Abstract
Smoking and physical inactivity contribute to disproportionate disease burden among underserved adults. Telephone-based interventions (quitlines) are becoming the standard care for addressing smoking. There is increasing interest to determine whether quitlines can be utilized to administer interventions for other unhealthy behaviors. This study aims to examine the proof-of-concept and potential efficacy of a telephone-based behavioral counseling intervention to boost daily low-to-moderate physical activity among low-income, physically inactive smokers. Participants (N = 101) were randomized to receive 4 weeks of counseling prior to their smoking quit day that included either standard smoking cessation counseling (control) or the Step-up to Quit (SUTQ) intervention. SUTQ promoted daily walking to foster physical activity as a primary smoking urge management strategy and facilitate incremental increases in daily steps with the goal of achieving 7500 steps/day by the quit day in week 4. Exploratory structural equation modeling tested SUTQ effects on six measures of low-to-moderate physical activity (primary outcome) and smoking cue reactivity (secondary outcome) simultaneously in a single multivariate model with controlling variables. The sample was 51 % female and 77 % African-American, with a mean age of 42.1 years (SD = 10.9). Compared to the control condition, SUTQ intervention was associated with greater physical activity at week 4 (b = 0.51, z = 1.71, p = 0.08), with between-group differences sustained at follow-up. At week 4, the SUTQ group had higher 7-day mean steps/day (M = 7,207.25, SD = 4,276.03) than controls (M = 3,947.03, SD = 3,655.03) (t = 3.35; p < .01); and had more participants reach the >7500 steps/day goal (49% vs. 11 %, c2 = 10.78; p < .01), a difference that was sustained at 1-month follow-up (X2 = 9.04, p < .01) Effects of SUTQ treatment on cue reactivity were in the hypothesized direction but not significant (b = −0.29; z = −1.09, p = 0.27). To our knowledge, this is the first study to promote physical activity using telephone counseling in an underserved population of smokers known to have greater challenges with physical activity adoption and smoking cessation. The SUTQ approach suggests that integration of physical activity advice and support within the context of smoking cessation treatment has the potential to promote physical activity among smokers intending to quit.
Keywords: Physical activity, Smoking cue reactivity, Telephone-based counseling, Low-income smokers
BACKGROUND
Telephone-based smoking cessation services, or quitlines, have become important components of comprehensive tobacco control and cessation programs [1]. Quitlines have intuitive appeal for delivering services because they are cost-efficient means to provide evidence-based smoking cessation counseling, eliminate potential barriers to treatment access (e.g., lack of transportation, inability to pay for support), and can effectively reach underserved or disparate groups of smokers who may have difficulties accessing traditional forms of treatment [2–4]. Given the emergence of quitlines as the standard of care for smoking cessation counseling in the United States along with evidence that smokers are more likely than the general population to engage in other unhealthy behaviors (e.g., more than 90 % of smokers maintain co-existing unhealthy behaviors [5]) public health professionals have shown increased interest in expanding telephone-based services to address other health behaviors than just smoking [6]. Physical inactivity is one such behavior that could be addressed via quitlines given its high occurrence among smokers [7, 8].
Well-established evidence points to the many benefits of routine physical activity (e.g., reducing cancer risk [9], enhancing cardiovascular health [10, 11]). Despite efforts to educate the public about the benefits of physical activity, many adults in the USA remain routinely inactive. Similar to smoking, physical inactivity is more likely in low-income populations compared to higher-income groups [12, 13]. Low-income populations also face greater health risks related to low physical activity than higher-income groups (e.g., hypertension, obesity) [14]. Therefore, combining elements of behavioral health treatment for addressing both physical activity and smoking in the same intervention could increase the impact of public health efforts to reduce disease risk in this vulnerable population of smokers.
Multiple health behavior change interventions attempt to treat two or more health behaviors, either sequentially or concurrently [15, 16], within a limited time period [17]. Testing such interventions is warranted because of potential efficiencies and synergistic effects that may occur when treating multiple behaviors in the same intervention as opposed to treating each target behavior separately [18]. Individuals making efforts to change one health behavior may be likely to change another if they understand the behaviors influence one another [19]. For example, physical activity could be engaged as a coping strategy or compensatory behavior during a smoking urge episode when someone is trying to quit smoking. Moreover, recent evidence suggests that success in changing one behavior may serve as a “gateway” to promote other health behavior change [17, 20], perhaps due to increased self-efficacy in the behavior change process.
Previous studies combining physical activity and smoking cessation treatment suggest potential to address both behaviors in tandem. A recent systematic review showed that short bouts of physical activity reduced smoking urges, withdrawal, and negative mood in smokers maintaining short-term abstinence [15, 21]. Other studies provide evidence supporting vigorous-intensity physical activity for long-term abstinence [22]. However, methodological differences (e.g., variability in length, intensity, and timing in relation to quitting) make it difficult to determine which physical activity intervention elements could facilitate both physical activity adoption and smoking behavior change in different sub-groups. Moreover, we are not aware of any studies that have targeted low-income, underserved smokers known to have greater challenges with health behavior adoption. Given that supervised vigorous physical activity programs lack external validity in low-income smokers [23], there is potential utility in strategies that integrate more realistic physical activity programming (low-to-moderate intensities) within the context of smoking treatment. Such strategies may be more acceptable for this population [24], thereby also reducing attrition [25]. Moreover, considering the acute positive effects of engaging in short bouts of physical activity on smoking urges and nicotine withdrawal [16], examining the effects of change in routine physical activity on withdrawal and cue-induced urge reactivity is warranted. Theoretically, using physical activity as an alternate reinforcement to smoking during urge episodes could facilitate the reduction in cue reactivity over time. Therefore, teaching smokers to practice engaging in physical activity to manage smoking urges when they occur [26], could be an ecologically valid and potentially effective approach to integrating physical activity as a counterconditioning strategy to facilitate health behavior change during smoking cessation treatment. To date, few studies have attempted to integrate physical activity efforts and urge management training in this manner [15, 27].
