic-risk women had a relatively high family history of breast cancer suggestive of possible positive genetic mutation status [37, 38]. We reasoned that these women would likely be considered candidates for genetic counseling in an HMO or other health care setting and, therefore, needed special attention.
Web site description
Project staff developed and maintained a specialized Web site that contained and delivered all informational materials pertaining to breast cancer, genetic risk, and associated issues and links. The content was based our successful counseling and tailored print message interventions that increased mammography and quality of life in women in the same age group as was recruited for this study [22, 24]. Each participant was assigned a unique username and password for accessing the site. This restricted access system ensured that personal risk information was only accessible by the participant, and it allowed for tailoring of the site and its interactive features to each participant.
Home page
The Web site’s home page highlighted new content and included several rotating features. The most recent health news article added to the Web site was featured on the home page. Participants saw a new breast cancer “tip of the day” each time they logged in to the Web site. Participants also saw a different “personal story” at each login. The personal stories were fictitious accounts of women’s experiences of learning about their risk. These accounts were based on composites of stories from real women.
Web site login sequence
The first time a participant logged on to the study Web site, she saw a welcome letter from the study staff. The letter provided an overview of what was on the site and included a photo of the study staff. The next page had three facts about breast cancer. For average risk participants, the three facts were: “Most women never get breast cancer. In fact, almost 90% of women will never develop the disease”; “Most women who do get breast cancer survive. In fact, the majority of women who get breast cancer are still alive five years after their diagnosis”; and “Heart disease, not breast cancer, is the major cause of death for women. Heart disease causes more than eight times as many deaths as breast cancer”. For mixed and genetic risk participants, the first fact above was replaced with the following: “Treatments are effective. Especially when the cancer is detected early, treatment of breast cancer is very effective.” The page after the three facts was the home page, which encouraged participants to first look at their personal risk sheet.
At the second login, participants were greeted with a check-in message from the health counselor. The message asked the participant if she had had a chance to view her risk sheet and what her thoughts were. The participant had the opportunity to send a question to the health counselor. If the participant had a question, she could submit it through the “ask a health expert form.” If she had no questions, the next page was the home page, with a reminder to look at her personal risk sheet. On the third login, participants saw the three facts page again, before going to the home page. The home page included a reminder to look at their personal risk sheets. On fourth and subsequent logins, participants went directly to the home page.
Average risk women were provided with information about breast cancer risk that included a personal risk estimate, access to health experts to answer questions, and the option to participate in group activities. Mixed risk women were provided with information about breast cancer risk, access to health experts, and invitations to attend a counseling session to discuss their risk. Genetic risk women were provided with information about breast cancer risk, access to health experts, and the option to participate in a genetic counseling session and receive a personal risk estimate.
Personal risk page/Gail score
For each participant we included an online personal risk page, based on the model developed by Gail to predict personalized lifetime risk estimates [39]. We used data from the baseline survey to generate estimates for each participant. These Gail scores were presented numerically and graphically, based on our risk sheet presentations used in the previous counseling project. Average risk women were able to access their personal risk page immediately after logging on to the site for the first time. Mixed and genetic risk women were prompted to sign up for a counseling session to review their risk page. Their risk pages, modeled after paper sheets used in our previous research project [40], were not viewable on the Web site until they completed their counseling session.
Content categories
The Web site had three main content categories: about breast cancer, early detection, and prevention. The breast cancer category included information on breast cancer and breast cancer risk. The early detection category included information on breast self-exams, clinical breast exams, and mammography. The prevention category included information on exercise, healthy eating, and Tamoxifen.
Interactive features
Participants were given the opportunity to make “breast health commitments” based on information they provided in the baseline survey. For example, a participant who reported never having a mammogram could commit to scheduling one in the next few months. A participant who reported already eating five servings of fruits and vegetables could commit to continue eating at least five servings per day and exceed that when possible. Participants could also complete interactive worksheets to make and review healthy goals. For example, participants could enter intermediate and long-term exercise goals. Their goals were saved so they could review them at any time. The Web site also contained quizzes for assessing patterns of behaviors and motivations. Participants could complete the quizzes and get scores that gave them feedback on how to improve their eating and exercise habits. Three additional interactive features allowed participants to select “concerns” that may have kept them from doing mammograms, clinical breast exams, and/or breast self-exams. The participant clicked a button next to the concern that sounded most like her, and a suggestion appeared for addressing that concern. Each of these interactive features was accessible through the appropriate Web site section as well as in a dedicated section called the Health Action Plan. The Health Action Plan combined all of the interactive features so a participant could easily review all of her goals and worksheets.
