Abstract
Background
We aimed to analyze the factors predicting the diagnostic performance of flexible bronchoscopy without guidance in peripheral lung lesions that are endoscopically invisible.
Methods
This was a retrospective study conducted in St. Paul's Hospital, The Catholic University of Korea, between January 2007 and March 2013. We included all patients who received bronchoscopy during this period. The analyzed variables were age, sex, the etiology of the lesion, lesion size, distance from the pleura, and presence of the bronchus sign. We used multiple logistic regression analysis to identify the significant independent factors associated with diagnostic yield.
Results
We included 151 patients in this study. The overall diagnostic yield was 58.3%. The sensitivity was 43.2% for malignant disease and 78.1% for benign disease. The benign lung lesions (p<0.001), lesion size (p=0.015), presence of the exposed type of bronchus sign (p<0.001), and presence of cavitary lung lesions (p=0.005) were factors influencing the yield of flexible bronchoscopy by univariate analysis. In a multivariate logistic regression analysis, the exposed type of bronchus sign and benign lung lesions were independent predicting factors (odds ratio [OR]: 27.95; 95% confidence interval [CI], 7.56–103.32; p<0.001 and OR, 4.91; 95% CI, 1.76–13.72; p=0.002).
Conclusion
The presence of the exposed type of bronchus sign and benign lung lesions are determining factors of the diagnostic yield in flexible bronchoscopy in evaluating peripheral lesions that are not endoscopically visible.
Keywords: Bronchoscopy, Lung, Bronchi, Multidetector Computed Tomography
Introduction
Flexible bronchoscopy is commonly performed to evaluate peripheral lung lesions. Several diagnostic innovations, including ultrathin bronchoscopy, virtual bronchoscopy, electromagnetic navigation, and radial endobronchial ultrasound, have been established to increase the diagnostic yield of bronchoscopy1,2,3,4. However, these modalities are costly and limited to specialized centers, causing difficulty of application in routine clinical practice. Although flexible bronchoscopy under fluoroscopic guidance is a widely accepted method and increases the diagnostic yield of nonendobronchial lung lesions, it has the disadvantages of causing radiation exposure in patients and clinicians5,6.
Many studies have analyzed the factors that affect the diagnostic performance of flexible bronchoscopy in the evaluation of peripheral lesions. These studies have demonstrated that the size, presence of the bronchus sign, and nature of the lesion are determinants of the diagnostic yield in bronchoscopy when evaluating peripheral lung lesions7,8,9. However, few studies have reported the diagnostic performance of conventional bronchoscopy without fluoroscopic guidance.
In a previous study, we demonstrated that the size and nature of the lesion were significant predictive factors for a higher diagnostic performance of flexible bronchoscopy without fluoroscopic guidance9. However, the bronchus sign, one of the reported predictors of the outcome of flexible bronchoscopy, was not included, and multivariate analytical techniques were not used. Therefore, we performed this study to analyze factors that influence the diagnostic performance of flexible bronchoscopy without fluoroscopic guidance in evaluating peripheral lung lesions.
Materials and Methods
1. Study subjects
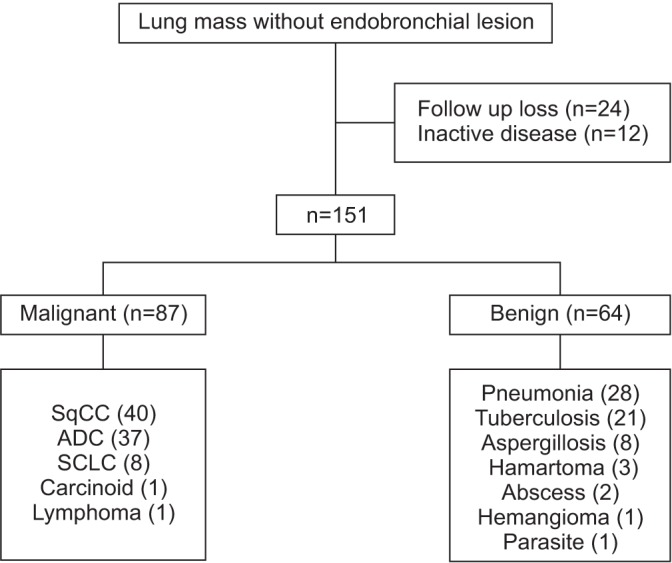
This was retrospective study conducted in St. Paul's Hospital, The Catholic University of Korea between January 2007 and March 2013. All patients received bronchoscopy during this period were included. Initially, 204 patients with peripheral lesions without endobronchial lesions who underwent conventional bronchoscopy were enrolled in the study. The nondiagnostic results of bronchoscopy were confirmed from following percutaneous transthoracic needle aspiration (TTNA) or surgical resection. Twelve patients with inactive lung lesions, 24 patients lost to follow-up, and 17 patients with lesions not confirmed by any diagnostic methods were excluded. The remaining 151 patients diagnosed with active lung lesions were registered for the final analysis (Figure 1). All data were collected from the medical records. The requirement for informed consent was waived by the ethical review board. This study was approved by the Institutional Review Board of St. Paul's Hospital (Seoul, Korea) (IRB No. PC16OASI0022).
