Abstract
A number of research studies have attempted to translate the behavioral lifestyle intervention delivered in the Diabetes Prevention Program (DPP). To compare the active interventions of two trials, Diabetes Prevention Program DPP and Healthy Living Partnerships to Prevent Diabetes (HELP PD), after 1 and 2 years of intervention. DPP included 3234 adults with prediabetes randomized to intensive lifestyle intervention, metformin, troglitazone, or placebo. The lifestyle intervention, professionally delivered to individuals in a clinical setting, focused on diet and increased physical activity. HELP PD, a community-based translation of DPP, included 301 adults randomized to receive intensive lifestyle intervention or enhanced usual care. Mean weight-losses at 1 year (6.9 kg in DPP, 6.4 kg in HELP PD) and 2 years (5.5 kg in DPP, 4.4 kg in HELP PD) were similar across studies. Reductions in glucose were also similar across studies at both time points (5.2 mg/dL in DPP and 4.1 mg/dL in HELP PD at 1 year; 1.8 mg/dL and 1.6 mg/dL at 2 years). HELP PD participants achieved larger reductions in triglycerides at 1 and 2 years (38.4 mg/dL and 34.9 mg/dL, respectively) than DPP participants (24.8 mg/dL and 22.4 mg/dL). High-density lipoprotein decreased in HELP PD participants at year 1 (−0.6 mg/dL) and increased in DPP (1.2 mg/dL) but there were no significant differences in year 2. HELP PD, a community model for diabetes prevention, was similar to DPP in reducing body weight and lowering blood glucose, both important risk factors that should be controlled to reduce risk for developing type 2 diabetes.
Keywords: Diabetes prevention, Translation research, Behavioral lifestyle intervention
Introduction
Type 2 diabetes prevalence has consistently increased in the USA in the last 30 years [1]. Efforts to intervene in people who present with prediabetes, specifically those who have impaired glucose tolerance or impaired fasting glucose, are essential in order to delay or prevent the onset of type 2 diabetes. Health care organizations, public health service agencies, and a variety of other community services and organizations can play an important role in reducing risk; however, it is imperative that evidence-based interventions be utilized in these settings to achieve real progress in diabetes prevention.
Community-based translations of the lifestyle intervention used in the landmark clinical trial, the Diabetes Prevention Program (DPP) [2], have been conducted in an effort to provide lower cost programs to the broader population [3–12]. According to a recent meta-analysis of 22 DPP translational studies, these programs have achieved modest success with an average 12-month weight loss of 2.32 kg [13]. The Healthy Living Partnerships to Prevent Diabetes (HELP PD) trial was one of the largest randomized controlled trials to translate the methods of the original DPP [14]. Previously we have shown that the HELP PD intervention was effective in reducing fasting glucose relative to an enhanced usual care condition [9, 10]. We also reported that the intervention could be done at a lower cost than the DPP intervention [15]. As the DPP lifestyle balance intervention is considered the “gold standard” of lifestyle approaches to favorably impact risk factors for type 2 diabetes, the current evaluation was conducted to compare participant data in the active interventions of the two trials, DPP and HELP PD, to show that they provide comparable results, illustrating that the lower cost, community-based delivery of the HELP PD intervention is a practical option. Additionally, we assessed weight change and change in fasting glucose, triglycerides and high-density lipoprotein (HDL) and in several subgroups (sex, age and race) found to impact success in achieving weight loss and physical activity goals in DPP [16].
Methods
The Diabetes Prevention Program (DPP)
DPP was a highly successful multi-center randomized clinical trial funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to determine whether intensive lifestyle modification (weight loss, increased physical activity), standard lifestyle recommendations and metformin, or standard lifestyle recommendations plus placebo could delay or prevent type 2 diabetes in 3234 adults with prediabetes [17]. The trial originally had four arms but the troglitazone arm was discontinued due to the potential liver toxicity of the drug. To qualify, candidates had to be 25 or older, have impaired fasting glucose of 95–125 mg/dl or a blood glucose of 140–199 mg/dl 2 h post oral glucose tolerance test [18]. Data from DPP was obtained via data use agreement from the NIDDK Central Repository and for the purpose of this evaluation, only data from participants in the lifestyle intervention arm are included. Baseline data collection for DPP began in 1996 and data collection concluded in 2001 when the trial was stopped early due to the beneficial impact of the lifestyle intervention on reducing the incidence of diabetes. Full details regarding the study outcomes have been published elsewhere [2].