To address these gaps in existing approaches, the current study reports the proof-of-concept and preliminary efficacy of an adoptable, translatable, and telephone-based multiple health behavior change intervention model, “Step-up to Quit (SUTQ)”. The purpose of the SUTQ intervention was to increase physical activity (primary outcome) among smokers intending to quit smoking and to test treatment effects on quit day smoking cue reactivity (secondary outcome). Specifically, the SUTQ counseling was structured to engage treatment-seeking smokers in low-to-moderate physical activity programming by promoting daily steps (walking) and linking short bouts of physical activity with urge management training, thus integrating evidence-based physical activity promotion- and smoking cessation-related health messaging. Given the efficacy and utility of telephone-based services or quitlines to promote smoking behavior change for disadvantaged populations [2, 4], this study used a proactive telephone counseling approach to deliver the multiple health behavior change intervention. We hypothesized that compared to a control group that received standard, evidence-based quitline counseling only, the SUTQ intervention group would have greater daily physical activity and lower smoking cue reactivity on their quit day. Exploratory outcomes included physical activity and smoking quit rates at 1 week and 1 month post-quit day follow-ups.
METHODS
Participants
Participants included treatment-seeking male and female smokers aged 18–59 who self-reported low levels of physical activity. Participants were recruited using fliers placed in local stores and laundromats in low-income, largely African-American neighborhoods in urban North Philadelphia (median annual income in recruitment areas ranged from US$16,500 to 29,000) [28]. Study inclusion criteria included smoking more than six cigarettes per day [29], intention to quit smoking within the next 6 months, and low physical activity. Low physical activity was measured via self-report using the International Physical Activity Questionnaire (IPAQ) [30] at the time of eligibility screening and was defined as <20 min of purposeful vigorous-intensity activity or <60 min of purposeful moderate-intensity activity, or <100 min of purposeful walking during a typical week [30]. The IPAQ captures three specific types of activity: walking, moderate intensity, and vigorous intensity. Exclusion criteria included pregnancy; substance abuse (e.g., alcohol, stimulants, or narcotics) within the past 12 months; current diagnosis or treatment for mental health disorders (e.g., bipolar, schizophrenia); use of psychiatric medications (e.g., lithium,); and non-English proficiency.
Design overview
The study used a two-group randomized controlled design with assessment time points at baseline, quit day (week 4), 1-week (week 5), and 1-month (week 8) follow-ups. The study procedures were reviewed and approved by the Institutional Review Board. Following telephone screening, eligible participants were instructed to attend a baseline session with three hours of smoking abstinence. During this session, participants completed informed consent, self-report assessments, and pre-treatment smoking cue-exposure task, followed by randomization procedures. Randomization was stratified by gender and conducted using values from Research Randomizer (http://www.randomizer.org/). Following randomization, participants met briefly with a health counselor where they received treatment orientation and an Omron™ Digital Pocket Pedometer (HJ-112) with instructions for usage specific to their assigned group described below. All participants were instructed to set their quit day during the fourth week post baseline. The published protocol describes the detail of study procedures [31]. Staff conducting baseline and follow-up assessments were blind to treatment assignment throughout all time points.
Intervention procedures
SUTQ counseling intervention group
Participants randomized to the SUTQ intervention group received a pedometer, written treatment materials, and weekly phone counseling sessions. Counselors’ primary goals were to (a) facilitate daily low-to-moderate physical activity following a tailored algorithm that gradually increased daily steps with the goal of achieving 7500 steps by their week 4 (quit day) (7500 steps is a minimum goal to realize health benefits [32]) and (b) adoption of physical activity as the primary smoking urge control strategy. Counseling during weeks 1 and 2 (pre-cessation) focused on establishing concurrent self-monitoring of cigarettes smoked and smoking urges, as well as initial adoption of routine physical activity with guidance to gradually increase daily steps. To explicitly intertwine physical activity within smoking cessation preparation efforts, SUTQ participants were encouraged to delay smoking at the onset of a smoking urge at least one time every day by taking a 10–15-min walk. During weeks 3 and 4 (pre-cessation), participants continued to increase the frequency and duration of daily walking. They were also introduced to additional cognitive-behavioral coping skills to prepare for their quit day with ongoing emphasis on using walking to delay urge-motivated smoking. The messaging around increasing physical activity also included an emphasis of its utility in managing negative affect, withdrawal, and weight concerns/weight gain post-smoking cessation [33, 34]. Adherence to walking was maximized by maintenance of daily exercise logs where participants recorded their end-of-day pedometer count and urge monitoring data which were reported to the counselors during each phone session. At their quit day session, counselors additionally provided participants with strategies to achieve a long-term goal of 10,000 steps/day as advised by the American College of Sports Medicine [35]. No counseling was provided after the week-4 quit day session and participants were only contacted to obtain follow-up assessments.
Standard smoking cessation counseling control group
The smoking cessation counseling (SCC) control group was designed to provide the same intensity of pre-quit telephone sessions as the SUTQ intervention, but the focus was on standard evidence-based cessation counseling only (no explicit focus on physical activity). Participants were provided with a pedometer, but counseling was centered on standard stimulus and urge control strategies (avoiding smokers, escaping high-risk situations, using gum or candy as substitutes) during the pre-quit preparatory period. SCC participants did not receive any encouragement or coaching to increase their physical activity. Similar to the SUTQ group, the SCC control did not receive any phone counseling after their quit day.
Baseline and quit day smoking cue-exposure assessment procedures
Participants completed one smoking cue-exposure trial during their baseline pre-treatment session and four trials on their quit day. Subjects were abstinent (verified using expired CO <10 ppm) [36] prior to initiation of the cue-exposure procedures at baseline and quit day. Each trial consisted of exposure to smoking cues (e.g., cigarettes, ashtray) accompanied by audio-taped instructions that directed participants through each trial. The instructions started with guided imagery focusing on multi-sensory cues associated with smoking, had the participant light and handle a cigarette, and mimic motor movements associated with smoking (without inhaling), and finally, extinguishing the lit cigarette. Each trial lasted approximately for 5–6 min. Self-report assessments of smoking urge and nicotine withdrawal were administered after each trial. For the baseline session, participants were presented with a single trial since the purpose of the task was to acquaint them to the lab environment. On the quit day, after observing overnight abstinence, participants were exposed to four massed trials. All trials and instructions were similar to the baseline session. Additional details of the intra-trial cue-exposure procedures can be found elsewhere [31, 37].