Figure 1. Study patients. SqCC: squamous cell carcinoma; ADC: lung adenocarcinoma; SCLC: small cell lung cancer.
2. Bronchoscopic procedures
Bronchoscopic examinations were performed without fluoroscopic guidance by five full-time faculty staffs with clinical experience of bronchoscopy at least 1 year in the pulmonology division. Before the procedure, the patient was given 0.5 mg of atropine sulfate to reduce oral secretions and bronchospasm, and 50 mg of pethidine hydrochloride to provide pain relief and suppress coughing intramuscularly, and took nebulized lidocaine (10 mL of 2% solution) for 5 minutes. The heart rates and saturation of peripheral oxygen (SpO2) were continuously monitored. Oxygen was applied via nasal cannula or mask and adjusted to keep saturation above 90%. Bronchoscopy (models BF-1T260; Olympus, Tokyo, Japan) was used for brushing, washing, and biopsy. Bronchoscopy was performed via the transnasal or transoral approach. Midazolam was administered to achieve moderate sedation. When the vocal cord is inspected, 2 mL of 4% lidocaine was sprayed to anesthetize the upper airway as well as the tracheobronchial tree. All segments of the bronchus were observed. Next, the bronchoscope was moved to the bronchial segment of a targeted lesion located on computed tomography (CT). The specimen obtained by cytology brush was smeared on three slides. These were air-dried and alcohol-fixed, and sent for acid-fast bacilli and Papanicolaou stain, respectively. At least three 1- to 3-mm-sized adequately sized specimens from transbronchial lung biopsies (TBLBs) were processed by first incubating in formalin and then sending to an external vendor for hematoxylin and eosin stain. About 15 mL of saline was instilled through the bronchoscope and aspirated at least three times after biopsy. A sample from bronchial washing (BW) was prepared for routine cytology, cell block cytology, acidfast bacilli and gram staining, and bacterial and fungal culture. For benign pulmonary lesions, cytologic findings associated with infectious etiologies such as inflammatory cells and/or microbial organisms and resolution of radiologic findings with antibiotics were considered pneumonia. Pulmonary lesion with the presence of necrotic debris or pus was diagnosed of abscess.
3. Radiographic assessments
Chest CT were performed in most patients within one month before flexible bronchoscopy. The radiologic findings were analyzed by a radiologist, and the size of the lesion was defined as the measurement of the longest dimension. The CT bronchus sign was divided into the exposed and unexposed types according to the definition of Tsuboi et al.10: (1) the bronchial lumen is exposed to the tumor and cut off or contained within the tumor, or (2) the bronchus is compressed by the tumor or constricted by peri-mucosal tumor spread, causing either smooth or irregular narrowing. When two or more bronchus signs were identified, the order of the more proximal bronchus was prioritized.
4. Definition of bleeding
There is no exact definition of minor and major bleeding. In our study, minor bleeding was defined as bleeding which stopped spontaneously or was controlled with local epinephrine. Major bleeding was defined when additional interventions such as embolization of blood vessel, surgical intervention or transfusion were required.
