Abstract
Thyroid hormone (T3) affects development and metabolism in vertebrates. We have been studying intestinal remodeling during T3-dependent Xenopus metamorphosis as a model for organ maturation and formation of adult organ-specific stem cells during vertebrate postembryonic development, a period characterized by high levels of plasma T3. T3 is believed to affect development by regulating target gene transcription through T3 receptors (TRs). While many T3 response genes have been identified in different animal species, few have been shown to be direct target genes in vivo, especially during development. Here we generated a set of genomic microarray chips covering about 8000 bp flanking the predicted transcription start sites in Xenopus tropicalis for genome wide identification of TR binding sites. By using the intestine of premetamorphic tadpoles treated with or without T3 and for chromatin immunoprecipitation assays with these chips, we determined the genome-wide binding of TR in the control and T3-treated tadpole intestine. We further validated TR binding in vivo and analyzed the regulation of selected genes. We thus identified 278 candidate direct TR target genes. We further provided evidence that these genes are regulated by T3 and likely involved in the T3-induced formation of adult intestinal stem cells during metamorphosis.
Introduction
Thyroid hormone (T3) regulates the formation and/or maturation of many organs into the adult form during vertebrate development and also affects the homeostasis and physiological function of many adult organs/tissues, such as the heart and muscles1–8. T3-deficiency during development causes severe defects in mammals, including cretinism in human3–5, 9, 10. T3 exerts its effect on vertebrate development mainly during the so-called postembryonic development when high levels of T3 are present in the plasma, a period around birth in mammals and spanning from a few months prior to birth to several months after birth in human3, 11. How T3 affects mammalian development has been difficult to study in part because of the difficulty to manipulate the uterus-enclosed mammalian embryos. In addition, it has been shown that maternal T3 can also influence embryonic development in mammals12–14, complicating the analysis of T3 action in vivo.
Anuran metamorphosis resembles the postembryonic development in mammals and is totally dependent on T34, 15. It can be easily manipulated by simply adding physiological levels of exogenous T3 to tadpole rearing water or blocking the synthesis of endogenous T3 in tadpoles. In addition, recent progress in genomic editing has also made it possible to analyze the function of endogenous genes in Xenopus 16–20. These and other properties have made anuran metamorphosis an excellent model to study the molecular basis of T3 action during vertebrate development. Studies on amphibian metamorphosis, especially in Xenopus laevis and tropicalis, have shown that T3 controls metamorphosis by regulating gene transcription through T3 receptors (TRs)8, 21–37.
Numerous studies have been carried out to identify genes regulated by T3 during metamorphosis. Early subtractive hybridization screening and subsequent gene expression microarray analyses have discovered many such genes in different organs/tissues during both natural and T3-induced metamorphosis4, 21, 38–45. These studies have revealed complex but informative global gene regulation patterns underlying organ transformations during metamorphosis. However, most of the genes thus identified are likely affected indirectly by T3, while only a few direct TR target genes have been characterized through traditional promoter analyses46–50. Here we have made use of the genomic sequence information available in Xenopus tropicalis (genome release version 4.1) to generate a tiled genomic probe set (479459 probes) of two microarray slides covering the sequences from 5.5 kb upstream to 2.5 kb downstream of the predicted transcriptional start sites of 17,355 Xenopus tropicalis genes (based on Ensembl release 46). This resulted in a probe-set that has about 40 probes (60-mer) tiled for each of the putative promoter regions at an average tiling distance of 205 bp. By using this genomic chip set and an antibody against Xenopus TRs, we then carried out a genome-wide chromatin immunoprecipitation (ChIP) assay on the intestine of premetamorphic tadpoles treated with or without T3. Nearly 300 genes were found to be bound by TR in vivo and more importantly, most were indeed found to be regulated by T3. Furthermore, bioinformatics analyses suggest that these genes are regulated during T3-dependent intestinal remodeling, implicating a role in the formation of adult intestinal stem cells during Xenopus metamorphosis, a period equivalent to the postembryonic development in mammals3, 4.
Materials and Methods
Experimental animals
Wild-type tadpoles of Xenopus tropicalis were obtained from NASCO, and developmental stages were determined according to Nieuwkoop and Faber51. Stage 54 tadpoles were treated for 2 days at 22 °C with 10 nM T3, close to the peak levels of T3 at the climax of metamorphosis in Xenopus laevis 52. All animal care and treatment were done as approved by Animal Use and Care Committee of Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), U.S. National Institutes of Health (NIH). The methods were carried out in accordance with relevant guidelines for the use of Xenopus tropicalis as a vertebrate model.