DPP lifestyle balance intervention
In DPP, the lifestyle intervention participants (n = 1051) were provided intensive education in dietary modification, physical activity and behavioral modification. The goal was to lose 7 % of body weight by 6 months and to maintain that weight loss over time. The physical activity goal was at least 150 min of moderate intensity physical activity per week. The DPP intervention was delivered primarily on an individual basis by a case manager, usually a registered dietitian or nurse educator, who was responsible for administering the 16-session core curriculum over a 24-week period. Participants were then seen face-to-face at least once every 2 months for the remainder of the trial [19].
Healthy living partnerships to prevent diabetes (HELP PD)
HELP PD was designed to translate key components of DPP for delivery in a real-world setting. Garfield, et al. [20] outlined a number of priority areas for diabetes translational research that we focused on to adapt HELP PD including, demonstrating effectiveness, understanding barriers/facilitators to translation, adopting chronic care paradigms, involving the community, developing organizational, financial, attitudinal, and cultural supports for sustaining gains at the individual patient level and program level, consideration of economic factors and conducting intervention research.
In HELP PD, we addressed financial, organizational, attitudinal and cultural supports for sustaining gains at the individual patient level and program level by locating the diabetes prevention program within an existing Diabetes Care Center (DCC), as DCCs have the financial and organizational supports to maximize the likelihood of sustainability. Eligibility criteria were simplified to utilize fasting glucose measures as routinely collected in primary care and graded exercise tests prior to participation were eliminated. At the individual level, we focused on delivering the intervention in group settings led by CHWs in conjunction with supervision and assistance from registered dietitians employed by the DCC. This approach provided organizational support in a cost-effective manner and enabled us to address long-term attitudinal support to individuals in a culturally appropriate manner. The delivery of HELP PD was cost-effective as reported by Lawlor et al.; per capita direct costs in 2010 dollars were $850 in HELP PD compared to $2631 for DPP [15, 21].
HELP PD was a single-center randomized controlled research trial involving 301 community dwelling adults. Briefly, to qualify for the trial, participants were required to be 21 years of age or older, overweight or obese (25–40 kg/m2) with fasting blood glucose 95–125 mg/dl. Participants were recruited from Forsyth County, North Carolina and those who enrolled were representative of the local community with regards to ethnicity and race [22]. Eligible candidates were randomized using a block randomization with block sizes of 2 and 4. The randomization was 1:1; participants had an equal probability of being randomized to either the control or intervention group. Eligible participants were randomized to a lifestyle weight loss intervention or to an enhanced usual care condition. Baseline characteristics of the study participants did not differ significantly between treatment groups. All participants signed informed consent and the Institutional Review Board of Wake Forest University Health Sciences approved the trial. Details regarding the HELP PD trial design, participant recruitment, outcomes, and cost have been published elsewhere [9, 10, 14, 15, 22–24]. For the purpose of this evaluation, only data from participants in the lifestyle intervention arm is included.
HELP PD lifestyle weight loss intervention (LWL)
The goal for the LWL participants (n = 151) was to achieve a ≥5 % weight loss at 6 months and to complete at least 150 min per week of moderate intensity physical activity. At study entry, all intervention participants were individually counseled by a registered dietitian employed by the local DCC. During this session, the participants were provided a daily calorie and physical activity goal. Calorie goals ranged from 1200 to 1800 cal depending on baseline weight. During the intensive phase (first 6 months) of the intervention, participants attended weekly group meetings facilitated by a CHW. CHWs were community members who had been diagnosed with T2DM and were successfully implementing lifestyle behaviors to manage their T2DM. CHWs received 36 h of training prior to facilitating group sessions. The training was provided by DCC dietitians who then managed the CHWs throughout the intervention [24]. To support the fidelity of the intervention delivery, key concepts of the weight-loss intervention were provided via a DVD series that was developed by the investigative team. HELP PD utilized the 16 session curriculum developed by the DPP lifestyle intervention and added eight additional sessions which incorporated presentations by local community partners and group trouble-shooting sessions. The maintenance phase, months 7–24, consisted of monthly CHW-facilitated group meetings and one monthly individual telephone contact with the CHW.
Data collection and measurement
HELP PD was modeled after DPP and therefore the study investigators intentionally utilized forms similar to those used in DPP. In both studies, self-reported information concerning demographic and health characteristics was collected via standardized questionnaires.
For both studies, height was measured using a wall-mounted stadiometer and weight was measured by digital scale. Body weight measurements were performed in light clothing (jackets, coats, shoes removed). BMI was calculated as body weight in kilograms divided by the square of height in meters.
For the HELP PD study, a research data collector and the General Clinical Research Center staff were trained to collect the study data. Although the study staff members were not blinded, none were involved in the delivery or conduct of the intervention and laboratory staff and GCRC staff were blinded to randomization assignment.