Breast cancer news and information
Participants had access to the latest breast cancer news and information through the Health News section, the Questions and Answers section, and the Other Websites section. The Health News section was updated approximately once a week with relevant health news articles. The Questions and Answers section was an archive of answers to questions asked in our previous breast cancer risk information study. This section was also updated with new questions and answers from the current study. The Other Websites section included links to other Web sites with related information on breast cancer risk, early detection and prevention.
Contact with others
Participants could contact study staff via the Web site using the following contact forms:
Ask an expert: Participants could use this form to send a question to the health expert. A response was sent within two business days. Participants could specify whether they wanted their response by e-mail or phone.
Counseling sign-up: This form was for study participants to sign up for a counseling session. Counseling was available to any participant who requested it. Mixed and genetic risk participants had to complete counseling in order to view their personal risk sheets.
Contact us: Participants could use this form to contact study staff for any reason. Participants could specify whether they wanted to receive a response by e-mail or phone. We also included the phone number of the study line, if the participant preferred to contact us by phone.
Regular cues to action
We sent a monthly newsletter to all participants in the intervention arm of the study. Each newsletter included a study update, a summary of health news articles added to the study Web site that month, and a description of a different Web site feature. We sent the newsletter by e-mail to participants who requested this medium as their preferred mode of contact. We sent the newsletter by conventional mail to participants who did not want e-mail contact.
We followed up with participants who had not logged into the study Web site within 3 weeks and then 3 months of receiving access. The follow-up reminded participants of the features of the Web site and how to log on. We also provided information on how to contact us if the participant was having difficulty accessing the Web site. We sent an e-mail follow-up to participants who requested e-mail as their preferred contact method. We conducted the follow-up by phone with those who preferred to be called. Eligible participants who did not sign up for counseling within 6 and 9 months of entering the study received a phone prompt in which we told the participants about the counseling sessions and invited them to attend.
Genetic counseling and testing
Women determined to be at elevated risk due to family history of breast cancer were invited to participate in introductory genetic counseling, based on our previous research. To be eligible, a woman had to meet the high-risk criteria. The genetic counseling protocol had four sessions, based on the standard model of multiple sessions for genetic susceptibility testing for breast cancer [40–42]. Each woman received her personal risk sheet and the counselor, and the participant reviewed the findings presented on the sheet. A major topic of discussion was genetic testing, including the genetics of breast cancer, the purpose of genetic testing, and the possible limitations and uses of testing and test results. The participants were informed that they were personally responsible for the cost of the genetic testing should they be eligible and choose to proceed with genetic testing within this study. The initial cancer risk genetic counseling session lasted approximately one and one-half hours. Eligible participants who decided to proceed with genetic testing were referred to their medical provider for a discussion of testing.
Measures
Screening behaviors
We assessed breast cancer-related screening behaviors and intentions using items adapted from the Community Mammography Trial [43]. To assess screening behaviors, we used specific items from the Cancer Risk Appraisal questionnaire to measure utilization of breast self-examination and mammography screening. The Cancer Risk Appraisal measures reported screening behavior over the previous year with a single question addressing each behavior. This study includes questions regarding the last time, how many times, and next time that the behavior will take place. For example, “How often have you had a mammogram in the past?” The response categories range from never to twice or more a year. Adherence to breast cancer screening guidelines was based on ACS recommendations.
Genetic testing
We measured interest in pursuing genetic testing in the future using four questions from previous research [14]. Three of the questions were “Do you consider yourself to be an appropriate candidate for (BRCA1/2 testing) given your family history?” “I intend to ask my health care provider more about genetic testing,” and “ I intend to get a genetic test for breast cancer risk.” The response structure for these three questions was a scale of 1–4, with 1 meaning definitely no and 4 meaning definitely yes. The fourth question was “At the present time, which of the following statements describes you?” (1= not considering/not thought about genetic testing, 2= considering having genetic testing, 3= probably will have genetic testing, 4= definitely will have genetic testing). We calculated a single testing intentions scale score by averaging the individual scores for each person. Cronbach’s alpha for this scale was .82, indicating high internal consistency.
Cancer worry
We measured breast cancer worry with a single four-item scale, used to assess the presence of breast cancer worries that interfere with daily functioning [44]. This scale has been shown to distinguish between persons at high and normal risk for breast cancer and to relate to screening behaviors. This widely used questionnaire also measures the frequency of worry about breast cancer in specific settings. The questions include, “During the past month, how often have thoughts about your chances of getting breast cancer affected your mood?” and “During the past month, how often have thoughts about your chances of getting breast cancer affected your ability to perform your daily activities?” The answers range from 1 (not at all or rarely) to 4 (a lot). The questions are summed with a range from 4 to 16. The alpha coefficient for this questionnaire was 0.71.