Measures
Physical activity (primary outcome)
Seven-day point prevalence mean steps per day, time spent in physical activity, and metabolic equivalent tasks (METs—a measure of exercise intensity) served as measures of physical activity at week 4 (quit day). Steps per day was obtained by pedometers, a reliable and valid device to measure physical activity [38–40]. The Omron™ pedometers in this study record the last 7 days’ total step counts. On the quit day, researchers recorded 7-day step counts directly from the pedometer, whereas participants reported their pedometer readings during telephone follow-up assessments (at 1 week and 1 month) following the same protocol as quit day data collection. Self-reported total time spent in physical activity and metabolic equivalent of tasks (METS) was obtained using the International Physical Activity Questionnaire (IPAQ) [41]. The IPAQ has adequate reliability and criterion validity with accelerometers [30, 42].
Quit day smoking cue reactivity (secondary outcome)
Smoking cue reactivity was obtained on the week 4 quit day and was measured using two single-item Likert format scales that assessed strength of smoking urge [37, 43] and the 10-item brief questionnaire of smoking urges [44]. Participants completed four sequential cue reactivity trials on their quit day, and difference scores (trial 4 urge–trial 1 urge) were calculated to assess changes in strength of urge across the four trials.
Controlling variables
Baseline controlling variables included (1) nicotine dependence as measured using the Fagerstrom Test for Nicotine Dependence (FTND). The FTND has good internal consistency and high test-retest reliability and has been extensively used to assess nicotine dependence [45]. (2) Body mass index (BMI) was calculated using self-reported height (in meters) and weight (in kilograms). (3) Impulsivity was assessed using the impulsivity subscale from the Adult ADHD Rating Scale [46]. (4) Sex (0 = male; 1 = female) was collected as part of the demographic assessment. Quit day controlling variables were (1) depressive symptoms assessed using the short-form of the Center for Epidemiological Scale Depression Scale [47]. (2) Smoking urge coping measured using a 12-item scale adapted from O’Connell et al. [48] that assessed use of cognitive and behavioral strategies for urge management. (3) Nicotine withdrawal prior to exposure to the cue-exposure trial procedures was assessed using the Minnesota Nicotine Withdrawal Scale [49].
Smoking status (exploratory follow-up outcome)
Smoking status at 1-week and 1-month follow-ups was assessed by self-report using standard, validated 7-day timeline follow back methods [50]. Participants were coded as quit if they reported smoking zero (0) cigarettes (not even a puff) during all 7 days prior to assessment.
Statistical analysis
Descriptive statistics, including means, standard deviations, and frequencies for selected variables were reported. We used multivariate exploratory structural equation modeling (ESEM) to test our primary hypothesis that at week 4, the SUTQ participants would demonstrate greater physical activity (primary outcome) and lower smoking cue reactivity (secondary outcome) compared to the SCC control group. ESEM is a hybrid structural equation modeling method that allows one to combine exploratory and confirmatory factor analysis in a single model [51, 52]. Unlike confirmatory factor analysis alone, which restricts items to load on a single factor, ESEM permits items to load on multiple factors, which is a more realistic assumption. [51] The process involves several steps. In the first, an exploratory factor analysis is conducted to identify the number of factors that best represent the relations among the measured variables. In the second step, theoretically relevant predictor variables are added to the model. In the third step, the effects of the predictor variables on the observed indicator variables are assessed. This is achieved by modeling direct paths from all predictors to all measured variables, and then testing the effects for significance through a chi-square difference test. Importantly, ESEM permitted us to assess intervention effects on physical activity and cue reactivity outcomes (six measures) simultaneously with theoretically relevant controlling variables in a single multivariate model. Therefore, all controlling variables included in the model affected variance explained on the outcome measures.
Secondary univariate analyses explored group differences in physical activity and quit status at 1 week and 1 month post quit day. Because a key focus of physical activity counseling was to promote daily steps (not time or intensity), we compared mean steps per day between groups as well as the proportion of participants in each group that achieved the 7500 steps/day goal at follow-up. Likewise, because cue reactivity was not tested post-quit day, we explored group differences in quit status at 1-week and 1-month follow-ups.
Given that this was a pilot study and in keeping with the small sample size, we set our a priori alpha at p = .10. SPSS 21 software was used to generate descriptive statistics and frequency distributions. We used Mplus 7.0 software [53] for the ESEM analysis. Model fit was evaluated with model chi-square, comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean residual (SRMR). Suggested criteria for model fit are non-significant model chi-square, CFI above .95, RMSEA below .05–.08, and an SRMR value below 8 [53–55].
RESULTS
Baseline sample characteristics
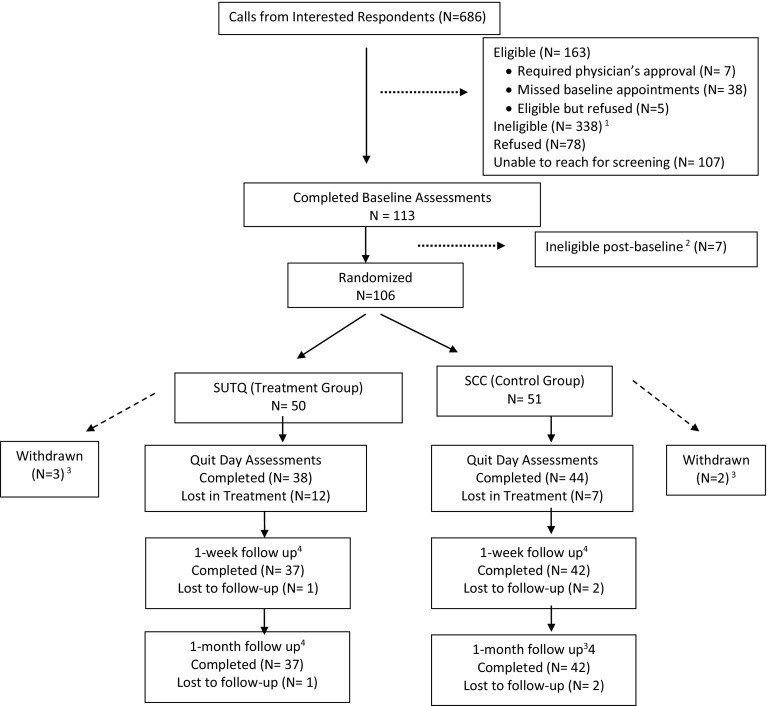
We screened 501 individuals for eligibility. Randomization was completed for 106 eligible smokers. Figure 1 shows the CONSORT (COnsolidated Standards of Reporting Trial) flow diagram [56]. The final study sample included 101 participants (N = 50 assigned to the SUTQ and N = 51 to the SCC). Descriptive characteristics by treatment condition are presented in Table 1. There were no significant baseline differences between treatment conditions in terms of demographics, smoking history, or other study measures. Overall, the sample was 51 % female, 94 % non-Hispanic, and over 70 % reported being Black/African-American. The average age was 42.1 years (SD = 10.91), 60 % had less than or equal to a high school education, and 78.6 % were single/never married. Over 75 % had a BMI ≥ 25 indicating that majority of the population was overweight. On an average, participants completed at least two (out of a possible three) counseling sessions (M = 2.46; SD = .83) and the average time for each phone session was 12.21 min (SD = 2.17). There were no significant differences in duration of counseling contact time between the SUTQ and SCC group (t df (55) = −1.46; p = .15).
