Abstract
Background
The first line of anti-tuberculosis (TB) drugs are the most effective standard of drugs for TB. However, the use of these drugs is associated with hepatotoxicity. Silymarin has protective effects against hepatotoxicity of anti-TB drugs in animal models. This study aims to investigate the protective effect of silymarin on hepatotoxicity caused by anti-TB drugs.
Methods
This is a prospective, randomized, double-blind and placebo-controlled study. Patients were eligible if they were 20 years of age or order and started the first-line anti-tuberculosis drugs. Eligible patients were randomized for receiving silymarin or a placebo for the first 4 weeks. The primary outcome was the proportion of patients who showed elevated serum liver enzymes more than 3 times the upper normal limit (UNL) or total bilirubin (TBil) > 2× UNL within the first 8 weeks of anti-TB treatment.
Results
We enrolled a total of 121 patients who silymarin or a placebo to start their anti-TB treatment, for the first 8 weeks. The proportions of elevated serum liver enzymes more than 3 times of UNL at week 2, week 4, and week 8 did not show any significant difference between the silymarin and placebo groups, at 0% versus 3.6% (p>0.999); 4.4% versus 3.6% (p>0.999); and 8.7% versus 10.8% (p=0.630), respectively. However, patients with TBil >2× ULN at week 8 were significantly low in the silymarin group (0% versus 8.7%, p=0.043).
Conclusion
Our findings did not show silymarin had any significant preventive effect on the hepatotoxicity of anti-TB drugs.
Keywords: Tuberculosis, Silymarin
Introduction
Major adverse reaction of the first-line anti-tuberculosis (TB) drugs can lead not only to the discontinuation of treatment but also to considerable morbidity, even mortality. Drug-induced liver injury (DILI) is one of the well-known major adverse reaction of the first-line anti-TB drugs. The overall risk of TB DILI has been reported from 5% to as high as 33%1.
Silymarin has been established that it increases the phagocytic activity of the Kupffer cells, prevents lipid peroxidation and reduces pathologically increased lipid metabolism, keeps the membranes and the membrane-bound enzymes functioning normally, prevents toxic metabolic liver damage and effective in inflammatory degenerative disease2,3. In animal models, silymarin showed protective effect against hepatotoxicity of anti-TB drugs4,5. However, the hepatoprotective effect of Silymarin during anti-TB treatment in human has not been studied enough6.
Therefore, we performed a randomized double-blinded prospective study to elucidate the preventive effect of silymarin on the development of DILI related to the first-line anti-TB drugs.
Materials and Methods
Patients who received the first line standard anti-TB treatment drugs including isoniazid, rifampicin and pyrazinamide and older than 35 years were enrolled. Exclusion criteria included patients who could not take the first line anti-TB drugs because of the abnormal baseline liver enzyme test including alanine aminotransferase (ALT), aspartate transaminase (AST), and total bilirubin (TBil), were pregnant or lactating women, and had a history of adverse effect to silymarin.
A double-blinded randomized controlled trial was performed between November 2011 and August 2013 in Boramae medical center in Korea. The study protocol was approved by the Institutional Review Board of Seoul Metropolitan Government-Seoul National University Boramae Medical Center (IRB No. 06-2011-75) and was performed in accordance with the Declaration of Helsinki. All the enrolled patients provided written informed consent.
Patients were randomly assigned in a 1:1 ratio to either silymarin group or placebo group at the time of starting anti-TB treatment. A computer-generated, permuted block randomization with a block of two and four was used. Only the pharmacologist knew the randomization result. Patients and doctors did not know the details of the study drugs that the patients received.
The first line anti-TB drugs including isoniazid, rifampicin, ethambutol, and pyrazinamide were started with the dosage according to Korean Guidelines for Tuberculosis7. Silymarin or placebo (with similar appearance with the study drug) were assigned to the study patient on the first day of anti-TB treatment. One tablet of silymarin (140 mg) or placebo was taken twice a day along with anti-TB drugs. The remaining tablets were counted on the days of follow-up to check patient's compliance and adherence. Patients were followed up for a liver function test (LFT) at week 2, week 4, and week 8 after the beginning of the study.