Generation of luciferase reporter constructs for transcription assay
The firefly luciferase reporter constructs containing Xenopus tropicalis Dot1L (Dot1-Like) promoter (pTRE(Dot1L)-luc or Xenopus tropicalis Dot1L promoter with a mutant TRE (pmTRE(Dot1L)-luc were made based on pGL4.10 firefly luciferase vector (Promega) as previously described53, 54. The promoter constructs containing a putative TRE of selective genes (Table 1) were generated through PCR-mediated mutagenesis on the pmTRE(Dot1L)-luc as described55. In brief, each TRE-luc construct was made with primer set 1: 5′-TGTTGGATGCTCATACTCGTCC-3′ (LZ631) and TRE_R for a putative TRE (Table 1), and primer set 2: 5′-TRE_F for the same TRE (Table 1) and 5′-GGTAATGTCCACCTCGATATGTGC-3′ (LZ632) in two different PCR reactions to produce about 1 kb and 500 bp fragments, respectively. The two PCR fragments were gel purified and then mixed together as the templates for a second round of PCR with primers LZ631 and LZ632 to produce a ~1.5 kb fragment. The 1.5 kb fragment was then subjected to restriction enzyme digestion with Kpn I and Hind III and gel-purified. The 1048 bp restricted fragment was then subcloned into Kpn I-Hind III digested firefly luciferase reporter construct pmTRE(Dot1L)-luc, whereby the Dot1L mTRE in pmTRE(Dot1L)-luc was replaced with the TRE of the respective gene (Table 1). All the firefly luciferase reporter constructs were confirmed by sequencing.
Table 1.
Putative TREs and primers for generating the firefly luciferase reporters.
| Gene | Putative TRE | Primers (TRE sequences in bold) |
|---|---|---|
| MBD3 | GGGTCAGATGGGGACA | TRE-F: 5′-CTAAGGGGTCAGATGGGGACACCCGCGGGATTATTTATTTTATTC-3′ TRE-R: 5′-GCGGGTGTCCCCATCTGACCCCTTAGCCTGAAGTCTGAGG-3′ |
| PPM1B | AGGTCATTTGAGGCCG | TRE-F: 5′-CTAAGAGGTCATTTGAGGCCGCCCGCGGGATTATTTATTTTATTC-3′ TRE-R: 5′-GCGGGCGGCCTCAAATGACCTCTTAGCCTGAAGTCTGAGG-3′ |
| PGPEP1 | GGTGCATGTCAGGACA | TRE-F: 5′-CTAAGGGTGCATGTCAGGACACCCGCGGGATTATTTATTTTATTC-3′ TRE-R: 5′-GCGGGTGTCCTGACATGCACCCTTAGCCTGAAGTCTGAGG-3′ |
| JUNB | GGGTAATGTAGGGTCA | TRE-F: 5′-CTAAGGGGTAATGTAGGGTCACCCGCGGGATTATTTATTTTATTC-3′ TRE-R: 5′-GCGGGTGACCCTACATTACCCCTTAGCCTGAAGTCTGAGG-3′ |
| BEND7 | AGTTCAGGGCAGGTCA | TRE-F: 5′-CTAAGAGTTCAGGGCAGGTCACCCGCGGGATTATTTATTTTATTC-3′ TRE-R: 5′-GCGGGTGACCTGCCCTGAACTCTTAGCCTGAAGTCTGAGG-3′ |
qRT-PCR
Total RNA was isolated from the intestine of tadpoles at premetamorphic stage 54, treated with or without 10 nM T3 at 22 °C for 2 days. The cDNA was prepared from 2.5 μg of total RNA using the Applied Biosystems’ High Capacity cDNA Archive kit according to the manufacturer’s instructions in a total volume of 50 μl. qRT-PCR based on SYBR Green detection was carried out to quantify gene expression levels on an ABI 7000 (Applied Biosciences) and EF1α (elongation factor 1α) was used as the normalization control as described previously56. The primers used for SYBR Green PCR were forward 5′-TCAAGCAACCAGTGACCAAG-3′ and reverse 5′-TTTCCCAGAAGAGCTGCCT-3′ for MBD3, forward 5′-GATGTCATGAGCAACGAGGA-3′ and reverse 5′-TCACGGCTTCCCTTATGTAAA-3′ for PPM1B, forward 5′-GCTGTGGTGGTGACTGGATTT-3′ and reverse 5′-GCCCAACTTTCCCAATTCCT-3′ for PGPEP1, forward 5′-GGTATTAGTACTGCCGCCCTC-3′ and reverse 5′-CATTCATTGTGGGCTCCGTG-3′ for TFG, forward 5′-CTATCCCCGCCAAACATCT-3′ and reverse 5′-CCATCTCAGCAGCTTCCTTC-3′ for EF1α56. All the primer pairs amplify fragments from cDNA of adjacent exons of the target genes, respectively.