Details for blood collection procedures and analyses for DPP have been described in detail [17]. Briefly, each DPP site followed a standardized manual of procedures for collecting and processing blood samples. Samples were stored at −20 °C prior to being shipped on dry ice to the Biochemistry Laboratory (University of Washington, Seattle, WA) where all analyses were performed. Plasma glucose was measured by a chemistry autoanalyzer using the glucokinase method [25]. Triglyceride measures were performed enzymatically [26] using standardized methods as described in the Centers for Disease Control and Prevention Reference Methods. HDL fractions for cholesterol analysis were obtained by the treatment of plasma with dextran sulfate-Mg2+ to precipitate all apo lipoprotein B -containing lipoproteins [27].
For the HELP PD study, all sample preparation was performed in the General Clinical Research Center laboratory and the prepared specimens were then sent to the Clinical Chemistry laboratories of Wake Forest Baptist Medical Center (Winston-Salem, NC) for analysis. Blood glucose was measured using a timed-end point method supplied by Beckman Coulter for the Synchron LX Analyzer, a method accepted by the Centers for Disease Control and Prevention. HDL cholesterol and triglycerides were analyzed using the Synchron LX system. Triglyceride GPO reagent was used to measure the triglyceride concentration by a timed endpoint method.
Statistical methods
The analyses described here were performed in 2015. DPP data was obtained via data use agreement from the NIDDK Central Repository. Descriptive statistics were calculated to show similarities and differences between the HELP PD and DPP cohorts. Chi-square tests (categorical measures) and t tests (continuous measures) were used to test for differences in baseline characteristics. At clinic visits where a participant reported having used a diabetes-related drug, the participant was removed from the analysis dataset but was added back to the dataset if the medication was not reported at subsequent visits. This removed data for two participants at 1 year and two participants at the2-year follow-up for HELP PD and2-year follow-up data for three DPP participants. For the analysis of change in triglycerides and HDL, visits during which participants reported using drugs targeting triglycerides or HDL were also excluded. The percent of participants in each study meeting weight loss targets (losing over 5, 7, 10, and 15 % of baseline weight) at 1 and 2 years were plotted, and logistic regression was used to assess study differences in the proportion of participants meeting those goals. Change in weight, fasting glucose, triglycerides, and high-density lipoprotein (HDL) were modeled using a mixed effects repeated measures analysis of covariance (SAS PROC MIXED) making use of both the 1- and2-year follow-up visits. The model adjusts for the baseline measure of the outcome, visit, study, baseline characteristics of sex (male, female), race (white, non-white), age (<50, 50–59, 60+), Beck depression index (0–10, 11+), marital status (never married, married, or living marriage like, divorced/widowed/separated), employment status (full or part time, other), the number living in the household (live by myself, live with one other person, live with two other people, live with three or more others). Additional covariates for the change in glucose, triglycerides, and HDL outcomes included baseline BMI (continuous), and a baseline BMI by sex interaction. Subgroup estimates were obtained by adding the study × visit × variable of interest interaction. p values for study differences were obtained by contrasting the least square (LS) means overall and within each subgroup at each study visit. Subgroup p values ≤ 0.01 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 151 participants from the HELP PD study intervention arm and 1051 participants from the DPP lifestyle intervention arm were available for baseline comparison between the studies. Study differences were observed for sex, race, age, employment status, the number in the household, and baseline body weight in females (Table 1). Compared to the DPP study, the HELP PD study had a smaller proportion of females (HELP PD, 58 % vs. DPP, 68 %), non-white (26 vs. 45 %), and full or part time employed participants (65 vs. 73 %). HELP PD participants were older than those enrolled in DPP (age <50, 28 % in HELP PD vs. 51 % in DPP). Females in the HELP PD study had a lower mean baseline body weight than females in DPP (89.2 kg in HELP PD vs. 92.6 kg in DPP). No differences were observed between the two study populations for the Beck depression index, marital status, or weight in males.
Table 1.