Fig. 1.
Participant flow. 1 Reasons for ineligibility: not meeting criteria for physical inactivity (n = 134), mental health diagnosis (n = 70), smoking history (n = 40), age (n = 31), not owning a phone (n = 24), drug/alcohol use (n = 30), disability (n = 5), pregnancy (n = 2), exclusive e-cig use (n = 2). 2 Seven participants were ineligible after completion of baseline (prior to being randomized); reasons included mental health diagnosis (n = 3), smoking <5 cigarettes per day (n = 2), and not meeting the criteria for physical inactivity (n = 1). 3 Reasons for withdrawal post-randomization included reported use of psychotropic medications, drug and alcohol use, and misreporting physical activity levels during screening, and moving outside service area. 4 One-week and 1-month assessments were only completed for participants who completed their quit day session
Table 1.
Baseline participant characteristics
| Variables | SUTQ (intervention) group (N = 50) | SCC (control) group (N = 51) | All (N = 101) |
|---|---|---|---|
| Age, mean (SD) | 41.7 (10.3) | 42.3 (11.5) | 42.1 (10.9) |
| Gender (%) | |||
| Male | 52.9 | 47.1 | 49.5 |
| Female | 46.1 | 52.9 | 50.5 |
| Education (%) | |||
| Less than high school | 18.4 | 12.2 | 15.3 |
| High school/GED | 42.9 | 44.9 | 43.9 |
| Above high school | 38.8 | 42.8 | 40.8 |
| Single/never married (%) | 49.4 | 50.6 | 78.6 |
| Body mass index, mean (SD) | 29.1 (6.9) | 30.6 (7.8) | 29.8 (7.4) |
| Cigarettes per day, mean (SD) | 12.2 (6.4) | 12.4 (5.3) | |
| Nicotine dependence (FTND) mean (SD) | 5.3 (1.3) | 5.3 (1.2) | 5.2 (1.2) |
| Self-efficacy, mean (SD) | 15.8 (9.8) | 13.6 (9.0) | 14.7 (9.4) |
| Depressive symptoms, mean (SD) | 9.8 (5.3) | 9.3 (5.6) | 9.5 (5.4) |
| Number of urge coping strategies, mean (SD) | 6.9 (3.2) | 6.8 (3.2) | 6.8 (3.5) |
Primary analyses: multivariate modeling of quit day physical activity and cue reactivity
Exploratory factor analysis
As expected, the exploratory model of six dependent measures supported the two-factor structure with one factor for physical activity (eigenvalue = 1.897; variance = 33 %) and a second factor for cue reactivity (eigenvalue = 2.284; variance = 38 %), χ 2 (n=81, df=4) = 5.733, p = .22; and CFI = .983, RMSEA = .073 (95 % CI = .000–.195), SRMR = .037.
ESEM analysis
We next added treatment and the seven control variables into the multivariate model. This model fit the data well with a non-significant chi-square, χ 2 (n=78, df=36) = 47.792, p = .09. Several of the other fit indexes diverged from the prevailing heuristic; CFI = 0.908, RMSEA = 0.065 (95 % CI = 0.000, 0.110), and SRMR = 0.118. These results suggest that the model complexity in relation to the sample size may be high. However, use of the ESEM method was necessary to assess the effects of treatment and covariates on the various observed outcomes simultaneously.
Quit day physical activity
The effects of our intervention was associated with greater physical activity, our primary outcome in the SUTQ group vs. controls (b = .51, z = 1.71, p = .08). Further, having greater impulsivity symptoms was also positively associated with physical activity (b = 0.26, z = 2.85, p < .01). There were no other significant baseline predictors of post-test physical activity (Table 2).
Table 2.
Results of exploratory structural equation modeling
| Outcome variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Predictor variables | Cue Reactivity | Physical Activity | ||||||
| Coefficient | SE | z-value | p-value | Coefficient | SE | z-value | p-value | |
| Treatment | −0.29 | 0.265 | −1.095 | 0.274 | 0.509 | 0.298 | 1.711 | 0.087 |
| Nicotine dependence (FTND) | 0.075 | 0.124 | 0.601 | 0.548 | 0.098 | 0.134 | 0.73 | 0.465 |
| Sex | −0.194 | 0.271 | −0.717 | 0.473 | −0.381 | 0.312 | −1.221 | 0.222 |
| Body mass index (BMI) | −0.015 | 0.017 | −0.876 | 0.381 | −0.028 | 0.021 | −1.334 | 0.182 |
| Depression symptoms | 0.005 | 0.027 | 0.171 | 0.864 | 0.012 | 0.034 | 0.359 | 0.72 |
| Impulsivity | −0.127 | 0.084 | −1.515 | 0.13 | 0.262 | 0.092 | 2.852 | 0.004 |
| Number of urge coping strategies | 0.08 | 0.04 | 1.982 | 0.047 | 0.028 | 0.044 | 0.638 | 0.523 |
| Nicotine withdrawal (WSCL) | 0.047 | 0.024 | 1.996 | 0.046 | -0.035 | 0.027 | −1.279 | 0.201 |
Quit day cue reactivity
The effects of the SUTQ treatment on cue reactivity were in the hypothesized direction but did not reach statistical significance (b = −.29; z = −1.09, p = .27). Greater nicotine withdrawal prior to the cue reactivity assessment (b = .04, z = 1.99, p = .04) and reporting greater number of coping strategies at end of treatment was significantly associated with increased reactivity (b = .08, z = 1.98, p = .04) (Table 2).