ChIP assay
ChIP assay on tadpole intestine was done as described previously57 with anti-TR (new PB), which recognized both TRα and TRβ in both Xenopus laevis and Xenopus tropicalis 24, 58. All ChIP experiments were done at least twice with similar results.
For immunoprecipitation, the DNA concentration of the chromatin was diluted to 10 ng/μl with ChIP dilution buffer (Millipore). After precleaning with salmon sperm DNA/protein A-agarose beads, input samples were taken, and 500 μl of each chromatin sample were immunoprecipitated with indicated antibodies and salmon sperm DNA/protein A-agarose beads. The mixtures were incubated overnight at 4 °C followed by spinning down the beads. The beads were washed with ChIP buffer I, ChIP buffer II, ChIP buffer III and TE (Millipore). After the last wash, 200 μl of elution buffer were added to the samples as well as the input controls and incubated at 65 °C overnight, and the immunoprecipitated DNA was purified. The DNA was then analyzed by qPCR on an ABI 7000 (Applied Biosciences) with the gene-specific primers. For the detection of exon 5 of Xenopus tropicalis TRβ, forward primer 5′-CCCCGAAAGTGAAACTCTAACTCT-3′, reverse primer 5′-CCACACCGAGTCCTCCATTTT-3′ and FAM-labeled Taqman probe CTGCCATCTCACCATTC were used. The following primers were used for the detection of the newly identified TRE regions with SYBR Green detection, forward primer 5′-ATCCCGCCTACTCTTTATTCCTCCAGCTGC-3′, reverse primer 5′-GAGAGAGAGTCAGTGTGGTGGTGGGTCAGA-3′ for MBD3; forward primer 5′-AGTTGTTTGTGTGACCTCGGCCTCA-3′, reverse primer 5′-GCCCTCTGCTCATTTAACTCACAACGGT-3′ for PPM1B; and forward primer 5′-ACGTTCTCCGCTCGCTTCCTTCGGA-3′, reverse primer 5′-TCCGGTGCATGTCAGGACAAGGGCA-3′ for PGPEP1.
The ChIP signals were expressed as the percentage of the total input DNA prior to immunoprecipitations.
ChIP-on-chip assay
The genomic sequence information for Xenopus tropicalis (genome release version 4.1) was used to generate a tiled genomic probe set (479459 probes) covering the sequences from 5.5 kb upstream to 2.5 kb downstream of the predicted transcriptional start site of 17,355 X. tropicalis genes (based on Ensembl release 46). This resulted in a probe-set that has about 40 probes (60-mer) tiled for each of the putative promoter regions at an average tiling distance of 205 bp. The Agilent microarray 244 k design was used for this and the genomic array probe-set contains 2 microarray slides (244 k each) for the complete coverage including control probes.
Xenopus tropicalis tadpole groups of 20 at stage 54 were treated with either 10 nM T3 for 48 h (treated group) or 0.1X MMR buffer (control group). Each of the groups was duplicated. They were not fed during this treatment. Chromatin immune-precipitation assays with antibody specific to TR (anti-TR(PB)) were performed above57. The enriched ChIP DNA was amplified by ligation mediated PCR (LM-PCR) using Agilent supplied adaptors, labeled with Cy5. The total genomic DNA isolated prior to antibody immunoprecipitation was similarly labeled with Cy3 (Input). Both Cy3 and Cy5 labeled DNAs were, hybridized to the microarray, washed, scanned, features extracted and primary scan data analyzed according to manufacturer’s instructions (Agilent Mammalian ChIP-on-chip, version 9.2).