Baseline characteristics of participants in HELP PD and DPP lifestyle intervention groups
| Characteristic | HELP PD (N = 151) N (%) |
DPP (N = 1051) N (%) |
p value |
|---|---|---|---|
| Sex | 0.016 | ||
| Male | 64 (42) | 341 (32) | |
| Female | 87 (58) | 710 (68) | |
| Race | <0.001 | ||
| White | 111 (74) | 580 (55) | |
| Non-white | 40 (26) | 471 (45) | |
| Age | <0.001 | ||
| <50 | 42 (28) | 539 (51) | |
| 50–59 | 51 (34) | 282 (27) | |
| 60+ | 58 (38) | 230 (22) | |
| Beck Depression Index (BDI) | 0.490 | ||
| 0–10 | 133 (88) | 918 (90) | |
| 11+ | 18 (12) | 103 (10) | |
| Marital status | 0.154 | ||
| Never married | 12 ( 8) | 139 (14) | |
| Married/living marriage-like | 105 (70) | 674 (66) | |
| Divorced/widowed/separated | 34 (23) | 211 (21) | |
| Employment | 0.037 | ||
| Full or part time | 98 (65) | 748 (73) | |
| Other | 53 (35) | 276 (27) | |
| Number in house | <0.001 | ||
| Live by myself | 26 (17) | 182 (18) | |
| 1 other | 84 (56) | 354 (35) | |
| 2 others | 20 (13) | 183 (18) | |
| 3+ others | 21 (14) | 305 (30) | |
| Body mass index (kg/m2) | |||
| Male | 0.070 | ||
| <30 | 19 (30) | 152 (45) | |
| 30–34.9 | 28 (44) | 109 (32) | |
| 35+ | 17 (27) | 78 (23) | |
| Mean (SD) | 32.1 (0.5) | 32.1 (0.3) | 0.939a |
| Female | 0.445 | ||
| <30 | 20 (23) | 187 (27) | |
| 30–34.9 | 31 (36) | 201 (29) | |
| 35+ | 36 (41) | 297 (43) | |
| Mean (SD) | 33.4 (0.4) | 34.9 (0.3) | 0.004a |
| Weight (kg) | |||
| Male mean (SD) | 101.5 (1.8) | 98.7 (1.1) | 0.191a |
| Female mean (SD) | 89.2 (1.4) | 92.6 (0.8) | 0.032a |
p values are from a chi-square test
a p values are from a t test
Follow-up clinical assessments (data available on one or more outcomes of interest) were conducted in 133 (88 %) HELP PD intervention participants at 12 months and 125 (83 %) at 24 months. DPP conducted follow-up visits in 998 intervention participants (95 %) at 12 months and 964 participants (92 %) at 24 months.
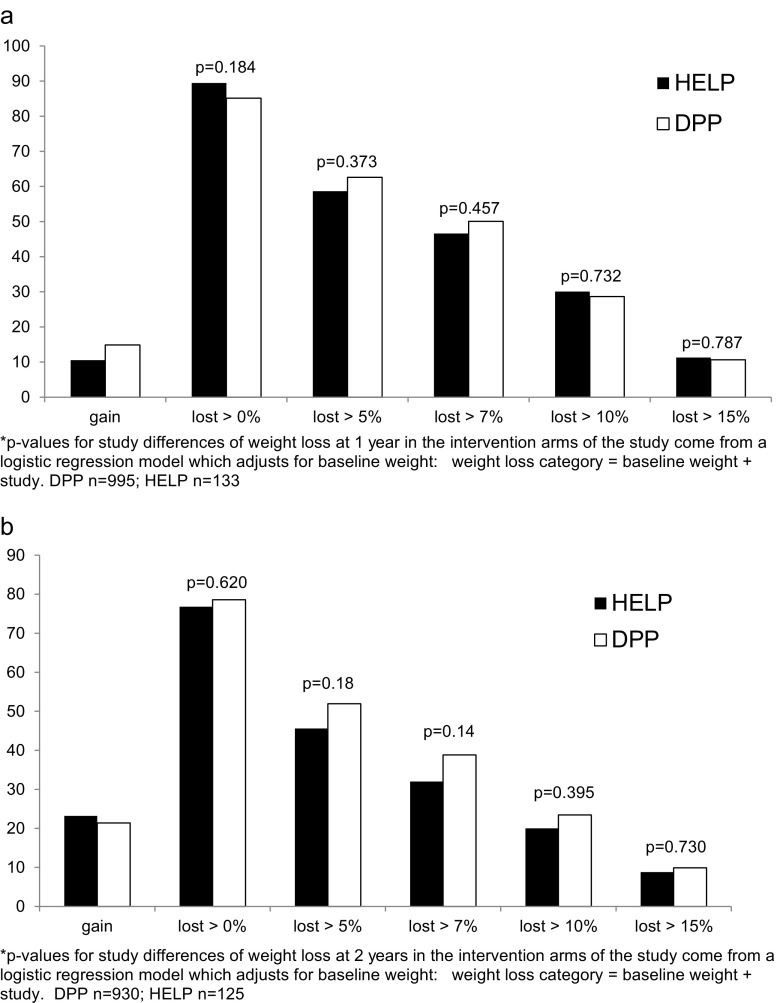
After 1 year of intervention (Fig. 1a), 89 % of the HELP PD study participants had lost weight with 11 % losing 15 % or more of their baseline weight. Similarly, in DPP 85 % of participants lost weight with 11 % losing 15 % or more of their baseline weight. The amount of weight lost after 2 years of intervention was also similar for the two studies (Fig. 1b).