Quality of life
We measured perceived quality of life using the SF-36 Health Survey for Perceived Quality of Life, a well-validated, reliable, and widely used instrument that includes 36 items [45]. This self-report scale measures the following eight health concepts: physical functioning, role-physical, role-emotional, bodily pain, general health, vitality, social functioning, mental health, and reported health transition. The higher the score, the more the participant has the attribute of the scale construct. We focused these analyses on the Mental Health subscale.
Perceived risk
We developed a three-question measure of perceived risk designed to measure a person’s perceptions of her risk for breast cancer [46, 47]. The questions included a numerical risk estimate (0–100 %), a set of seven categories from very low to very high, and a question about risk relative to other women. The perceived risk scale was the average of the score from these three questions.
Knowledge of breast cancer and genetic testing
We developed questions in our previous research to measure knowledge of breast cancer and genetic testing (n = 10 and 5, respectively), with response scales of 1–4. The knowledge questions regarding breast cancer included questions on treatment efficacy, prevention options, and survival rates. The genetic testing questions included questions on the purpose of genetic testing, limitations of the test, and possible information provided by the current testing options. We performed separate factor analyses on each of the two potential knowledge scales (breast cancer and genetic testing) and found them to each contain one factor, with reasonable factor scores (all scores over .7). Cronbach’s alpha’s for the scales were 0.76 and 0.63, respectively, for breast cancer and genetic test knowledge. Scale items were averaged for each scale for analysis purposes.
Medical risk
We asked six questions modified from the Women’s Health Initiative and Breast Cancer Detection Demonstration Project about risk factors for breast cancer. Questions included age of menarche, age at first live birth, history of breast biopsy, and current menstrual status. We estimated risk for breast cancer from these questions using the Gail model for risk appraisal [39]. The Gail algorithm results in a probability of developing breast cancer by a specified age or in the woman’s lifetime on a scale of 0–100 %. The Gail algorithm used all of the above listed data plus any first-degree female relatives diagnosed with breast cancer, as provided in the participants’ family history. We counted half-sisters as first-degree relatives if they were on the same side of the family as another first or second-degree relative who had been diagnosed with breast cancer. We gathered information on family history of cancer using questions modified from our previous research. For each blood relative with cancer, we asked participants the following: their relationship to the relative (mother, aunt, grandfather, etc.), whether the relative was on their mother’s or father’s side of the family, and the type of cancer and age at diagnosis for the first cancer and second cancer (if applicable).
Dosage of intervention
The Web site tracked use patterns, linked to individual identities of participants, allowing us to calculate use of all Web site components. All participant telephone calls and e-mails to the project, all requests for counseling, and actual counseling contacts were recorded.
Demographic data
We measured demographic variables including age, race/ethnic category, education/degree, current religious affiliation, marital status, employment status, income, household size, and Jewish background. These variables were used to describe the study sample, to evaluate the comparability of the control and intervention groups, and to serve as covariates, as needed, in the analyses.
Statistical analysis
The analyses consisted first of descriptive analyses to describe the sample and to determine the extent to which the randomization had been successful. We also compared the demographic data of participants who provided follow-up data with data from participants who had dropped out of the study before the follow-up period to determine the effects of any dropouts on study integrity. We analyzed results using both linear and logistical regression, depending on the properties of the outcome variable under study. The main adjustment variables in the model were age, race, education, income, medical risk, and date of recruitment, selected as common background variables. We looked for main effects of intervention using the intention-to-treat principle and then identified the intervention effect for subgroups using levels of dosage of intervention and levels of baseline theoretical model variables (perceived risk, cancer worry, knowledge of breast cancer, and genetic testing).
RESULTS
A total of 5021 letters were mailed to potential participants. We screened 2518 women, yielding 1452 eligible participants. We used the methods recommended by a leading survey research group (www.aapor.org, method #4) to calculate response rate. Basically, the response rate is the number of completed contacts over the number of contacts plus the number of eligible refusals and noncontacts, and an estimated proportion of the potential contacts that were eligible. Specifically, we used data from our previous study and from other telephone interviews to estimate the proportion of potential participants not screened that were eligible as 30 %, and used this figure in the calculation. Using this procedure, we calculated an 81 % response rate for this survey. We randomized 1354 participants, and the follow-up rate was calculated as the number of follow-up surveys collected over the number randomized. Using this formula, we achieved an 89 % follow-up rate at 12 months.
Table 2 presents background and demographic data from the present sample and comparisons to census data from the year 2000 census.
Table 2.