Transcription assay in Xenopus laevis oocytes
The plasmids containing GFP (green fluorescent protein), Xenopus laevis TRα and RXRα were linearized and transcribed in vitro using an mMESSAGE mMACHINE SP6 Transcription kit (Ambion). The cytoplasm of stage VI Xenopus laevis oocytes was injected with totally 46 pg per oocyte of the green fluorescent protein (GFP) mRNA or TR and RXR mRNAs. Two hours later, the firefly luciferase reporter construct (345 pg per oocyte) and the control phRG-TK Renilla luciferase (Promega) (34.5 pg per oocyte) were co-injected into the oocyte nucleus. After overnight incubation at 18 °C in the presence or absence of 100 nM T3, the injected oocytes were prepared for luciferase assay by using the Dual-Luciferase Reporter Assay system according to the manufacture’s protocol (Promega). Three oocytes per sample were lysed in 45 μl of 1X Passive buffer (Promega), and 10 μl of lysate were used for the luciferase assays. Three independent samples were used for each injection at the same time. The relative expression level of firefly luciferase to Renilla luciferase was determined. Each data point represents the average of the 5 samples with the standard error.
Bioinformatics analyses
The ChIP-on-Chip data were analyzed to identify peaks of TR binding in 8 kb promoter region for each gene (Personal Diagnostix Inc., Gaithursburg, MD). The data were pretreated by removing low quality probes that were flagged in the ChIP-on-chip analysis with statistical analysis using R package Limma. In brief, background intensities were subtracted from the ChIP intensities and within array normalization was carried out with Loess normalization to balance M (log-ratios) and A (average intensities)-values. Normalization was performed between arrays of the same treatment to ensure that A-values had the same empirical distribution across arrays leaving the M-values unchanged. Average intensities for the same treatment were calculated and smoothened by using a sliding window of genomic region of 1400 bp. The enriched regions of binding events were identified with the criterion of at least 3 consecutive probes having ChIP signal intensities of more than 2.5 times of standard deviation. The identities of putative promoters on the ChIP-on-chip microarray slides were determined based on their ENSEMBL identification numbers.
Results
Identification of TR-binding sites in premetamorphic intestine by ChIP-on-chip assay
A genomic microarray chip set consisting of two chips was generated to cover the sequences from 5.5 kb upstream to 2.5 kb downstream of the 5′-end of the about 17,355 known and predicted Xenopus tropicalis genes in the genomic database (Fig. 1A). Each gene had 40 probes of 60 bases in length (60-mer) at about 205 bp apart, spanning the entire 8 kb of the genomic sequence. To identify genes that are bound by TR in the intestine, we treated premetamorphic, stage 54, Xenopus tropicalis tadpoles with 10 nM T3 for 2 days. The intestine was isolated from the control and T3 treated tadpoles and subjected to anti-TR antibody ChIP-on-chip assay by using genomic microarray chip set. Two duplicated samples were done for each of the control and T3-treated groups. Initial analysis of the ChIP-on-chip data by aligning the ChIP signals to the partially annotated Xenopus tropicalis genomic scaffolds identified very limited number of candidate TR-bound genes, likely due to the poor annotation (data not shown). Thus, we next analyzed the ChIP-on-chip data against only the tiling sequences from 5.5 kb upstream to 2.5 kb downstream of the 5′-end of the 17,355 genes used for preparing the genomic microarray chips. The analyses led to the identification of 78 genes with significant TR binding in the control tadpole intestine and 253 genes in the T3-treated tadpole intestine, with 53 genes identified in both the control and T3-treated group (Figs 1 and 2) (Supplemental Table 1).
Figure 1.