5. Statistical analysis
Statistical analysis was performed using the Pearson's chisquare test and Fisher exact test for discrete variables, and the Mann-Whitney U test for continuous variables. McNemar's test was performed as a test of univariate association between diagnostic method and the outcomes. Multiple logistic regression analysis was used to identify the significant independent factors associated with diagnostic yield of conventional bronchoscopy. Two-sided p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (IBM SPSS Statistics 20.0; IBM Corp., Armonk, NY, USA).
Results
One hundred fifty-one patients were included in the study. Among the lesions of these patients, 87 were malignant (40 squamous cell carcinomas, 37 adenocarcinomas, eight small cell carcinomas, one carcinoid, and one lymphoma), and 64 were benign (28 related to pneumonia, 21 to tuberculosis, eight to aspergillosis, three to hamartoma, two to an abscess, one to sclerosing hemangioma, and one to parasite infestation) (Figure 1). In patients with pneumonia, microorganism was isolated in 11 (35.7%) patients. Pathogens detected with bronchoscopic cultures were Klebsiella pneumoniae (6) and Streptococcus pneumoniae (1), Enterobacter aerogenes (1), S. aureus (1), and Proteus mirabilis (1). The mean patient age was 65±14 years. The mean size of the pulmonary lesions was 3.68±1.92 cm. The mean distance from the lesions to the pleural surface was 1.02±1.09 cm. Most of the lesions were located in the upper lobes (44.3%, 67/151) and lower lobes (39.0%, 59/151) (Table 1).
Table 1. Patient demographics and characteristics of the pulmonary lesions.
| Characteristic | No. (%) (n=151) |
|---|---|
| Male sex | 100 (66.2) |
| Age, yr | 64.69±13.74 |
| Lesion size, cm | 3.68±1.92 |
| Lesion location | |
| LUL | 32 (21.2) |
| LLL | 29 (19.2) |
| RUL | 42 (27.8) |
| RML | 18 (11.9) |
| RLL | 30 (19.9) |
| Type of lesion | |
| Malignancy | 87 (57.6) |
| Benign | 64 (42.4) |
| Presence of cavity | 29 (19.2) |
| Presence of bronchus sign | 69 (45.7) |
| Exposed/unexposed | 55/14 |
| Distance from the pleura, cm | 1.02±1.09 |
LUL: left upper lobe; LLL: left lower lobe; RUL: right upper lobe; RML: right middle lobe; RLL: right lower lobe.
The overall diagnostic yield of bronchoscopy was 58.3%. The sensitivity was 43.2%, 78.1% in malignant and benign disease, respectively. All patients received TBLB, bronchial brushing (BB) and BW. The diagnostic performance from TBLB was higher than that from BB and BW. In malignant lesions, the diagnostic yield from TBLB was 40.2% (35/87), and the yield from BB and BW was 12.6% (11/87) and 3.4% (3/87), respectively. A similar tendency was seen in benign lesions. However, the diagnostic yield from TBLB and BW was higher in the benign lesions (Table 2). There was no inconsistency in diagnostic results among TBB, BB, and BW. Sixty-two patients were non-diagnostic with bronchoscopy. These patients received additional diagnostic method (one pleural biopsy, eight surgical biopsies, and 53 TTNA).
Table 2. Diagnostic yield of the three different diagnostic modalities.
| Variable | TBB*,† | BB | BW |
|---|---|---|---|
| Benign | 42/64 (65.6)‡ | 7/64 (10.9) | 14/64 (21.9)§ |
| Malignant | 35/87 (40.2) | 11/87 (12.6) | 3/87 (3.4) |
| Total | 77/151 (51.0) | 18/151 (11.9) | 17/151 (11.3) |
Values are presented as number (%).
*p<0.001, McNemar's test between diagnostic yields of TBB vs. BB. †p<0.001, McNemar's test between diagnostic yields of TBB vs. BW. ‡p=0.003, chi-square analysis between diagnostic yields for malignant vs. benign lesions. §p=0.001, chi-square analysis between diagnostic yields for malignant vs. benign lesions.
TBB: transbronchial biopsy; BB: bronchial brushing; BW: bronchial washing.