Background and demographic variables for the present study
| Background variable | Study value | N | Y2000 census data King County, WA |
|---|---|---|---|
| Percent under 40 | 43 % | 1354 | 38 % |
| Percent Caucasian | 85 % | 1354 | 89 % |
| Percent with high school education only | 16 % | 1345 | 21 % |
| Average Gail score ( X , SD) | 12 (1.3) | 1350 | unknown |
| Percent with family history | 35 % | 1349 | unknown |
Randomization was successful in that there were no significant differences in demographic variables between arms. Also, there were no significant baseline differences between participants who dropped out of the study and participants who provided some 12 month follow-up data. Table 3 presents changes from baseline to follow-up for key main outcome variables.
Table 3.
Changes in main outcomes of the present study
| Main outcomes | Baseline value | 12-month value | Change | Adjusted change1 | Intervention effect (difference of changes) | Confidence intervals |
|---|---|---|---|---|---|---|
| Percent | Percent | |||||
| Mammography in past year | +13** | 5.3–20.0 | ||||
| Intervention n = 334 | 69 % | 82 % | +13 % | +13 % | ||
| Control n = 338 | 71 % | 70 % | −1 % | 0 % | ||
| BSE once per month | +19** | 13.1–27.7 | ||||
| Intervention n = 655 | 40 % | 62 % | +22 % | +18 % | ||
| Control n = 653 | 41 % | 41 % | 0 % | −1 % | ||
| Genetic test interest (x) | −1.0* | −0.32–2.1 | ||||
| Intervention n = 655 | 3.1 | 1.5 | −1.6 | −1.6 | ||
| Control n = 653 | 3.1 | 2.9 | −0.1 | −0.1 | ||
| Quality of life (x) | +7.1 | 2.3–10.5 | ||||
| Intervention n = 655 | 73.2 | 86.2 | +13.0 | +7.1 | ||
| Control n = 653 | 73.9 | 74.2 | +0.3 | +0 |
*p < 0.05; **p < 0.01
1Adjusted for age, race, education, income, medical risk, and time of recruitment
Variables included in the adjustment were age, race, education/income, Gail score, time of recruitment, and baseline value of outcome variable. The key outcome variables included mammography in the last year (1= obtained mammography screening), frequency of breast self-examination (BSE) (1= BSE in the last month), mental health score from the quality of life measurement (continuous score), and interest in genetic testing (continuous score). We restricted the mammography analysis to women over 40 (n = 672). In general, mammography and BSE increased significantly in the intervention group relative to the control group, as did quality of life values. An average of 70 % of women in the intervention and comparison groups reported obtaining mammography screening at baseline. Women in the intervention group reported an increase of 13 % from baseline to follow-up while women in the control group reported a decrease of 1 %. These patterns were significantly different, in both adjusted and unadjusted analyses. A similar pattern of change occurred for BSE. Intervention women increased the irrate of BSE from 40 to 62 %. Women in the control group reported no average change from a baseline value of 41 %, and the lack of change was significantly different from intervention women. Women in the intervention group reported statistically significant decreases in interest in genetic testing, compared to control women, in both unadjusted and adjusted analyses (intervention decrease of 1.6 scale points versus control decrease of 0.1). Finally, quality of life scores improved significantly for intervention women, compared to control women. Values for intervention women on mental health scales increased from 73.2 to 86.2, while control values increased from 73.9 to 74.2 from baseline to follow-up.
We defined the dosage of intervention obtained during the study period as how much participants used the Web site and how much they participated in the counseling options. The Web site use patterns varied considerably among the 676 intervention participants. The participants logged onto the Web site an average of 1.6 times, with a range of 0–8 times during the study intervention period. For each successful login period, defined as being logged in for more than 15 s, the participants hit an average of ten pages, with a range of 2–30 pages per login. The most frequently hit pages were the home page and the personal risk information page. Of the 102 mixed-risk participants eligible for counseling, 80 % participated in counseling. Of the 81 participants eligible for the first session of genetic counseling, 92 % engaged in the session. We used these multiple dosage indicators to calculate two simple aggregate dosage variables, Web use and counseling participation. Intensive Web use was calculated as logging onto the Web site at least twice, and minimal use was defined as logging on one or zero times.
Counseling participation was a dichotomous variable (1= yes and 0= no), indicating whether or not the participant engaged in either form of counseling.
Table 4 presents the results for the breast health outcomes by knowledge change, cancer worry change, and dosage of intervention for intervention participants only.
Table 4.