The primary outcome of the study was to compare the development of anti-TB treatment related DILI defined by serum AST or ALT >3× upper normal limit (UNL) or TBil (TB) >2× UNL8.
Data were analyzed based on intention to treat analysis approach. Student's t test was used to compare means between two groups. Linear mixed effects model was used to compare the liver enzyme according to the time change and between groups. All the statistical analyses were performed using R version 3.3.0.
Results
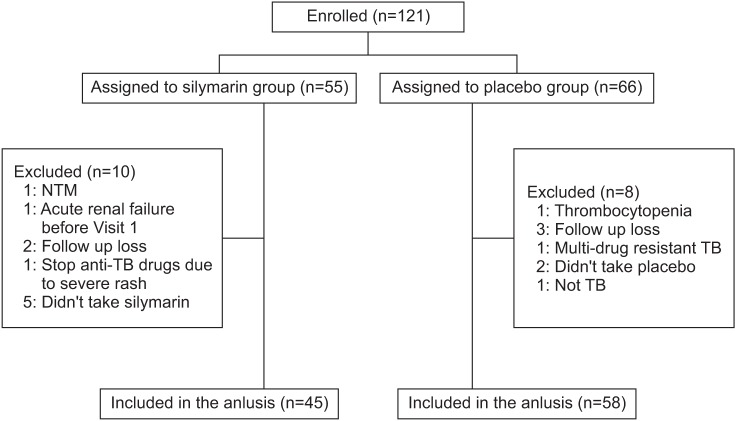
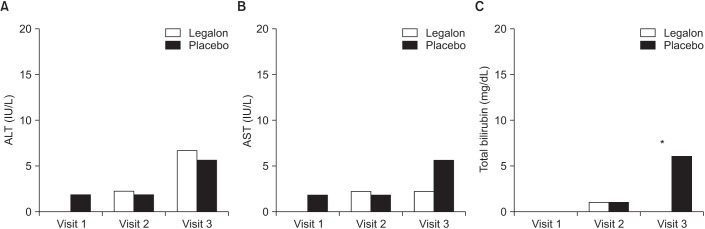
A total of 121 patients underwent randomization (Figure 1). After the secondary exclusion of 18 patients who could not take the study drug, 108 patients were included in the final analysis. A total of 45 patients were assigned to silymarin group and 58 to placebo group. The baseline characteristics of the patients were similar in silymarin and placebo groups (Table 1). Mean levels of AST, ALT, or TBil according to the time change were not different significantly between two groups. The incidence of an increase in serum ALT or AST that was greater than three times of the UNL was not different significantly between study groups (Figure 2). The proportions of elevated serum liver enzymes more than three times of UNL at week 2, week 4, and week 8 between silymarin and placebo group, were 0% versus 3.6% (p=1.0), 4.4% versus 3.6% (p>0.999), 8.7% versus 10.8% (p=0.630), respectively. However, patients with TBil >2× ULN at week 8 were significantly low in silymarin group (Figure 2). Five patients (8.6%) had TBil >2× UNL in placebo group and no patient in silymarin group (p=0.043). Regardless of silymarin treatment, serum AST and ALT increased significantly over time. For AST, average 2.61 IU/L increased weekly (p=0.014) and average 2.45 IU/L of ALT increased weekly (p=0.015).
Figure 1. Flow chart. NTM: nontuberculous mycobacteria; TB: tuberculosis.