Given the relatively small sample size and the mixed model fit statistics (i.e., CFI and SRMR), we assessed the power associated with our primary research questions, the effect of SUTQ intervention on physical activity and smoking cue reactivity. Using 1000 replications in a Monte Carlo simulation study, the population parameter value for the effect of treatment on physical activity was recovered (95 % CI) 93 % of the time, with a power (1-β) of .48. Only when the simulation sample was raised to n = 180 did 95 % coverage surpass the expected 95 % (.954), and was power >.80 (.84). Thus, the results of this simulation suggest that the group differences may be robust, but that a sample size greater than twice that of the present study may be necessary to have the effect reach p < .05 significance level, and that sample size may be the reason for the lower CFI value.
Secondary analyses: group comparison of average steps and quit status
Seven-day point prevalence mean steps and steps goal achievement
Seven-day point prevalence data on steps was obtained for 81.8 % of participants who completed their quit day (and 83.5 % of 1-week and 85 % of 1-month follow-up). At week 4, a significantly greater proportion of participants in the SUTQ group (80 %) compared to the control group (20 %) achieved the goal of 7500 steps/day (p < .01). Effects were sustained at 1 week (81 vs 19 %, p < .01) and 1-month follow-up (80 vs 20 %, p < .01). The SUTQ group also had significantly greater number of steps per day compared to the SCC control group at week 4: SUTQ [(M = 7207.24; SD = 4276.03) vs. SCC (M = 3974.03; SD 3655.27) (p = <.01). The group difference was maintained at 1-week post-quit day: SUTQ (M = 8482.82; SD = 5843.57) vs. SCC (M = 3932.24; SD = 3150.02) (p < .001). At 1-month post-treatment, the SUTQ group showed an increase in mean daily steps: SUTQ (M = 7506.78; SD = 5142.47) compared to the SCC control group (M = 3700.50; SD = 3089.51); (p < .001).
Seven-day point prevalence quit status
We examined differences in quit rates at 1 week and 1 month post-treatment as exploratory outcomes. There were no significant differences in 7-day point prevalence quit rates at 1 week and 1 month between the two groups. Thirty-eight percent of the SUTQ group reported quitting compared to 33.3 % in the SCC control group (p = .67) at 1 week. At 1 month, 32.4 % of SUTQ reported quitting (vs 40 % in the SCC group) (p = .34).
DISCUSSION
To our knowledge, this is the first study that has tested proof-of-concept and preliminary efficacy of a telephone-based counseling intervention promoting physical activity during smoking cessation treatment among underserved, physically inactive smokers. Consistent with our hypothesis, the SUTQ intervention was associated with increased physical activity during the 4-week pre-quit preparatory period that was sustained at 1 week and 1 month post-treatment, and with reduced quit day smoking cue reactivity (although the trend was not significant with our small sample size). Our results also suggest our intervention promotes adoption of daily walking, and this emphasis on promoting physical activity during the pre-quit period did not undermine SUTQ participants’ efforts to quit smoking compared to control group participants.
Our encouraging results and similar between-group attrition rates suggests that our approach was acceptable, feasible, and potentially efficacious in promoting physical activity in a low-income, underserved population known to have low intervention uptake and less successful response to physical activity and smoking interventions than the general population—major contributors to health disparities [57–61]. A review of counseling treatments in low-income populations underscored the need to establish enhanced interventions for this high-risk group, including more effective telephone-based counseling services (e.g., quitlines) [62].
Results suggest treatment effects on cue reactivity in the hypothesized direction that, with a larger sample size, could reflect reliable group differences. These results, coupled with evidence that reactivity to smoking cues during abstinence may predict of smoking relapse [63], suggest that further examination of strategies to encourage physical activity to help smokers manage their smoking urges is warranted. Future research could help determine whether our pre-quit intervention could foster physical activity to become an effective alternate reinforcer to smoking. Practicing this strategy during short periods of abstinence, along with increasing daily physical activity, could promote greater pre-quit urge management skills and reduce quit day, abstinence-induced cue reactivity..5
Nicotine withdrawal was a significant predictor of reactivity with greater withdrawal symptoms associated with increased quit day smoking cue reactivity. This finding is also consistent with the literature indicating that overnight abstinence can increase reactivity to smoking-related cues [64, 65]. The nicotine withdrawal syndrome [66] that includes irritability, difficulty concentrating, and restlessness are observed within two hours after tobacco use and usually peak between 24 and 48 hours after cessation. [67] All participants in the study provided a measure of expired CO (CO <10 ppm) to bioverify abstinence prior to initiating the reactivity procedures and since abstinence results in withdrawal, it elucidates the significant association between withdrawal and reactivity.
The positive association between quit day cue reactivity and greater number of coping strategies for urge management is contrary to the hypothesis that developing an increased number of coping skills can facilitate extinction of conditioned reactivity to cues. Social learning theory suggests that engaging in coping skills practice during high-risk situations increases the effectiveness of those skills when in the presence of cues. In one study that examined effectiveness of urge coping strategies in reducing alcohol-related cue reactivity [68], coping skills training was introduced in a graduated fashion whereby participants were introduced to one coping strategy at a time, encouraged to practice it during exposure to each drinking trigger, and the practice ended when the urge had reduced. Thus, while developing urge coping strategies can help attenuate cue reactivity and relapse in the long-run, the utility of coping skills training may have less to do with developing greater number of strategies as opposed to focusing on relevant/fewer key strategies that may be specific to the individuals’ high-risk situations.
Another interpretation is that the context for testing smoking cue reactivity may not have been best equipped to assess the potential association between pre-quit day training to use physical activity for urge management and quit day cue-elicited urge reactivity. Future studies could examine whether engaging in walking immediately following the cue-exposure trial on the quit day would mitigate urge reactivity in SUTQ participants compared to controls. Given evidence that short bouts of physical activity mitigates nicotine withdrawal [69], modifying the cue reactivity test as such would provide a greater opportunity to observe the degree to which physical activity training prior to quitting could attenuate quit day abstinence-induced cue reactivity.
Future research could also examine whether the type of urge coping strategies, as opposed to the number of strategies, may better predict quit day urge reactivity. Evidence of the differential effectiveness of coping on smoking cessation outcome is mixed. Some studies compared cognitive and behavioral coping in general [70] and suggest that while the number of coping strategies or the combination of the two was associated with reduced urges, no differential effects of cognitive and behavioral coping exist. Others studies revealed clear differential effects of behavioral and cognitive coping strategies (e.g., [71, 72]). This suggests that the distinction between cognitive and behavioral coping requires more examination and future studies in this area could benefit from exploration of other potential classification frameworks (e.g., stress and temptation coping paradigm [73], engagement or problem-focused coping, emotion-focused or disengagement coping) [74].