Intervention effects at 12 months for key predictor variables
| Predictor1 n = 665 | Annual monthly mammography (n = 334) | Genetic testing BSE (n = 665) | Interest (n = 665) |
|---|---|---|---|
| Breast cancer knowledge change | |||
| High mean effect | 16 % | 9 % | – |
| Confidence Intervals | 8–21** | 3–11** | |
| Low mean effect | 9 % | 4 % | – |
| Confidence intervals | 4–18** | 2–9* | |
| Genetic testing knowledge change | |||
| High mean effect | – | – | −2.4 |
| Confidence intervals | −1.3–5.8** | ||
| Low mean effect | – | – | −.3 |
| Confidence intervals | 1.2–1.8 | ||
| Cancer worry change | |||
| High mean effect | −14 % | 10 % | −1.4 |
| Confidence intervals | −9–15* | 5–17** | −.6- -2.8* |
| Low mean effect | −9 % | 2 % | −1.3 |
| Confidence intervals | −7–15* | .9–8 | −.5–1.9 |
| Dosage of intervention | |||
| Intensive mean effect | 13 % | 7 % | −1.3 |
| Confidence intervals | 6–29* | 4–19* | −.6–2.1** |
| Minimal mean effect | 11 % | 6 % | −1.4 |
| Confidence intervals | 5–25* | 3–16* | −.4–2.3** |
*p < 0.05; **p < 0.01
1Adjusted for age, race, education, income, medical risk, and time of recruitment
Key predictor variables included change in knowledge of breast cancer and genetic testing (high versus low using median splits), change in cancer worry scale score (high versus low using median split), and dosage of intervention (intensive versus minimal). Variables included in the adjustment were age, race, education/income, Gail score, time of recruitment, and baseline value of outcome variable. As seen in Table 4, the intervention changes for both screening behaviors differed for different knowledge change subgroups; women who reported more breast cancer knowledge increase also reported higher rates of mammography and BSE at 12 months, compared with women who reported smaller increases in breast cancer knowledge. Screening outcomes differed for women in differing cancer worry change subgroups as well; women who reported larger reductions in cancer worry from baseline to follow-up reported higher mammography and BSE rates, compared with women who reported smaller decreases in cancer worry. Dosage of intervention did not differentially affect reported screening rates for the intervention participants; screening changes were similar for participants in the minimal and intensive dosage subgroups. Genetic testing interest by subgroup of participants is also shown in Table 4. Subgroups defined by differential change in knowledge regarding genetic testing differed in their genetic testing interest outcomes; women who reported higher gains in knowledge about genetic testing also reported less interest in genetic testing after intervention. Subgroup status regarding change in cancer worry and dosage of intervention did not differentially affect interest in genetic testing.
DISCUSSION
This study was designed to test the efficacy of a web-based intervention designed to improve breast health behaviors. The intervention was successful in increasing screening behaviors, both mammography and BSE, over the 1 year intervention period. The screening effects are in line with the results of other interventions to improve breast health choices [4–6, 8] and are roughly equal to the effects found in our previous intervention using paper and in-person contact [22]. We were surprised by the intensity of the effects of this intervention. Although we expected that this intervention would be successful, we did not hypothesize similar size effects to our previous public health intervention [22]. The CONSORT flow chart is available in the Appendix.
Women in the intervention group reduced interest in genetic testing compared to control women. Again, this is exciting because women report inappropriately high interest in genetic testing, compared to their actual medical risk. Our previous intervention research has shown reductions in genetic testing interest [4], even when delivered through a primary care setting [48].
This result provides support for future research that rigorously evaluates these types of interventions. Not only is it possible to conduct efficacy studies in this setting, but it is necessary to do so to enable later dissemination of efficacious interventions to public health and clinical settings. We should use the same high standards of evaluation for electronic and interactive interventions that we use for print and other interventions. The positive effects of the intervention presented here indicate that web-based interventions can change health behavior; this lends support for design, development, and evaluation of future similar interventions in other applications and settings.
There are many limitations to the present study that limit the interpretation and generalization. First, the study recruited only women with access to the Internet at home. This limits the generalization of possible benefit to women with home Internet access. We know nothing about women who do not have access, and our next line of research is to study how to provide support and aid to these women. Undoubtedly, they differ in many ways from women with convenient home Internet access. Also, the findings are based on self-reported screening and other variables. We do know that self-reported screening is reasonably associated with screening accounts collected via medical records, but the chance exists for differential reporting by intervention group that could incur bias in outcome measure. The sampling methods and response rates were reasonable, but still the sample does not completely represent the defined population of the region. We were not able to include individuals without Internet access, so it is likely that we underrepresented lower income women. The intervention was most easily used by competent and experienced computer/Web users. Even with home access to the Internet, less computer-savvy women might have found it difficult to participate fully. Women who did not appear on the initial telephone lists would be excluded from the study. The lists contained known biases. The list under-sampled poor households due to characteristics such as higher mobility, frequent address changes, and lack of land telephone; therefore, very poor women would not be represented in this study. Women without land telephones (about 3 % of the population at the time of the study) were excluded because of the data collection methodology and therefore were not represented in the sample.