As shown in Table 3, the diagnostic yield of bronchoscopy in peripheral lung lesions was associated to the presence of the bronchus sign and cavity, lesion size, and etiology of the lung lesion by univariate analysis. The size of lesion >3 cm was based on dividing point between mass and nodule. The distance from pleura >2 cm was defined according to the previous study, and the lesion >2 cm from the pleura was known to be risk factor of pneumothorax after TTNA11,12.
Table 3. Logistic regression analysis of factors influencing the diagnostic yield of conventional bronchoscopy.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Sex | ||||
| Male | 1.09 (0.55–2.16) | 0.801 | 0.73 (0.29–1.84) | 0.503 |
| Female | ||||
| Age | 0.96 (0.94–0.99) | 0.005 | 0.97 (0.94–1.01) | 0.091 |
| Etiology | ||||
| Malignant | ||||
| Benign | 4.61 (2.22–9.54) | <0.001 | 4.91 (1.76–13.72) | 0.002 |
| Size, cm | ||||
| ≤3 | ||||
| >3 | 2.3 (1.17–4.37) | 0.015 | 1.32 (0.52–4.94) | 0.565 |
| Distance from pleura, cm | ||||
| ≤2 | ||||
| >2 | 0.96 (0.30–1.91) | 0.556 | 1.53 (0.47–4.94) | 0.478 |
| Cavity | ||||
| Presence | 4.35 (1.56–12.15) | 0.005 | 1.14 (0.27–4.82) | 0.861 |
| Absence | ||||
| Bronchus sign | ||||
| Exposed type | 19.9 (6.56–60.46) | <0.001 | 27.95 (7.56–103.32) | <0.001 |
| Unexposed type | 0.87 (0.27–2.82) | 0.814 | 1.77 (0.45–6.94) | 0.414 |
| Absent | ||||
OR: odds ratio; CI: confidence interval.
The location of lesion and distance from the pleura did not affect the diagnostic performance of bronchoscopy. Multivariate analysis after adjusting for sex, age, lesion size, distance from pleura, and the presence of cavity identified the exposed type of bronchus sign (p<0.001) and benign lung lesions (p=0.002) as the variables conditioning the diagnostic yield of bronchoscopy with an odds ratio (OR) of 27.95 (95% confidence interval [CI], 7.56–103.32) and 4.91 (95% CI, 1.76– 13.72), respectively.
Bronchoscopy was diagnostic in 92.7% of cases (51/55) in which the exposed type of bronchus sign was identified on chest CT but in only 39.0% (32/82) without an identifiable bronchus sign and in only 35.7% (5/14) with an unexposed type of bronchus sign. The unexposed type of bronchus sign was not different from the absence of the bronchus sign in affecting the diagnostic yield of bronchoscopy for peripheral lung lesions (OR, 1.5; 95% CI, 0.4–5.7; p=0.544).
During the study period, life threatening major complications did not occur. Sixteen patients (10.6%) had minor bleeding, and five patients (3.3%) had pneumothorax following bronchoscopy. Of patients with pneumothorax, four patients recovered by oxygen therapy and one patient required chest tube insertion.
Discussion
The present study analyzed factors that influence the diagnostic performance of flexible bronchoscopy without guidance in evaluating peripheral lung lesions not visible endoscopically. Benign lung lesions, a lesion size of >3 cm, and the presence of a bronchus sign were determining factors increasing the yield of flexible bronchoscopy by univariate analysis. In multivariate logistic regression analysis, the exposed type of bronchus sign and benign lung lesions were predicting factors.
The diagnostic performance of flexible bronchoscopy in peripheral lung lesions without endobronchial lesions was not high, varying from 19% to 77% in malignant lesions and 27% to 74% in benign lesions13,14,15. Many researchers have sought to determine factors that improve the diagnostic yield of bronchoscopy. Several factors that affect the diagnostic yield of conventional bronchoscopy in patients with peripheral lung lesions without endobronchial lesions have been suggested: the CT bronchus sign, size of the lesion, etiology of the lesion, and location of the lesion16,17,18,19,20. A point that claims our attention is that most of the studies routinely used fluoroscopy as guidance to increase the sensitivity of bronchoscopy. Few studies were conducted with non-guidance conventional bronchoscopy, and these were conducted for the cases confined to malignancies, those mixed with peripheral lung lesions visible bronchoscopically, and those performed with only brushing or washing8,21,22,23,24.