Exploratory analyses showed that at the 1-week and 1-month follow-up, there were equivalent quit rates in both groups with the overall quit rate being relatively high for the target population. We did not expect differences in quit rates between the two groups because (a) this was a proof-of-concept study primarily focused on increasing physical activity and reducing cue reactivity by week 4, (b) behavioral counseling was not provided beyond the quit day to focus on relapse prevention efforts, (c) no nicotine replacement therapies were provided, and (d) the short timeline of the study and resource constraints did not allow us to look at long-term bioverified quit rates. Importantly, compared to the control group, the SUTQ intervention group was significantly more likely to maintain physical activity during follow-up, suggesting that SUTQ counseling was effective at promoting physical activity adoption and maintenance among treatment-seeking smokers. Thus, continuing counseling to focus on smoking relapse prevention, physical activity maintenance, and provision of smoking cessation medications would be appropriate in a follow-up fully scaled treatment outcome study.
Limitations
The primary limitation of the study was the absence of a no-treatment control group. While this was a proof-of-concept study, we were interested in examining the preliminary evidence of the SUTQ intervention on adoption of daily physical activity in comparison to a standard care, smoking cessation-only control—despite the relative, potential potency of proactive SCC on short-term quit rates. However, this study provides pilot data for larger trials, perhaps a three-group randomized controlled trial, which can examine impact of the SUTQ intervention on long-term cessation and relapse. Secondly, given the pilot nature and the focus of the study on smoking cue reactivity, cessation outcomes at follow-up were not bioverified. While validated assessments were used to assess self-reported abstinence [50], future studies could benefit from using validated biochemical verification of smoking abstinence. Related to this, while safeguards were in place to maximize accurate reporting of step counts from pedometers, future studies may also benefit from utilizing nuanced and sophisticated measures of assessment of physical activity (e.g., Fitbit) that enables regular uploading of recorded data for enhanced objective measurement of physical activity. The study was also not designed to compare the influence of different intensities of exercise on smoking behavior change or compare the effects of adoption of daily physical activity relative to a quit attempt (e.g., sequential vs simultaneous). However, the area of multiple behavior change is a relatively new and emerging area and while some empirical questions need to be addressed, we believe that our results can guide theory and data related to promoting multiple behavior change efforts, especially in an underserved sample of smokers. Finally, the design may not have been the best equipped to examine the relation between pre-quit urge management practice and quit day urge reactivity. Future studies can utilize nuanced cue reactivity test strategies to better ascertain this association by including 5–10 min of walking immediately after eliciting smoking urges—similar to the counseling instructions during the 4 weeks prior to the quit day.
CONCLUSION
This study tests the effects of a novel multiple health behavior change intervention approach among underserved populations that bear a disproportionate burden of unhealthy lifestyle (i.e., tobacco use and physical inactivity) related death and disease rates. The SUTQ intervention that integrates an evidence-based non-pharmacological strategy for urge management within a smoking cessation context, making it seem complementary rather than burdensome, is appealing since it promotes broader health gain among people who have a cluster of unhealthy lifestyle behaviors. To our knowledge, this is the first study to deliver a telephone-based counseling intervention to increase physical activity among a group of low-income smokers. Our model has intuitive appeal since it is an approach that can be adopted by community based service providers such as quitlines. State quitlines are in a prime position to reach disparate populations (e.g., low-income, underserved smokers) and our telephone-based approach that promotes physical activity within the context of nicotine treatment can be adopted and disseminated through quitlines and perhaps deliver behavioral health promotion efforts that have high potential for reducing health disparities.
Acknowledgements
The authors thank the following staff and colleagues who contributed to this project: Salini Inangati, MPH, Shannon McGinnis, BS, Sean McCormick PhD, and Rachel Comly, BS.
Compliance with ethical standards
Funding information
This study was funded by the American Heart Association (13CRP14560028). AHA is not involved in the study design, data collection, analysis or interpretation of data, and in the decision to submit the article for publication. Research reported in this publication was also supported by an Institutional Development Award (IDeA) Center of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM113125 (FP).
Statement on human rights
All procedures were performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Conflict of interests
None of the authors have any conflicts of interest associated with this manuscript.
Informed consent
Informed consent was obtained from all individual participants included in the study.
IRB approval
The study was approved by Temple University’s Institutional review Board (Project # 21109).
Footnotes
Implications
Policy: Resources should be devoted to utilizing quitlines to promote multiple health behaviors along with smoking cessation efforts
Research: Telephone-based interventions may be utilized to promote physical activity among physically inactive smokers, thereby reducing overall unhealthy lifestyle-related disease burden.
Practice: A telephone-based counseling intervention that integrates multiple health behavior change within the context of nicotine treatment can be adopted and disseminated through quitlines
Trial Registration NCT02220465 (clinicaltrials.gov)
Findings from this study have not been previously published and the manuscript is not being simultaneously submitted elsewhere. Preliminary data from this study was presented as a poster at the Annual Conference for the Society for Research in Nicotine and Tobacco (2015). All co-authors have approved the manuscript for publication and agree to its submission. As corresponding author, I have full control of all primary data and agree to allow the journal to review the data if requested.
References
- 1.Centers for Disease Control and Prevention. Best practices for comprehensive tobacco control programs—2014; 2014. http://www.cdc.gov/tobacco/stateandcommunity/best_practices/index.htm?source=govdelivery.
- 2.Platt S, Tannahill A, Watson J, Fraser E. Effectiveness of antismoking telephone helpline: follow up survey. BMJ. 1997;314(7091):1371–1371. doi: 10.1136/bmj.314.7091.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummins SE, Bailey L, Campbell S, Koon-Kirby C, Zhu S-H. Tobacco cessation quitlines in North America: a descriptive study. Tob Control. 2007;16(Suppl 1):i9–i15. doi: 10.1136/tc.2007.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu SH, Anderson CM, Johnson CE, Tedeschi G, Roeseler A. A centralised telephone service for tobacco cessation: the California experience. Tob Control. 2000;9(Suppl 2):II48–II55. doi: 10.1136/tc.9.suppl_2.ii48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27:25–33. doi: 10.1016/j.amepre.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein, E., Zhu, S-H., Tedeschi G. J. (2010). Smoking cessation quitlines: an underrecognized intervention success story. Am Psychol, 65(4), 252–261. doi:10.1037/a0018598. [DOI] [PMC free article] [PubMed]
- 7.Kaczynski AT, Manske SR, Mannell RC, Grewal K. Smoking and physical activity: a systematic review. Am J Health Behav. 2008;32(1):93–110. doi: 10.5993/AJHB.32.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Furlanetto KC, Mantoani LC, Bisca G, et al. Reduction of physical activity in daily life and its determinants in smokers without airflow obstruction. Respirology. 2014;19(3):369–375. doi: 10.1111/resp.12236. [DOI] [PubMed] [Google Scholar]
- 9.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Glazer NL, Lyass A, Esliger DW, et al. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med Sci Sports Exerc. 2013;45(1):109–115. doi: 10.1249/MSS.0b013e31826beae5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murtagh EM, Murphy MH, Boone-Heinonen J. Walking: the first steps in cardiovascular disease prevention. Curr Opin Cardiol. 2010;25(5):490–496. doi: 10.1097/HCO.0b013e32833ce972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Behavioral Risk Factor Surveillance System (BRFSS); 2002.