This intervention package was simple to implement and could certainly fit well within an existing breast clinic, mammography facility, HMO setting, or primary care group. In fact, many health care organizations have their own Web-based supports for patient/client information, scheduling, and discussion with their providers. Referrals to a breast health Web site such as ours to answer patients’ questions and support dialog with their providers might work well in such a system. Support for disseminating this type of intervention might be found in health care organizations that want to increase use of appropriate services, while helping the public to understand more about services that are only appropriate for higher-risk women or other subgroups.
Appendix
Fig. 1.
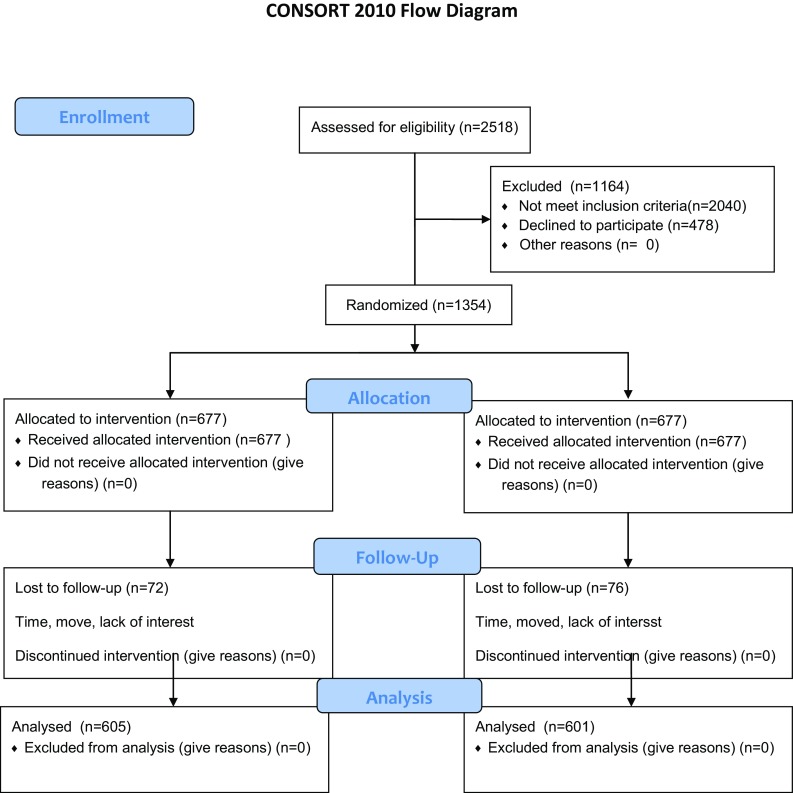
CONSORT 2010 flow diagram
Footnotes
Deborah J. Bowen, Nigel Bush, Hendrika Meischke, and Jean Wooldridge, Cancer Prevention Program, Fred Hutchinson Cancer Research Center. Robert Robbins, Information Technology, Fred Hutchinson Cancer Research Center. This research was supported by a grant (CA82894) from the National Cancer Institute.
Implications
Practice: As interventions like this one are evaluated, they can be put into practice to improve breast health behaviors by insurance systems, hospital systems, and provider networks.
Policy: Health care reform should be required to include evidence-based health communication strategies in its list of funded activities in new health care systems. Increasing cost-effective behaviors through tested health communication systems such as this one will improve health outcomes.
Research: Research can be conducted on the use of the Web as a delivery system for other types of health communications in a variety of diverse populations.