The bronchus sign on CT denotes the presence of a bronchus leading directly to a peripheral lung lesion18. The bronchus sign is grouped into two categories according to the presence or absence of exposure of the tumor mass to the bronchial lumen10. In a previous prospective study, there was no difference in the diagnostic yield between TBLB and TTNA in lesions with the exposed type of CT bronchus sign25. In a study of the determinants of diagnostic BW in peripheral lung cancers, the exposed type of bronchus sign was the most important factor8. Our results correspond with these earlier studies 8,25. A revealing fact is that the unexposed type of bronchus sign was not different from the absence of the bronchus sign in affecting the diagnostic yield of bronchoscopy for peripheral lung lesions. A study by Gaeta et al.18 supports this result: in the unexposed type of bronchus sign, tumor cells could not be obtained by TBLB or BB in evaluating peripheral carcinoma of the lung.
The diagnostic yield from TBLB for benign lesions is generally known to be lower than that for malignant lesions17,26,27. However, these studies used results from fluoroscopy-guided bronchoscopy. Fluoroscopy-guided TBLB significantly increases the diagnostic yield of non-endobronchial lung masses6. According to Labbe et al.21, the sensitivity of non-guided bronchoscopy for the diagnosis of peripheral malignant lesions is limited. In our study, the higher diagnostic yield in benign lesions than in malignant lesions may be due to the frequency of positive culture findings for tuberculosis obtained from BW (11/21, 52.4%) and BB (5/21, 23.8%) in our study. Additionally, in general, benign lung lesions present diffuse infiltrates, whereas malignant lung lesions present localized infiltrates28. Descombes et al.29 reported that the diagnostic yield of TBLB was better for lung lesions with diffuse pulmonary infiltrates than those with localized lung lesions or peripheral neoplasia.
Although the diagnostic yield of conventional bronchoscopy without guidance does not provide the highest performance, this procedure has some advantages. First, conventional bronchoscopy without guidance can be performed relatively safely without additional expenses16,26. Fluoroscopy has been used to identify the position of the sheath and forceps, confirm that the biopsy was conducted as planned, and check for complications such as pneumothorax. However, this method has the shortcomings of radiation exposure, a longer procedure time, and occupying a large amount of space8,30. According to a registry-based study, an advanced diagnostic technique such as electromagnetic navigation or radial endobronchial ultrasound does not improve the diagnostic yield compared with conventional bronchoscopy31. Second, the incidence rates of complications such as bleeding or pneumothorax are lower with conventional bronchoscopy than with TTNA32.
Our study has limitations inherent in a retrospective research. Additionally, our study was based on a single center; thus, broad application of our results is limited. Finally, direct comparison of the diagnostic yield was not possible because there was no fluoroscopy-guided control group. However, current study is meaningful in that few studies concerning the diagnostic performance of bronchoscopy without guidance have been conducted for peripheral lung lesions that are invisible endoscopically, and predicting factors for diagnostic yield of conventional bronchoscopy has clinical importance in deciding the diagnostic modalities for peripheral lung lesions.