Table 1. Comparison of baseline characteristics and liver enzyme in Legalon and placebo groups.
| Legalon (n=45) | Placebo (n=58) | p-value | |
|---|---|---|---|
| Male sex | 28 (62.22) | 40 (68.97) | 0.474 |
| Age, yr | 57.73±13.94 | 58.53±14.71 | 0.778 |
| BMI, kg/m2 | 21.73±0.409 | 21.00±0.402 | 0.209 |
| HBsAg | 1 (2.22) | 3 (5.17) | 0.442 |
| Anti-HCV | 2 (4.44) | 1 (1.72) | 0.415 |
| Baseline | |||
| T.Bil, mg/dL | 0.65±0.336 | 0.67±0.263 | 0.719 |
| ALP, IU/L | 86.67±26.51 | 92.47±15.19 | 0.227 |
| AST, IU/L | 21.42±8.09 | 24.258±15.19 | 0.227 |
| ALT, IU/L | 20.266±13.69 | 18.655±9.746 | 0.506 |
| GGT, IU/L | 30.568±56.95 | 27.31±34.71 | 0.738 |
| Week 2 | |||
| T.Bil, mg/dL | 0.753±0.242 | 0.733±0.224 | 0.657 |
| ALP, IU/L | 93.067±29.636 | 96.67±29.796 | 0.543 |
| AST, IU/L | 24.956±8.790 | 30.448±24.376 | 0.153 |
| ALT , IU/L | 21.867±12.302 | 24.293±19.234 | 0.438 |
| GGT, IU/L | 38.533±31.583 | 36.879±30.205 | 0.805 |
| Week 4 | |||
| T.Bil, mg/dL | 0.756±0.230 | 0.8±0.295 | 0.406 |
| ALP, IU/L | 90.227±27.355 | 89.52±29.039 | 0.901 |
| AST, IU/L | 31.289±20.499 | 33.07±25.605 | 0.697 |
| ALT, IU/L | 27.444±3.144 | 27.175±22.421 | 0.95 |
| GGT, IU/L | 39.068±27.286 | 34.491±24.302 | 0.383 |
| Week 8 | |||
| T.Bil, mg/dL | 0.756±0.252 | 0.777±0.277 | 0.684 |
| ALP, IU/L | 83.222±25.971 | 83.075±27.253 | 0.978 |
| AST, IU/L | 36.756±25.414 | 42.340±78.974 | 0.627 |
| ALT , IU/L | 38.511±44.001 | 36.981±61.967 | 0.887 |
| GGT, IU/L | 42.156±40.880 | 46.622±79.706 | 0.722 |
Values are presented as number (%) or mean±SD.
BMI: body mass index; HBsAg: hepatitis B surface antigen; HCV: hepatitis C virus; T.Bil: total bilirubin; ALP: alkaline phosphatase; AST: aspartate aminotransferase; ALT: alanine transaminase; GGT: gamma glutamyl transferase.
Figure 2. Number of patients with greater than 3× upper limit of normal (ULN) liver enzyme (A, B) and 2× ULN total bilirubin (C). *p=0.043.
Finally, three (5.2%) stopped anti-TB treatment in placebo group and one (2.2%) stopped in silymarin group due to hepatotoxicity of anti-TB drugs (p=0.442) during the study period.
Discussion
DILI is one of the most common and important adverse effects of anti-TB drugs9.
Silymarin has antifibrotic, immunomodulating, anti-inflammatory as well as antioxidant properties by scavenging free radicals and increasing the glutathione concentrations3. In animal model, silymarin showed protective effects against hepatotoxicity of anti-TB drugs4. However, there is only limited data about the protective effect of silymarin on hepatotoxicity in human.
In this randomized controlled study, silymarin was used during the first 2 months of treatment. Silymarin decreased the incidence of DILI by preventing the increased of TBil >2× ULN during the first 2-month of anti-TB treatment. Hoewever, it did not show any preventive effect on the elevation of AST or ALT >3× ULN and did not prevent clinically meaningful hepatotoxicity leading to treatment interruption or regimen change.
According to the recent randomized controlled study of silymarin from Thailand, receiving silymarin showed 0.28 risk reduction ratio for anti-TB–DILI than placebo (0.10, 0.47)10. But in this study, only 55 patients were enrolled and received silymarin during the first 4 weeks.