A randomized trial to test a web-based intervention on women’s breast health behaviors
References
- 1.Bowen D, et al. Results of an adjunct dietary intervention program in the Women’s health initiative. J Am Diet Assoc. 2002;102(11):1631–1637. doi: 10.1016/S0002-8223(02)90347-0. [DOI] [PubMed] [Google Scholar]
- 2.Bowen D, et al. Participation in breast cancer risk counseling among women with a family history. Cancer Epidemiol Biomark Prev. 1999;8(7):581–585. [PubMed] [Google Scholar]
- 3.Bowen DJ, Beresford SA. Dietary interventions to prevent disease. Annu Rev Public Health. 2002;23:255–286. doi: 10.1146/annurev.publhealth.23.100901.140555. [DOI] [PubMed] [Google Scholar]
- 4.Bowen DJ, et al. Effects of risk counseling on interest in breast cancer genetic testing for lower risk women. Genet Med. 2002;4(5):359–365. doi: 10.1097/00125817-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bowen DJ, et al. Effects of counseling and ethnic identity on perceived risk and cancer worry in African American women. J Clin Psychol Med Settings. 1998;05(3):365–379. doi: 10.1023/A:1026262321756. [DOI] [Google Scholar]
- 6.Bowen DJ, et al. Predicting breast cancer screening intentions and behavior with emotion and cognition. J Soc Clin Psychol. 2003;22(2):212–232. doi: 10.1521/jscp.22.2.213.22875. [DOI] [Google Scholar]
- 7.Bowen DJ, et al. Early experience with a web-based intervention to inform risk of breast cancer. J Health Psychol. 2003;8(1):175–186. doi: 10.1177/1359105303008001455. [DOI] [PubMed] [Google Scholar]
- 8.Bowen DJ, et al. Jewish identity and intentions to obtain breast cancer screening. Cultur Divers Ethnic Minor Psychol. 2003;9(1):79–87. doi: 10.1037/1099-9809.9.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Esplen MJ, et al. Supportive-expressive group intervention for women who test positive for BRCA 1/2. Psycho-Oncology. 2000;9(5 Suppl):S1–106. doi: 10.1002/1099-1611(200005/06)9:3<243::aid-pon457>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Esplen MJ, et al. A group therapy approach to facilitate integration of risk information for women at risk for breast cancer. Can J Psychiatr. 1998;43(4):375–380. doi: 10.1177/070674379804300405. [DOI] [PubMed] [Google Scholar]
- 11.Esplen MJ, et al. A supportive-expressive group intervention for women with a family history of breast cancer: results of a phase II study. Psycho-Oncology. 2000;9(3):243–252. doi: 10.1002/1099-1611(200005/06)9:3<243::AID-PON457>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Lerman C, Audrain J, Croyle RT. DNA-testing for heritable breast cancer risks: lessons from traditional genetic counseling. Ann Behav Med. 1994;16(4):327. [Google Scholar]
- 13.Lerman C, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer Inst. 1997;89(2):148–157. doi: 10.1093/jnci/89.2.148. [DOI] [PubMed] [Google Scholar]
- 14.Lerman C, et al. Racial differences in testing motivation and psychological distress following pretest education for BRCA1 gene testing. Cancer Epidemiol Biomark Prev. 1999;8(4 Pt 2):361–367. [PubMed] [Google Scholar]
- 15.Lerman C, Kash K, Stefanek M. Younger women at increased risk for breast cancer: perceived risk, psychological well-being, and surveillance behavior. J Natl Cancer Inst Monogr. 1994;16:171–176. [PubMed] [Google Scholar]
- 16.Lerman C, et al. Effects of individualized breast cancer risk counseling: a randomized trial. J Natl Cancer Inst. 1995;87(4):286–292. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]
- 17.Lerman C, et al. A randomized trial of breast cancer risk counseling: interacting effects of counseling, educational level, and coping style. Health Psychol. 1996;15(2):75–83. doi: 10.1037/0278-6133.15.2.75. [DOI] [PubMed] [Google Scholar]
- 18.Taplin S, Anderman C, Grothaus L. Breast cancer risk and participation in mammographic screening. Am J Public Health. 1989;79(11):1494–1498. doi: 10.2105/AJPH.79.11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taplin SH, et al. Testing reminder and motivational telephone calls to increase screening mammography: a randomized study. J Natl Cancer Inst. 2000;92(3):233–242. doi: 10.1093/jnci/92.3.233. [DOI] [PubMed] [Google Scholar]
- 20.Kendall C, Hailey BJ. The relative effectiveness of three reminder letters on making and keeping mammogram appointments. Behav Med. 1993;19(1):29–34. doi: 10.1080/08964289.1993.9937562. [DOI] [PubMed] [Google Scholar]
- 21.Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat. 1993;28(2):145–155. doi: 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- 22.Bowen, D.J., et al. (2010) Effects of a public health intervention on women’s breast health behaviors. Health Education and Behavior, xxx, xxx-xxx. [DOI] [PubMed]
- 23.Abrams DB, et al. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: a combined stepped-care and matching model. Ann Behav Med. 1996;18(4):290–304. doi: 10.1007/BF02895291. [DOI] [PubMed] [Google Scholar]