In conclusion, we documented that the exposed type of bronchus sign and benign lung lesions were influencing factors in the diagnostic performance of conventional bronchoscopy without guidance in evaluating peripheral lung lesions that are invisible bronchoscopically. Thus, detailed analysis of pre-procedure radiologic findings is needed for peripheral lesions, and non-guidance conventional bronchoscopy could be considered when the exposed type of bronchus sign is present and a benign lesion is suspected.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Asano F, Shinagawa N, Ishida T, Shindoh J, Anzai M, Tsuzuku A, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy: a randomized clinical trial. Am J Respir Crit Care Med. 2013;188:327–333. doi: 10.1164/rccm.201211-2104OC. [DOI] [PubMed] [Google Scholar]
- 2.Boonsarngsuk V, Raweelert P, Juthakarn S. Endobronchial ultrasound plus fluoroscopy versus fluoroscopy-guided bronchoscopy: a comparison of diagnostic yields in peripheral pulmonary lesions. Lung. 2012;190:233–237. doi: 10.1007/s00408-011-9359-3. [DOI] [PubMed] [Google Scholar]
- 3.Bose S, Ghatol A, Eberlein M, Yung RC. Ultrathin bronchoscopy in the diagnosis of peripheral cavitary lung lesions. J Bronchology Interv Pulmonol. 2013;20:167–170. doi: 10.1097/LBR.0b013e3182904987. [DOI] [PubMed] [Google Scholar]
- 4.Chee A, Stather DR, Maceachern P, Martel S, Delage A, Simon M, et al. Diagnostic utility of peripheral endobronchial ultrasound with electromagnetic navigation bronchoscopy in peripheral lung nodules. Respirology. 2013;18:784–789. doi: 10.1111/resp.12085. [DOI] [PubMed] [Google Scholar]
- 5.Jain P, Fleming P, Mehta AC. Radiation safety for health care workers in the bronchoscopy suite. Clin Chest Med. 1999;20:33–38. doi: 10.1016/s0272-5231(05)70124-6. [DOI] [PubMed] [Google Scholar]
- 6.Rittirak W, Sompradeekul S. Diagnostic yield of fluoroscopy-guided transbronchial lung biopsy in non-endobronchial lung lesion. J Med Assoc Thai. 2007;90(Suppl 2):68–73. [PubMed] [Google Scholar]
- 7.Gaeta M, Russi EG, La Spada F, Barone M, Casablanca G, Pandolfo I. Small bronchogenic carcinomas presenting as solitary pulmonary nodules. Bioptic approach guided by CT-positive bronchus sign. Chest. 1992;102:1167–1170. doi: 10.1378/chest.102.4.1167. [DOI] [PubMed] [Google Scholar]
- 8.Lee HS, Kwon SY, Kim DK, Yoon HI, Lee SM, Lee JH, et al. Determinants of diagnostic bronchial washing in peripheral lung cancers. Int J Tuberc Lung Dis. 2007;11:227–232. doi: 10.4046/trd.2007.62.3.227. [DOI] [PubMed] [Google Scholar]
- 9.Rhee CK, Kang HH, Kang JY, Kim JW, Kim YH, Park SA, et al. Diagnostic yield of flexible bronchoscopy without fluoroscopic guidance in evaluating peripheral lung lesions. J Bronchology Interv Pulmonol. 2010;17:317–322. doi: 10.1097/LBR.0b013e3181f552a5. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi E, Ikeda S, Tajima M, Shimosato Y, Ishikawa S. Transbronchial biopsy smear for diagnosis of peripheral pulmonary carcinomas. Cancer. 1967;20:687–698. doi: 10.1002/1097-0142(1967)20:5<687::aid-cncr2820200521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Hsia DW, Jensen KW, Curran-Everett D, Musani AI. Diagnosis of lung nodules with peripheral/radial endobronchial ultrasound-guided transbronchial biopsy. J Bronchology Interv Pulmonol. 2012;19:5–11. doi: 10.1097/LBR.0b013e31823fcf11. [DOI] [PubMed] [Google Scholar]
- 12.Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 13.Hergott CA, Tremblay A. Role of bronchoscopy in the evaluation of solitary pulmonary nodules. Clin Chest Med. 2010;31:49–63. doi: 10.1016/j.ccm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Joos L, Patuto N, Chhajed PN, Tamm M. Diagnostic yield of flexible bronchoscopy in current clinical practice. Swiss Med Wkly. 2006;136:155–159. doi: 10.4414/smw.2006.11344. [DOI] [PubMed] [Google Scholar]