We may need to expand the study duration to confirm the preventive effect of silymarin during anti-TB treatment. In this study, silymarin was added during the first 2 months, the intensive phase of anti-TB treatment. Because DILI is relatively common in early phase and periodic monitoring of LFT is recommend at an early stage11,12. According to the Korean tuberculosis cohort of 1,031 patients, 108 patients (10.5%) developed drug-induced hepatotoxiity a mean of 39.6±43.7 days after treatment initiation. Twenty-eight patients (25.9%) developed antituberculous drug-induced hepatotoxicity within 7 days, 73 (67.6%) within 30 days13. But, another study showed median interval from treatment initiation of drug to development of clinical symptoms is 16 weeks (range, 6 weeks–6 months)14. Actually, serum AST/ALT has been continuously increased until week 8 of anti-TB treatment in our study. Therefore, longer period study of silymarin is needed beyond the intensive phase of anti-TB treatment.
Once DILI was developed, changes of anti-TB treatment could be considered according to the physician's clinical decision, including drug interruption, discontinuation or change the regimen15. Any could extend the treatment duration, induce the drug resistance, poor adherence, or treatment failure after all. Therefore, further study is warranted in order to enhance the prevention of DILI.
From this study, silymarin has not shown significant efficacy to prevent DILI. But, it showed less increase of TBil and a tendency to decrease the rate of anti-TB drug interruption due to DILI. Therefore, a larger and longer study is needed to confirm the preventive effect of silymarin.
Footnotes
Conflicts of Interest: Placebo was kindly donated by Bukwang Pharmaceutical Co.
References
- 1.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 2.Sherif IO, Al-Gayyar MM. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw. 2013;24:114–121. doi: 10.1684/ecn.2013.0341. [DOI] [PubMed] [Google Scholar]
- 3.Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci. 2011;14:308–317. [PMC free article] [PubMed] [Google Scholar]
- 4.Eminzade S, Uraz F, Izzettin FV. Silymarin protects liver against toxic effects of anti-tuberculosis drugs in experimental animals. Nutr Metab (Lond) 2008;5:18. doi: 10.1186/1743-7075-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahan S, Khan M, Imran S, Sair M. The hepatoprotective role of Silymarin in isoniazid induced liver damage of rabbits. J Pak Med Assoc. 2015;65:620–622. [PubMed] [Google Scholar]
- 6.Liu Q, Garner P, Wang Y, Huang B, Smith H. Drugs and herbs given to prevent hepatotoxicity of tuberculosis therapy: systematic review of ingredients and evaluation studies. BMC Public Health. 2008;8:365. doi: 10.1186/1471-2458-8-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joint Committee for the Revision of Korean Guidelines for Tuberculosis; Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 8.Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380–1387. doi: 10.1038/ajg.2012.138. [DOI] [PubMed] [Google Scholar]
- 9.Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–1477. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 10.Luangchosiri C, Thakkinstian A, Chitphuk S, Stitchantrakul W, Petraksa S, Sobhonslidsuk A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 2015;15:334. doi: 10.1186/s12906-015-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agal S, Baijal R, Pramanik S, Patel N, Gupte P, Kamani P, et al. Monitoring and management of antituberculosis drug induced hepatotoxicity. J Gastroenterol Hepatol. 2005;20:1745–1752. doi: 10.1111/j.1440-1746.2005.04048.x. [DOI] [PubMed] [Google Scholar]
- 12.Saukkonen JJ, Powell K, Jereb JA. Monitoring for tuberculosis drug hepatotoxicity: moving from opinion to evidence. Am J Respir Crit Care Med. 2012;185:598–599. doi: 10.1164/rccm.201112-2174ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CM, Lee SS, Lee JM, Cho HC, Kim WS, Kim HJ, et al. Early monitoring for detection of antituberculous drug-induced hepatotoxicity. Korean J Intern Med. 2016;31:65–72. doi: 10.3904/kjim.2016.31.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramappa V, Aithal GP. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013;3:37–49. doi: 10.1016/j.jceh.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Xia Y, Lv X, Tang S, Yang Z, Zhang Y, et al. Preventive use of hepatoprotectors yields limited efficacy on the liver toxicity of anti-tuberculosis agents in a large cohort of Chinese patients. J Gastroenterol Hepatol. 2015;30:540–545. doi: 10.1111/jgh.12717. [DOI] [PubMed] [Google Scholar]