Fig. 1.
a Weight loss in HELP and DPP at 1 year. p values for study differences of weight loss at 1 year in the intervention arms of the study come from a logistic regression model which adjusts for baseline weight: weight loss category = baseline weight + study. DPP n = 995; HELP n = 133. b Weight loss in HELP and DPP at year 2. p values for study differences of weight loss at 2 years in the intervention arms of the study come from a logistic regression model which adjusts for baseline weight: Weight loss category = Baseline weight + Study. DPP n = 930; HELP n = 125
HELP PD participants lost an average 6.4 (SE = 0.6) and 4.4 (0.6) kilograms at 1- and 2-year assessment visits, respectively, whereas DPP participants lost 6.9 (0.2) and 5.5 (0.2) kg. These differences were not significantly different at either time point (year 1 difference [95 % confidence interval], −0.5 [−1.8, 0.8] p = 0.47; year 2, −1.1 [−2.4, 0.2] p = 0.10). The weight loss in both studies was fairly consistent across sex, race and age subgroups (Table 2). In HELP PD, the participants who did not have data (defined as having a valid weight) at 12 months, whether missing due to missed visit or excluded from the analyses as they reported using a diabetes medication at the visit, differed in terms of age (those missing data were of younger average age), and number living in the household (of those missing data, a higher proportion had 3+ others in the house). At 24 months, only the number in the household showed a significant difference between those with and without a visit. The covariates that differed between those with and without dropout were included in the mixed models.
Table 2.
LS mean (SE) weight change (in kilogram) from baseline in HELP PD and DPP at years 1 and 2
| Year 1 | Year 2 | |||||
|---|---|---|---|---|---|---|
| HELP PD n = 133 |
DPP n = 995 |
p value | HELP PD n = 125 |
DPP n = 930 |
p value | |
| Overall | −6.4 (0.6) | −6.9 (0.2) | 0.468 | −4.4 (0.6) | −5.5 (0.2) | 0.102 |
| Sex | ||||||
| Male | −6.2 (1.0) | −7.2 (0.4) | 0.335 | −4.5 (1.0) | −6.0 (0.4) | 0.160 |
| Female | −6.7 (0.8) | −6.8 (0.3) | 0.911 | −4.3 (0.8) | −5.2 (0.3) | 0.306 |
| Race | ||||||
| White | −6.8 (0.7) | −7.7 (0.3) | 0.277 | −4.3 (0.7) | −5.8 (0.3) | 0.057 |
| Non-white | −6.0 (1.2) | −5.9 (0.4) | 0.896 | −5.2 (1.2) | −5.0 (0.4) | 0.848 |
| Age | ||||||
| <50 | −7.9 (1.2) | −6.6 (0.4) | 0.290 | −4.7 (1.2) | −5.0 (0.4) | 0.809 |
| 50–59 | −6.1 (1.1) | −7.3 (0.4) | 0.308 | −4.7 (1.1) | −5.6 (0.4) | 0.454 |
| 60+ | −6.2 (1.0) | −7.1 (0.5) | 0.376 | −4.3 (1.0) | −6.4 (0.5) | 0.050 |
p values are based on differences of LS means
The reduction in glucose (in milligram per deciliter) from baseline to the year 1 clinic assessment in HELP PD was 4.1 mg/dL (SE = 1.0) and 1.6 mg/dL (1.0) at the2-year visit; DPP had a reduction in glucose at the year 1 clinic assessment of 5.2 mg/dL (0.4) and 1.8 mg/dL (0.4) at the year 2 assessment (Table 3). The difference in glucose reduction overall was not significantly different between the two studies at year 1 (−1.1 [−3.2, 1.0] p = 0.31) or year 2 (−0.2 [−2.4, 2.0] p = 0.84). This non-significant difference was observed in adjusted models for all the subgroups.
Table 3.