- 13.CDC. Behavioral Risk Factor Surveillance System (BRFSS); 2001.
- 14.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat 10. 2012;(252):1–207. http://www.ncbi.nlm.nih.gov/pubmed/22834228. [PubMed]
- 15.Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation (review) Cochrane Libr. 2012;1:1–39. doi: 10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology. 2001;158(1):66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- 17.Prochaska JJ, Prochaska JO. A review of multiple health behavior change interventions for primary prevention. Am J Lifestyle Med. 2011;5:208–221. doi: 10.1177/1559827610391883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nigg CR, Long CR. A systematic review of single health behavior change interventions vs. multiple health behavior change interventions among older adults. Transl Behav Med. 2012;2(2):163–179. doi: 10.1007/s13142-012-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson SS, Paiva AL, Cummins CO, et al. Transtheoretical model-based multiple behavior intervention for weight management: effectiveness on a population basis. Prev Med (Baltim). 2008;46(3):238–246. doi: 10.1016/j.ypmed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmons K, Marcus B, Linnan L, Rossi J, Abrams DB. Mechanisms in multiple risk factor interventions: smoking, physical activity, and dietary fat intake among manufacturing workers. Prev Med (Baltim) 1994;23:481–489. doi: 10.1006/pmed.1994.1066. [DOI] [PubMed] [Google Scholar]
- 21.Taylor, A., Katomeri, M., & Ussher, M. (2006). Effects of walking on cigarette cravings and affect in the context of the Nesbitt’s paradox and the circumplex model. J Sport Exerc Psychol, 28, 18–31.r
- 22.Marcus BH, Albrecht AE, King TK, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999;159(11):1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- 23.Spring B, Moller AC, Coons MJ. Multiple health behaviours: overview and implications. J Public Health (Oxf) 2012;34(Suppl 1):i3–i10. doi: 10.1093/pubmed/fdr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 25.Kovelis D, Zabatiero J, Furlanetto KC, Mantoani LC, Proença M, Pitta F. Short-term effects of using pedometers to increase daily physical activity in smokers: a randomized trial. Respir Care. 2012 doi: 10.4187/respcare.01458. [DOI] [PubMed] [Google Scholar]
- 26.Aveyard P. A commentary on “exercise and smoking cessation: and the verdict is ...”. Ment Health Phys Act. 2012;5:101–102. doi: 10.1016/j.mhpa.2012.04.005. [DOI] [Google Scholar]
- 27.Taylor AH, Thompson T, Greaves CJ, et al. A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost-effectiveness of exercise assisted reduction then stop (EARS) among disadvantaged smokers. Health Technol Assess (Rockv) 2014;18(4):1–32. doi: 10.3310/hta18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Income Statistics. Current income statistics for zip codes in the United States. https://www.incomebyzipcode.com/. Accessed 1 Sept 2016.
- 29.Okuyemi KS, Ahluwalia JS, Richter KP, Mayo MS, Resnicow K. Differences among African American light, moderate, and heavy smokers. Nicotine Tob Res. 2001;3:45–50. doi: 10.1080/14622200020032097. [DOI] [PubMed] [Google Scholar]
- 30.Biernat E. SRLBJL. Assessment of physical activity by applying IPAQ questionnaire. Phys Educ Sport. 2008;52(1):46–52. doi: 10.2478/v10030-008-0019-1. [DOI] [Google Scholar]
- 31.Nair US, Collins BN, Patterson F, Rodriguez D. Promoting pre-quit physical activity to reduce cue reactivity among low-income sedentary smokers: a randomized proof of concept study. Contemp Clin Trials. 2015;42:158–166. doi: 10.1016/j.cct.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Houle J, Valera B, Gaudet-Savard T, Auclair A, Poirier P. Daily steps threshold to improve cardiovascular disease risk factors during the year after an acute coronary syndrome. J Cardiopulm Rehabil Prev. 2013;33:406–410. doi: 10.1097/HCR.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 33.Nair US, Collins BN, Napolitano M. Differential effects of a body image exposure session on smoking urge between physically active and sedentary female smokers. Psychol Addict Behav. 2013;27:322–327. doi: 10.1037/a0031367. [DOI] [PubMed] [Google Scholar]
- 34.Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2008;4 doi: 10.1002/14651858.CD002295.pub3. [DOI] [PubMed] [Google Scholar]
- 35.American College of Sports Medicine Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2006. http://books.google.com/books/about/ACSM_s_Guidelines_for_Exercise_Testing_a.html?id=8cRfd7GFZjMC&pgis=1. Accessed 13 Feb 2014.