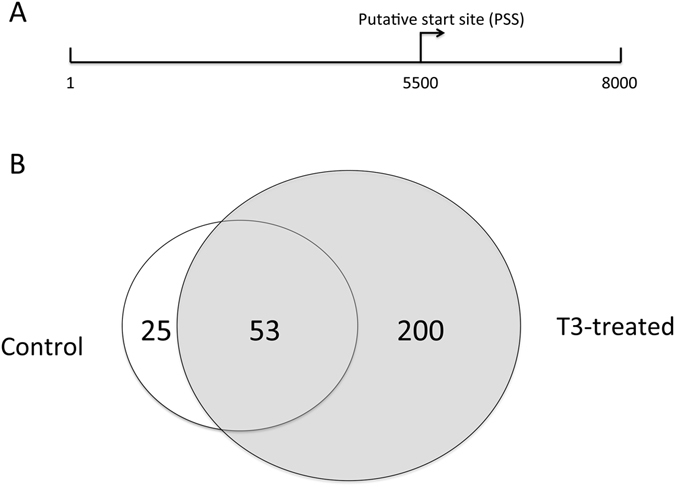
ChIP-on-chip assay identified putative TR targets in the tadpole intestine. (A) Schematic representation of a gene in the genomic chips. About 5500 bp upstream and 2500 bp downstream of the 5′-end of the cDNA sequence in the databank for each putative gene in the Xenopus tropicalis genome database were used to design a 60 bp oligonucleotide probe at an average tiling distance of 205 bp, covering the entire 8000 bp. The probes were custom-printed onto the genomic chips. PSS: putative transcription start site. (B) Venn-diagram showing the genes with TR binding sites as identified from the ChIP-on-chip assay with the intestine samples from control and T3-treated stage 54 premetamorphic tadpoles. Note that vast majority of the genes bound by TR in the control tadpoles were also found in the T3-treated animals.
Figure 2.
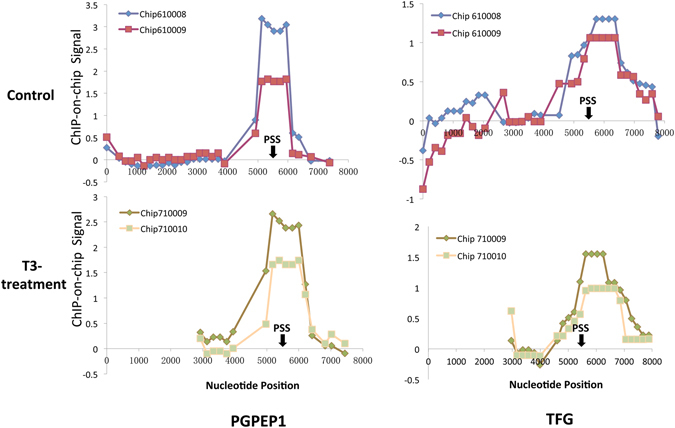
The ChIP signals across the 8000 bp promoter region for PGPEP1 and TFG1 from the control and T3 treated tadpole intestine. Both the control and T3-treated groups had two independent samples as shown. The arrow points to the putative start site (PSS).
To validate the ChIP-on-chip findings independently, we selected 8 genes (Dot1L, MBD3, PPM1B, PGPEP1, JUNB, BEND7, PUM2, and TGFα, see Supplemental Table 1) based on the analyses of ChIP signals against both the genomic scaffolds and the sequences used for preparing the genomic microarray chips as well as the presence of putative TREs within the tiling sequences from a bioinformatics analysis (see below). We designed two PCR primers around the putative TRE(s) for each gene and carried out regular ChIP assays by using the TR antibody and the intestine from stage 54 premetamorphic Xenopus tropicalis tadpoles treated with or without 10 nM T3 for 2 days. The results showed that all 8 genes analyzed, including the MBD3 (Methyl-CpG binding domain protein 3), PPM1B (Protein phosphatase, Mg2+/Mn2+ dependent 1B) and PGPEP1 (Pyroglutamyl-Peptidase I), were indeed bound by TR in the intestine (Fig. 3 and data not shown).
Figure 3.
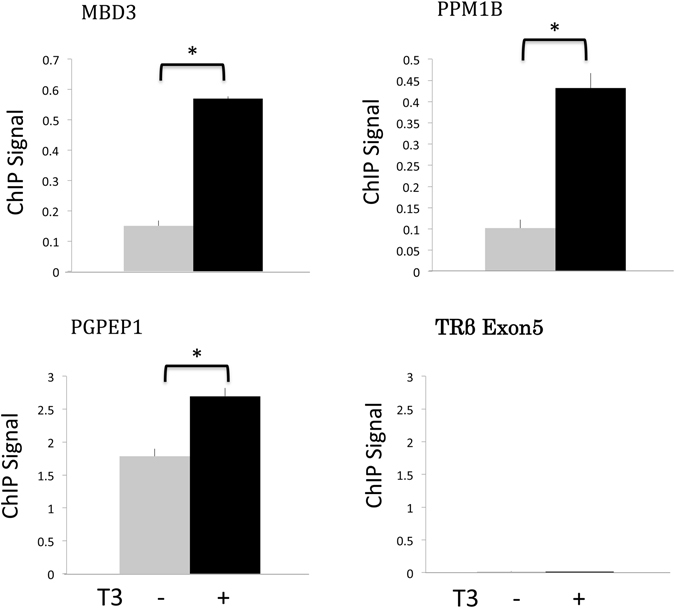
ChIP confirmation of the TR binding to target sites identified from the ChIP-on-chip assay. The intestine from stage 54 tadpoles treated with or without T3 was isolated and subjected to anti-TR antibody ChIP assay and the region around the putative TR binding sites as identified from the ChIP-on-chip assay was PCR amplified. Note that no TR binding was found in the control gene (exon 5 of the TRβ gene) while all three newly identified target genes had TR binding in the absence of T3 and this binding was enhanced upon T3 treatment, in agreement with the ChIP-on-chip data. * indicates pairs of samples with significant differences (p < 0.05).