- 15.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–e165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 16.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 17.Botana-Rial M, Nunez-Delgado M, Pallares-Sanmartin A, Leiro-Fernandez V, Torres-Duran M, Represas-Represas C, et al. Multivariate study of predictive factors for clearly defined lung lesions without visible endobronchial lesions in transbronchial biopsy. Surg Endosc. 2010;24:3031–3036. doi: 10.1007/s00464-010-1080-4. [DOI] [PubMed] [Google Scholar]
- 18.Gaeta M, Pandolfo I, Volta S, Russi EG, Bartiromo G, Girone G, et al. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol. 1991;157:1181–1185. doi: 10.2214/ajr.157.6.1950861. [DOI] [PubMed] [Google Scholar]
- 19.Naidich DP, Sussman R, Kutcher WL, Aranda CP, Garay SM, Ettenger NA. Solitary pulmonary nodules: CT-bronchoscopic correlation. Chest. 1988;93:595–598. doi: 10.1378/chest.93.3.595. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123(1 Suppl):115S–128S. doi: 10.1378/chest.123.1_suppl.115s. [DOI] [PubMed] [Google Scholar]
- 21.Labbe C, Beaudoin S, Martel S, Delage A, Joubert P, Drapeau C, et al. Diagnostic yield of non-guided flexible bronchoscopy for peripheral pulmonary neoplasia. Thorac Cancer. 2015;6:517–523. doi: 10.1111/1759-7714.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonsarngsuk V, Raweelert P, Sukprapruet A, Chaiprasithikul R, Kiatboonsri S. Factors affecting the diagnostic yield of flexible bronchoscopy without guidance in pulmonary nodules or masses. Singapore Med J. 2010;51:660–665. [PubMed] [Google Scholar]
- 23.Sing A, Freudenberg N, Kortsik C, Wertzel H, Klosa B, Hasse J. Comparison of the sensitivity of sputum and brush cytology in the diagnosis of lung carcinomas. Acta Cytol. 1997;41:399–408. doi: 10.1159/000332531. [DOI] [PubMed] [Google Scholar]
- 24.Roth K, Hardie JA, Andreassen AH, Leh F, Eagan TM. Predictors of diagnostic yield in bronchoscopy: a retrospective cohort study comparing different combinations of sampling techniques. BMC Pulm Med. 2008;8:2. doi: 10.1186/1471-2466-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilaceroglu S, Kumcuoglu Z, Alper H, Osma E, Cagirici U, Gunel O, et al. CT bronchus sign-guided bronchoscopic multiple diagnostic procedures in carcinomatous solitary pulmonary nodules and masses. Respiration. 1998;65:49–55. doi: 10.1159/000029237. [DOI] [PubMed] [Google Scholar]
- 26.Chechani V. Bronchoscopic diagnosis of solitary pulmonary nodules and lung masses in the absence of endobronchial abnormality. Chest. 1996;109:620–625. doi: 10.1378/chest.109.3.620. [DOI] [PubMed] [Google Scholar]
- 27.Tamiya M, Sasada S, Kobayashi M, Uehara N, Okamoto N, Morishita N, et al. Diagnostic factors of standard bronchoscopy for small (≤15 mm) peripheral pulmonary lesions: a multivariate analysis. Intern Med. 2011;50:557–561. doi: 10.2169/internalmedicine.50.4275. [DOI] [PubMed] [Google Scholar]
- 28.Fraire AE, Cooper SP, Greenberg SD, Rowland LP, Langston C. Transbronchial lung biopsy. Histopathologic and morphometric assessment of diagnostic utility. Chest. 1992;102:748–752. doi: 10.1378/chest.102.3.748. [DOI] [PubMed] [Google Scholar]
- 29.Descombes E, Gardiol D, Leuenberger P. Transbronchial lung biopsy: an analysis of 530 cases with reference to the number of samples. Monaldi Arch Chest Dis. 1997;52:324–329. [PubMed] [Google Scholar]
- 30.Cox ID, Bagg LR, Russell NJ, Turner MJ. Relationship of radiologic position to the diagnostic yield of fiberoptic bronchoscopy in bronchial carcinoma. Chest. 1984;85:519–522. doi: 10.1378/chest.85.4.519. [DOI] [PubMed] [Google Scholar]
- 31.Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions: results of the AQuIRE Registry. Am J Respir Crit Care Med. 2016;193:68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Boiselle PM, Shepard JO, Trotman-Dickenson B, McLoud TC. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol. 1996;167:105–109. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]