- 36.Benowitz NL, Ahijevch K, Hall SM, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2001;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 37.Collins BN, Nair US, Komaroff E. Smoking cue reactivity across massed extinction trials: negative affect and gender effects. Addict Behav. 2011;36(4):308–314. doi: 10.1016/j.addbeh.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Tudor-locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing convergent validity. Sport Med. 2002;32(12):795–808. doi: 10.2165/00007256-200232120-00004. [DOI] [PubMed] [Google Scholar]
- 39.Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity. Sport Med. 2004;34:281–291. doi: 10.2165/00007256-200434050-00001. [DOI] [PubMed] [Google Scholar]
- 40.Holbrook EA, Barreira TV, Kang M. Validity and reliability of omron pedometers for prescribed and self-paced walking. Med Sci Sports Exerc. 2009;41(3):669–673. doi: 10.1249/MSS.0b013e3181886095. [DOI] [PubMed] [Google Scholar]
- 41.Hallal PC, Victora CG. Reliability and validity of the international physical activity questionnaire (Ipaq) Med Sci Sport Exerc. 2004;36(3):556. doi: 10.1249/01.MSS.0000117161.66394.07. [DOI] [PubMed] [Google Scholar]
- 42.Oyeyemi AL, Adegoke BO, Oyeyemi AY, Fatudimu BM. Test-retest reliability of IPAQ environmental- module in an African population. Int J Behav Nutr Phys Act. 2008;5(1):38. doi: 10.1186/1479-5868-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ussher M, Beard E, Abikoye O, Hajek P, West R. Urge to smoke over 52 weeks of abstinence. Psychopharmacology. 2013;226:83–89. doi: 10.1007/s00213-012-2886-7. [DOI] [PubMed] [Google Scholar]
- 44.Sanderson-Cox L, Tiffany S. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- 45.Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine Tob Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 46.Barkley RA. Barkley Adult ADHD Rating Scale-IV (BAARS-IV). New York. 2011.
- 47.Van Dam NT, Earleywine M. Validation of the Center for Epidemiologic Studies Depression Scale-Revised (CESD-R): pragmatic depression assessment in the general population. Psychiatry Res. 2011;186(1):128–132. doi: 10.1016/j.psychres.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 48.O’Connell KA, Fears B, Cook MR, Gerkovich M, Zechmann A. Overcoming the urge to smoke: the strategies of long-term abstainers and late relapsers. Psychol Addict Behav. 1991;5:1–8. doi: 10.1037/h0080579. [DOI] [Google Scholar]
- 49.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Curr Med Res Opin. 2005;21(5):749–760. doi: 10.1185/030079905X43712. [DOI] [PubMed] [Google Scholar]
- 50.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the alcohol timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-X. [DOI] [PubMed] [Google Scholar]
- 51.Asparouhov T, Muthen B. Exploratory structural equation modeling. 2009; 16. doi:10.1080/10705510903008204.
- 52.Marsh HW, Morin AJS, Parker PD, Kaur G. Exploratory structural equation modeling: an integration of the best features of exploratory and confirmatory factor analysis. Annu Rev Clin Psychol. 2014;10(Mimic):85–110. doi:10.1146/annurev-clinpsy-032813-153700. [DOI] [PubMed]
- 53.Muthén L, Muthén B. Mplus user’s guide (5th Ed.).; 2012.
- 54.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model A Multidiscip J. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 55.Loehlin JC. Latent variable models: an introduction to factor, path, and structural equation analysis (4th Ed.).; 2004. doi:10.4324/9781410609823.
- 56.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 57.Richter DL, Wilcox S, Greaney ML, Henderson KA, Ainsworth BE. Environmental, policy, and cultural factors related to physical activity in African American women. Women Health. 2002;36(2):91–109. doi: 10.1300/J013v36n02_07. [DOI] [PubMed] [Google Scholar]
- 58.Kotz D, West R. Explaining the social gradient in smoking cessation: it’s not in the trying, but in the succeeding. Tob Control. 2009;18(1):43–46. doi: 10.1136/tc.2008.025981. [DOI] [PubMed] [Google Scholar]
- 59.Fernander A, Resnicow K, Vishwanath K, Perez-Stable J. Cigarette smoking interventions among diverse populations. Am J Health Promot. 2011;25(5):1–4. doi: 10.4278/ajhp.25.5.c1. [DOI] [PubMed] [Google Scholar]
- 60.Bryant J, Bonevski B, Paul C, O’Brien J, Oakes W. Developing cessation interventions for the social and community service setting: a qualitative study of barriers to quitting among disadvantaged Australian smokers. BMC Public Health. 2011;11:493. doi: 10.1186/1471-2458-11-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart MJ, Greaves L, Kushner KE, Letourneau NL, Spitzer DL, Boscoe M. Where there is smoke, there is stress: low-income women identify support needs and preferences for smoking reduction. Health Care Women Int. 2011;32(5):359–383. doi: 10.1080/07399332.2010.530724. [DOI] [PubMed] [Google Scholar]
- 62.North American Quitline Consortium (NAQC). The use of quitlines among priority populations in the U.S.: lessons from the scientific evidence.; 2011. https://c.ymcdn.com/sites/www.naquitline.org/resource/resmgr/Issue_Papers/IssuePaperTheUseofQuitlinesA.pdf.
- 63.Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- 64.Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96(10):1419–1432. doi: 10.1046/j.1360-0443.2001.961014196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson NL, Carpenter MJ, Saladin ME, Gray KM, Upadhyaya HP. Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addict Behav. 2010;35(7):673–677. doi: 10.1016/j.addbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. 1986; 43. doi:10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed]
- 67.Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 68.Monti PM, Rohsenow DJ, Rubonis AV, et al. Cue exposure with coping skills treatment for male alcoholics: a preliminary investigation. J Consult Clin Psychol. 1993;61(6):1011–1019. doi: 10.1037/0022-006X.61.6.1011. [DOI] [PubMed] [Google Scholar]
- 69.Daley AJ, Oldham AR, Townson M. The effects of acute exercise on affective responses and desire to smoke in sedentary temporarily abstaining smokers: a preliminary study. J Sports Sci. 2004;22(3):303. [Google Scholar]
- 70.O’Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation? Health Psychol. 2007;26(1):77–84. doi: 10.1037/0278-6133.26.1.77. [DOI] [PubMed] [Google Scholar]
- 71.Shiffman S, Paty JA, Gnys M, JA K, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. doi: 10.1037/0022-006X.64.2.366. [DOI] [PubMed] [Google Scholar]
- 72.Stöffelmayr B, Wadland W, Pan W. An examination of the process of relapse prevention therapy designed to aid smoking cessation. Addict Behav. 2003;28:1351–1358. doi: 10.1016/S0306-4603(02)00250-2. [DOI] [PubMed] [Google Scholar]
- 73.Wills TA, Shiffman S. Coping and substance use: a conceptual framework. In: Shiffman S, Wills TA, editors. Coping and substance use. London: Academic Press; 1985. pp. 3–24. [Google Scholar]
- 74.Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wadsworth ME. Coping with stress during childhood and adolescence: problems, progress, and potential in theory and research. Psychol Bull. 2001;127(1):87–127. doi: 10.1037/0033-2909.127.1.87. [DOI] [PubMed] [Google Scholar]