Regulation of TR target genes by T3 in the intestine
To determine if the newly identified candidate TR target genes are regulated by T3 in the tadpole intestine, stage 54 premetamorphic Xenopus tropicalis tadpoles were treated with or with 10 nM T3 for 2 days and total RNA was isolated from the intestine. The expression of the 8 genes validated by ChIP assay above was determined by qRT-PCR. Of the 8 genes analyzed, 6 genes were found to be induced by T3 in the tadpole intestine (Fig. 4 and data not shown), suggesting that most of the newly identified genes bound by TR were T3 response genes. The expression levels of the other two were too low to determine their regulation by T3. It is possible that these two genes are still T3-regulated genes in vivo or under different treatment conditions.
Figure 4.
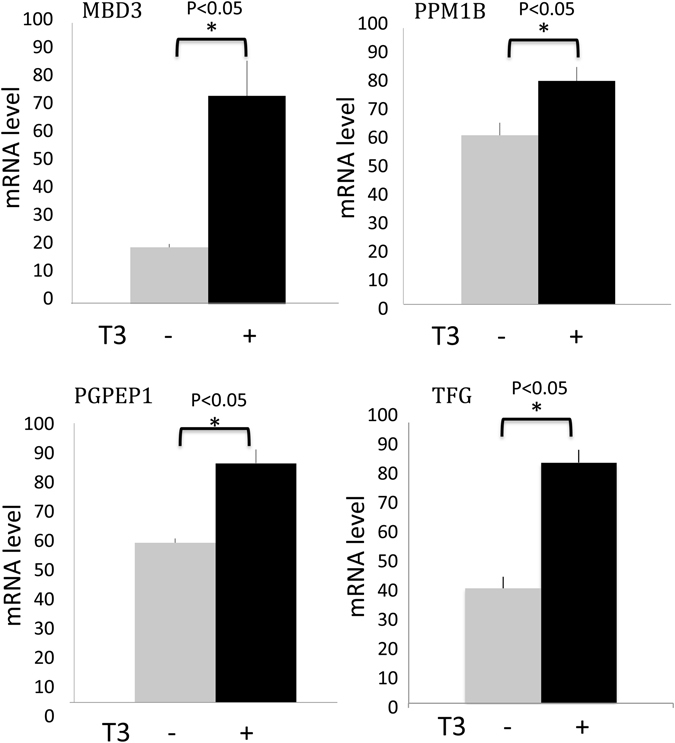
RT-PCR analysis confirming the regulation of newly identified TR targets as T3 response genes. The RNA was isolated from the intestine of stage 54 tadpoles treated with or without T3 was isolated and subjected to RT-PCR analysis for gene expression. Note that all three genes were found to be induced by T3 treatment. *Indicates pairs of samples with significant differences (p < 0.05).
The TR target genes contain functional TREs
Among the 278 TR target genes identified by ChIP-on-chip assay, 191 genes had 1 or more putative TRE, as revealed by NHR SCAN analysis (http://www.cisreg.ca/cgi-bin/NHR-scan/nhr_scan.cgi), within the 8 kb sequences covered on the ChIP-on-chip slides (Data not shown). To investigate if these TREs were functional TREs, we chose several TREs from the 8 selected genes whose binding by TR was confirmed (Fig. 3 and data not shown) and analyzed their function in the reconstituted Xenopus laevis oocyte transcription system, where the reporter DNA is chromatinized24, 58. It is well-known that in this system or in tissue culture transfection studies, TREs that are located about 1 kb or further away from the site has little effect on the promoter activity59. Thus, we test the function of the putative TREs in a heterologous reporter. The Xenopus tropialis Dot1L promoter has been shown to be regulated by TR during Xenopus development53, and it is also among the candidate target genes identified by our ChIP-on-chip assay. The Dot1L promoter was cloned to drive firefly luciferase gene expression in frog oocytes and showed to be regulated directly by T3 (Fig. 5). This T3-regulation of the Dot1L promoter is mediated by a functional TRE in the proximity of transcriptional start site and mutation within the TRE sequences abolished T3-regulation on the promoter53. Importantly, placing the putative TREs from the selected genes in place of the Dot1L TRE also enabled the promoter to be activated by T3 in the presence of TR/RXR (Fig. 5), suggesting that they are likely functional TREs mediating the response of the endogenous genes to T3.