LS mean (SE) fasting glucose change from baseline
| Year 1 | Year 2 | |||||
|---|---|---|---|---|---|---|
| HELP PD n = 133 |
DPP n = 996 |
p value | HELP PD n = 125 |
DPP n = 930 |
P value | |
| Overall | −4.1 (1.0) | −5.2 (0.4) | 0.310 | −1.6 (1.0) | −1.8 (0.4) | 0.836 |
| Sex | ||||||
| Male | −1.9 (1.6) | −4.5 (0.7) | 0.121 | −2.2 (1.6) | −0.9 (0.7) | 0.450 |
| Female | −5.5 (1.3) | −5.5 (0.5) | 0.999 | −0.9 (1.4) | −2.2 (0.5) | 0.364 |
| Race | ||||||
| White | −3.8 (1.2) | −6.0 (0.5) | 0.081 | −0.6 (1.2) | −2.1 (0.5) | 0.253 |
| Non-white | −5.3 (2.0) | −3.9 (0.6) | 0.484 | −4.7 (2.0) | −1.3 (0.6) | 0.099 |
| Age | ||||||
| < 50 | −5.5 (2.0) | −5.2 (0.6) | 0.893 | −2.4 (2.1) | −1.0 (0.6) | 0.532 |
| 50–59 | −4.5 (1.7) | −5.8 (0.7) | 0.465 | −2.5 (1.8) | −2.9 (0.7) | 0.822 |
| 60+ | −2.9 (1.6) | −4.2 (0.9) | 0.456 | −0.3 (1.7) | −1.9 (0.9) | 0.357 |
p values are based on differences of LS means
For triglycerides, the two studies differed in terms of change from baseline. After 1 year of intervention, HELP PD had a significantly (13.6 [2.3, 24.9] p = 0.02) larger reduction in triglycerides from baseline (38.4 mg/dL, SE = 5.4) than DPP (24.8 mg/dL, SE = 1.9). After 2 years of intervention, a significant difference (12.5 [0.9, 24.0] p = 0.03) was still observed in the reduction in triglycerides between the studies (HELP PD: 34.9 mg/dL, SE = 5.5; DPP: 22.4 mg/dL, SE = 1.9). None of the subgroups showed a significant difference in triglyceride levels at the 0.01 level at either the year 1 or year 2 assessments.
The change from baseline in HDL was significantly different between HELP PD and DPP at year 1 (1.8 [0.5, 3.1] p = 0.01), but not at year 2 (0.6 [−0.7, 2.0] p = 0.36). At 1 year, HELP PD had a decrease in HDL of 0.6 mg/dL (SE = 0.6) while DPP had an increase of 1.2 mg/dL (SE = 0.2). By year 2, HDL change became more similar with both studies showing small increases from baseline (HELP-PD—0.1 mg/dL, SE = 0.7; DPP—0.8 mg/dL, SE = 0.2). After 1 year in the study, subgroups showing significant differences in HDL between studies included whites (2.1 [0.5, 3.7] p < 0.01) and those 60 or older (3.0 [0.9, 5.2] p < 0.01).
Discussion
In this evaluation, we compared the HELP PD lifestyle intervention data to those of the DPP lifestyle balance intervention to determine whether utilizing the lower cost, community-based, CHW-led intervention resulted in reduced effectiveness. We report here that the HELP PD results were similar to those of the DPP.
HELP PD sought to translate the DPP lifestyle intervention for community-based settings and utilized several key translational elements, including a group-based intervention format, the inclusion of CHWs as intervention facilitators, and the use of fasting glucose as the primary outcome. Both the DPP and HELP PD study interventions were successful in facilitating participant weight loss and significant reductions in fasting glucose levels. The studies were similar in that both utilized calorie restrictions, exercise goals, and tool box strategies to achieve weight loss and change in biomarkers. Both studies were able to retain participants over time with 83 % in HELP PD and 92 % in DPP returning for assessment visits at year 2. The better retention in DPP may be related to the face-to-face visits with health care providers. Importantly, body weight and blood glucose were lowered in both studies in a similar fashion even though the HELP PD participants were encouraged to lose 5 % of their baseline weight compared to DPP participants who were encouraged to lose 7 % of their baseline weight.
As an estimated 86 million adults in the US have prediabetes [1]; it is critical to provide a variety of intervention approaches to meet the demand placed on the health care system to intervene early to prevent the onset of diabetes. Many healthcare providers are struggling to identify ways to facilitate weight loss in their patients who are increasingly suffering from the multiple-morbidities of obesity, including prediabetes. The HELP PD study intervention should be considered for inclusion in the arsenal of evidence-based approaches to prevent diabetes. The HELP PD intervention activities were purposefully linked to an established health care provider, as staff in the Diabetes Care Center managed the CHW activities. This is a unique aspect of HELP PD as most other diabetes prevention translational studies include either medical professionals or community-based programs, but few studies have included a partnership between community-based resources and established medical care facilities [28, 29]. Potential benefits from this partnership when delivering future diabetes prevention programming include an established referral process for potential participants, access to an existing patient population that can serve as CHWs, personnel with the necessary expertise to oversee the intervention, and additional medical oversight as needed. HELP PD outcomes were achieved at a fraction of the cost by utilizing CHWs to facilitate the intervention groups, and we were able to obtain similar results to trained professionals. Lawlor et al. [15] reported that the cost of delivering the HELP PD intervention was approximately one-third the per capita cost of delivering the DPP intervention. Furthermore, the HELP PD intervention curriculum has been approved by the CDC for use in national diabetes prevention programming.