- 24.Bowen DJ, et al. Breast cancer risk counseling improves women’s functioning. Patient Educ Couns. 2004;53(1):79–86. doi: 10.1016/S0738-3991(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 25.Rabin BA, et al. Dissemination and implementation research on community-based cancer prevention: a systematic review. Am J Prev Med. 2010;38(4):443–456. doi: 10.1016/j.amepre.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Marcus BH, et al. Physical activity interventions using mass media, print media, and information technology. Am J Prev Med. 1998;15(4):362–378. doi: 10.1016/S0749-3797(98)00079-8. [DOI] [PubMed] [Google Scholar]
- 27.Oenema A, Brug J, Lechner L. Web-based tailored nutrition education: results of a randomized controlled trial. Health Educ Res. 2001;16(6):647–660. doi: 10.1093/her/16.6.647. [DOI] [PubMed] [Google Scholar]
- 28.Cassell MM, Jackson C, Cheuvront B. Health communication on the internet: an effective channel for health behavior change? J Health Commun. 1998;3(1):71–79. doi: 10.1080/108107398127517. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson, T. (1995). Consumer health informatics. The Healthcare Forum Journal, 38(1). [PubMed]
- 30.Green MJ, Fost N. An interactive computer program for educating and counseling patients about genetic susceptibility to breast cancer. J Cancer Educ. 1997;12(4):204–208. doi: 10.1080/08858199709528490. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman-Goetz, L., & Clarke, J. N. (2000). Quality of breast cancer sites on the World Wide Web. Can Journal Public Health, 91(4), 281–284. [DOI] [PMC free article] [PubMed]
- 32.Wootton JC. The quality of information on women’s health on the Internet. Journal of women’s health / the official publication of the Society for the Advancement of Women’s Health Research. 1997;6(5):575–581. doi: 10.1089/jwh.1997.6.575. [DOI] [PubMed] [Google Scholar]
- 33.Atienza AA, et al. E-health research and patient-centered care examining theory, methods, and application. Am J Prev Med. 2010;38(1):85–88. doi: 10.1016/j.amepre.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Pew Internet and American Life Project, The social life of health information. 2009, http://www.webcitation.org/5iDHSOJLE,http://www.pewinternet.org/~/media//Files/Reports/2009/PIP_Health_2009.pdf.
- 35.Leventhal H. Behavioral theories and the problem of compliance. 1987. [Google Scholar]
- 36.Decruyenaere M, et al. Cognitive representations of breast cancer, emotional distress and preventive health behaviour: a theoretical perspective. Psychooncology. 2000;9(6):528–536. doi: 10.1002/1099-1611(200011/12)9:6<528::AID-PON486>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Hoskins KF, et al. Assessment and counseling for women with a family history of breast cancer: a guide for clinicians. JAMA. 1995;273(7):577–585. doi: 10.1001/jama.1995.03520310075033. [DOI] [PubMed] [Google Scholar]
- 38.Shattuck-Eidens D, et al. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995;273(7):535–541. doi: 10.1001/jama.1995.03520310033026. [DOI] [PubMed] [Google Scholar]
- 39.Gail MH, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 40.Burke W, et al. Genetic counseling for women with an intermediate family history of breast cancer. Am J Med Genet. 2000;90(5):361–368. doi: 10.1002/(SICI)1096-8628(20000228)90:5<361::AID-AJMG4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Biesecker BB, et al. Genetic counseling for families with inherited susceptibility to breast and ovarian cancer. JAMA. 1993;269(15):1970–1974. doi: 10.1001/jama.1993.03500150082032. [DOI] [PubMed] [Google Scholar]
- 42.Botkin JR, et al. A model protocol for evaluating the behavioral and psychosocial effects of BRCA1 testing. J Natl Cancer Inst. 1996;88(13):872–882. doi: 10.1093/jnci/88.13.872. [DOI] [PubMed] [Google Scholar]
- 43.Andersen MR, Urban N. Physician gender and screening: do patient differences account for differences in mammography use? Women Health. 1997;26(1):29–39. doi: 10.1300/J013v26n01_03. [DOI] [PubMed] [Google Scholar]
- 44.Lerman C, et al. Psychological side effects of breast cancer screening. Health Psychol. 1991;10(4):259–267. doi: 10.1037/0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 45.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 46.Hannon, M., et al. Measuring perceptions of breast cancer risk. Health Psychology, Under Review.
- 47.Weinstein, N.D Taking care: understanding and encouraging self-protective behavior. Cambridge; New York: Cambridge University Press.
- 48.Helmes AW, Bowen DJ, Bengel J. Patient preferences of decision-making in the context of genetic testing for breast cancer risk. Genet Med. 2002;4(3):150–157. doi: 10.1097/00125817-200205000-00009. [DOI] [PubMed] [Google Scholar]