Figure 5.
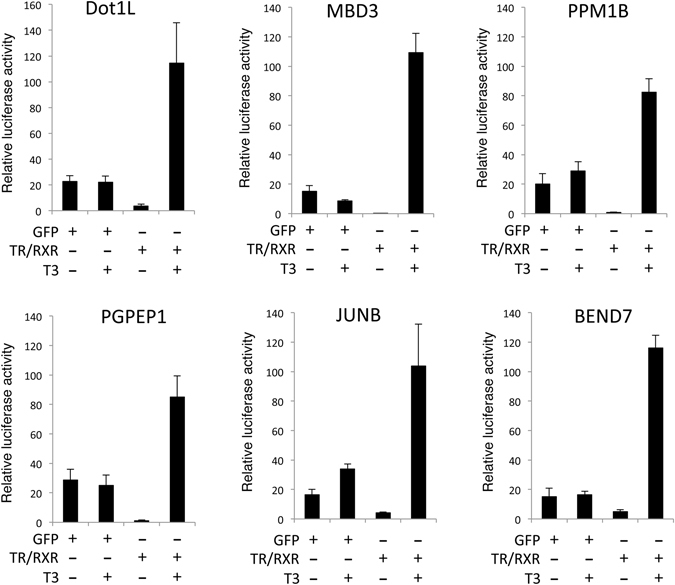
The putative TREs in the candidate TR target genes can mediate transcriptional activation by T3 in frog oocytes. The luciferase reporter construct containing the TREs of Dot1L, MBD3, PPM1B, PGPEP1, JUNB, BEND7, respectively, was co-injected with the control Renilla luciferase construct phRG-TK into the nuclei of Xenopus oocytes with or without prior cytoplasmic injection of Xenopus laevis TRα and RXRα mRNAs or GFP mRNA as negative control. The oocytes were incubated at 18 °C overnight in the presence or absence of 100 nM T3 and then used for dual luciferase assays. The relative activities of the firefly luciferase to Renilla luciferase were plotted. Note that all the reporters responded to T3 in the presence of TR/RXR.
Many of the newly identified TR-bound genes are regulated during intestinal metamorphosis
We have previously carried out a gene expression microarrays to identify genes whose expression is altered in the intestinal epithelium (EP) or the non-epithelium (Non-EP, or the rest of the intestine) during natural metamorphosis in Xenopus laevis 43, a highly related species whose metamorphosis resembles that in Xenopus tropicalis with essentially identical morphological and molecular properties24, 53, 60–63. Of the 278 TR target genes identified by ChIP-on-chip assay, 95 or 34% are also found on the Xenopus laevis microarray chips used for the expression study (Note that the actually overlap might be much higher as most genes may have different names on the microarray chips used in the expression study vs. the genomic chips here, therefore showing up as non-overlapping). Of these 95 genes present in the gene expression microarray, 38 genes or 40% were found to be regulated during metamorphosis in the intestine in either the epithelium (EP), non-epithelium (Non-EP) or both (Fig. 6) (Supplemental Table 1). As the microarray studies were done with only 3 developmental stages, it is very likely that many developmentally regulated genes were not identified by the microarray analysis. Thus, it is possible that most of the genes identified here are regulated in the intestine during metamorphosis, as suggested by the above RT-PCR expression analysis of selected genes in the intestine of premetamorphic tadpoles treated with or without T3.
Figure 6.