Limitations
There are differences that warrant discussion. The main outcome for HELP PD was change in fasting blood glucose as opposed to the development of diabetes as assessed in DPP; therefore, we were unable to directly compare the diabetes incidence rates between studies. In the HELP PD intervention, we increased the number of intervention sessions during the initial 6-months (16 in DPP, 24 in HELP PD), and the sessions in HELP PD were conducted using a group format rather than one-on-one as delivered in DPP. It is possible that if DPP had included 24 sessions in the core curriculum, weight loss and change in biomarkers could have been even better for DPP participants. In addition, the studies were not conducted during the same time frame as the DPP began in 1996 while HELP PD began in 2007.
Conclusions
Overall, the HELP PD lifestyle intervention results were similar to those of the DPP lifestyle balance intervention providing convincing evidence that utilizing this lower cost, community-based intervention did not result in reduced effectiveness.
Kahn and Davidson [30] stated that limitations in the evidence base of community diabetes prevention programs support not providing funding for these types of interventions. To address this point of concern, HELP PD and other community translations of the DPP should be willing to test their interventions in randomized controlled trials and provide results comparable to the DPP before the intervention is further disseminated. The HELP PD intervention produced results comparable to those of the gold standard DPP pre-diabetes intervention and is an effective approach to lifestyle change to reduce body weight and lower blood glucose, both important risk factors for developing T2DM, in the community setting.
Acknowledgments
The authors would like to thank the participants in the HELP PD and DPP trials, the research staff of both trials, and the Joslin Diabetes Center of Wake Forest Baptist Medical Center for their work in delivering the HELP PD lifestyle intervention. The Healthy Living Partnerships to Prevent Diabetes was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, R18DK069901) and conducted by researchers at Wake Forest School of Medicine. NIDDK had no role in the design of HELP PD; the collection, analysis and interpretation of study data; the preparation of this manuscript; and the decision to submit it for publication. The Diabetes Prevention Program (DPP) was conducted by the DPP Research Group and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the General Clinical Research Center Program, the National Institute of Child Health and Human Development (NICHD), the National Institute on Aging (NIA), the Office of Research on Women’s Health, the Office of Research on Minority Health, the Centers for Disease Control and Prevention (CDC), and the American Diabetes Association. The data from the DPP were supplied by the NIDDK Central Repositories. This manuscript was not prepared under the auspices of the DPP and does not represent analyses or conclusions of the DPP Research Group, the NIDDK Central Repositories, or the NIH. No financial disclosures were reported by the authors of this paper.
Compliance with ethical standards
Conflict of interest
No conflicts of interest, financial or otherwise, were reported by the authors of this paper.
Previous reporting of data
This manuscript has not been previously published and is not under consideration in the same or substantially similar form in any other peer-reviewed media. Other manuscripts from the HELP PD study include: design, recruitment and baseline characteristics, one and2-year fasting glucose, insulin, and adiposity outcomes, and cost, with an additional manuscript under review reporting 2-year outcomes related to the metabolic syndrome.
Data access
The authors of this manuscript had full control of the data used to perform the analyses described herein and agree to allow Translational Behavioral Medicine to review the data if so requested.
Ethics approval and consent to participate
The HELP PD study was reviewed and approved by the Institutional Review Board (IRB) no. 2 of Wake Forest University Health Sciences (IRB00000613) and all participants were required to sign an informed consent document prior to participation. All procedures were conducted in accordance with the ethical standards of the IRB of Wake Forest University Health Sciences and all local, state, and national regulations regarding the protection of human subjects.
Welfare of animals
Not applicable. The studies described in this manuscript were conducted only in humans; no data was collected from animals.
Footnotes
Implications
Research: This evaluation demonstrates that the Healthy Living Partnerships to Prevent Diabetes (HELP PD) lifestyle intervention results were similar to those of the Diabetes Prevention Program (DPP) lifestyle balance intervention providing convincing evidence that utilizing this lower cost, community-based intervention did not result in reduced effectiveness and future translational studies related to DPP should be assessed in a similar way.
Practice: HELP PD was similar to DPP in reducing body weight and lowering blood glucose, both clinically important risk factors that should be controlled to reduce risk for developing type 2 diabetes.
Policy: The use of community health workers to deliver the HELP PD intervention effectively supports the development of reimbursement policies to implement and sustain evidence-based, lay-led programs, like HELP PD, within the health care system.
Trial Registration: NCT00631345; Clinicaltrials.gov, registered March 5, 2008
IRB: Wake Forest University Health Sciences; IRB00000613; initial approval 10/10/2006
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report: estimates of diabetes and its burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Absetz P, Valve R, Oldenburg B, Heinonen H, Nissinen A, Fogelholm M, Ilvesmaki V, Talja M, Uutela A. Type 2 diabetes prevention in the “real world”: one-year results of the GOAL implementation trial. Diabetes Care. 2007;30:2465–2470. doi: 10.2337/dc07-0171. [DOI] [PubMed] [Google Scholar]
- 4.Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, Fogelholm M, Talja M, Uutela A. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care. 2009;32:1418–1420. doi: 10.2337/dc09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the diabetes prevention program into the community. The DEPLOY pilot study. Am J Prev Med. 2008;35:357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann RT, Liss DT, Finch EA, Schmidt KK, Hays LM, Marrero DG, Saha C. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015;105:2328–2334. doi: 10.2105/AJPH.2015.302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract. 2008;14:29–32. doi: 10.1097/01.PHH.0000303410.66485.91. [DOI] [PubMed] [Google Scholar]
- 8.Davis-Smith YM, Boltri JM, Seale JP, Shellenberger S, Blalock T, Tobin B. Implementing a diabetes prevention program in a rural African-American church. J Natl Med Assoc. 2007;99:440–446. [PMC free article] [PubMed] [Google Scholar]
- 9.Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, Goff DC., Jr One-year results of a community-based translation of the diabetes prevention program: healthy-living partnerships to prevent diabetes (HELP PD) project. Diabetes Care. 2011;34:1451–1457. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katula JA, Vitolins MZ, Morgan TM, Lawlor MS, Blackwell CS, Isom SP, Pedley CF, Goff DC., Jr The healthy living partnerships to prevent diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med. 2013;44:S324–S332. doi: 10.1016/j.amepre.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, Siminerio LM, Solano FX, Orchard TJ. Translating the diabetes prevention program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the diabetes prevention program to primary care: a pilot study. Nurs Res. 2009;58:2–12. doi: 10.1097/NNR.0b013e31818fcef3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, Khunti K. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37:922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 14.Katula JA, Vitolins MZ, Rosenberger EL, Blackwell C, Espeland MA, Lawlor MS, Rejeski WJ, Goff DC. Healthy living partnerships to prevent diabetes (HELP PD): design and methods. Contemp Clin Trials. 2010;31:71–81. doi: 10.1016/j.cct.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Diabetes Prevention Program Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin RR, Fujimoto WY, Marrero DG, Brenneman T, Charleston JB, Edelstein SL, Fisher EB, Jordan R, Knowler WC, Lichterman LC, Prince M, Rowe PM. The diabetes prevention program: recruitment methods and results. Control Clin Trials. 2002;23:157–171. doi: 10.1016/S0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 18.Diabetes Prevention Program (DPP) Research Group The diabetes prevention program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garfield SA, Malozowski S, Chin MH, Venkat Narayan KM, Glasgow RE, Green LW, et al. Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26:2670–2674. doi: 10.2337/diacare.26.9.2670. [DOI] [PubMed] [Google Scholar]
- 20.Herman WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, Lachin JM, Engelgau MM. Diabetes prevention program research group costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26(1):36–47. doi: 10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackwell CS, Foster KA, Isom S, Katula JA, Vitolins MZ, Rosenberger EL, Goff DC., Jr Healthy living partnerships to prevent diabetes: recruitment and baseline characteristics. Contemp Clin Trials. 2011;32:40–49. doi: 10.1016/j.cct.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katula JA, Blackwell CS, Rosenberger EL, Goff DC., Jr Translating diabetes prevention programs: 1tions for dissemination and policy. N C Med J. 2011;72:405–408. [PMC free article] [PubMed] [Google Scholar]
- 23.Lawlor MS, Blackwell CS, Isom SP, Katula JA, Vitolins MZ, Morgan TM, Goff DC., Jr Cost of a group translation of the diabetes prevention program: healthy living partnerships to prevent diabetes. Am J Prev Med. 2013;44:S381–S389. doi: 10.1016/j.amepre.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sydell, J., Kernodle, D., Blackwell, C. S., & Vitolins, M. Z. (2015). Identifying, training, and monitoring community health Workers in a Community-Based Diabetes Prevention Study. JJ Community Medicine, 1.
- 25.McCraw EF, Peterson MJ, Yarnell G, Ashmore J. Autoregulation of glucose in the isolated perfused rat liver. Adv Enzym Regul. 1968;6:57–65. doi: 10.1016/0065-2571(68)90007-1. [DOI] [PubMed] [Google Scholar]
- 26.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 27.Warnick GR, Mayfield C, Benderson J, Chen JS, Albers JJ. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. Am J Clin Pathol. 1982;78:718–723. doi: 10.1093/ajcp/78.5.718. [DOI] [PubMed] [Google Scholar]
- 28.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the diabetes prevention program? Health Aff (Millwood) 2012;31:67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 29.Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med. 2011;1:480–491. doi: 10.1007/s13142-011-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes Care. 2014;37:943–949. doi: 10.2337/dc13-1954. [DOI] [PubMed] [Google Scholar]