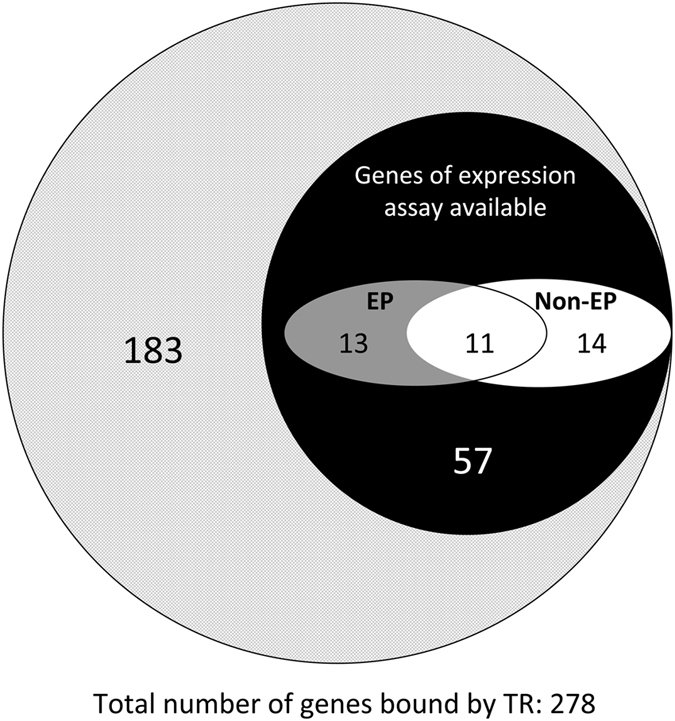
Venn diagram showing overlap of the TR target genes identified by ChIP-on-chip assay with known developmentally regulated genes identified from an early expression microarray study. Of the 278 TR target genes identified by ChIP-on-chip assay, 95 or 34% were also found on the microarray used for the expression study (Note that the actually overlap might be higher as most genes may have different names on the microarray chips used in the expression study and on the genomic chips used here, therefore showing up as non-overlapping). Of the 95 genes present in the gene expression microarray, 38 were found to be regulated during metamorphosis in the intestine in either the epithelium (EP, 13 genes), non-epithelium (Non-EP, 14 genes) or both (11 genes), suggesting that about 40% of the genes identified here are regulated by T3 during intestinal metamorphosis.
Discussions and Conclusions
T3 is known to regulates gene transcription by binding to TRs, which bind to T3 response elements (TREs) in their target genes as either homodimers or heterodimers formed with 9-cis retinoic acid receptors (RXRs)1, 2, 64, 65. Many T3 response genes have been identified over the years by using various approaches in cell cultures and in vivo, relatively few have been shown to be direct TR target genes in vivo, especially during vertebrate development. Here, using an unbiased genome-wide approach, we have identified nearly 300 genes bound by TR in the premetamorphic tadpole intestine and provided evidence to support the involvement of these binding sites in mediating the regulation of these genes by T3 during intestinal metamorphosis.
The intestinal metamorphosis has been used as a model to investigate the in vivo mechanism of gene regulation by TR during vertebrate development. The vertebrate intestine is one of the best-studied organs where self-renewal is an integral part of their physiological function66, 67. The establishment and/or maturation of this self-renewal system in all vertebrates takes place during the postembryonic period when plasma T3 concentrations are high4, 68–71. In amphibians such as Xenopus laevis and Xenopus tropicalis, this takes place during metamorphosis and involves T3-dependent apoptotic degeneration of most of the larval epithelial cells and concurrent de novo formation of adult epithelial stem cells via dedifferentiation of some larval cells through yet-unknown mechanism67, 72–81. This makes intestinal metamorphosis an excellent model system to study the formation of adult organ-specific stem cells during vertebrate development.
Earlier studies have shown that TR plays an essential role in regulating premetamorphic development and mediating T3-effect on metamorphosis8, 22, 23, 25–34, 36, 37. T3 is believed to induce a gene regulation cascade that is responsible for metamorphic transformation of individual organs/tissues and the immediate early T3 response genes, i.e., those regulated by T3 directly at the transcription level when T3 first becomes available, are expected to play critical roles in this cascade. Toward understanding how T3 induces the formation of adult intestinal stem cells, many T3 response genes have been isolated over the years by using various means39, 43–45. However, few of the genes identified so far are shown to be direct TR target genes in vivo. On the other hand, our studies suggest that most genes that we have identified here are such immediate early T3 response genes regulated by TR at the transcription level. First, by definition, all genes identified from our ChIP-on-chip assay are bound by TR in the intestine either directly or indirectly, a prerequisite of immediate early T3 response genes. Of the 8 genes that we chose to validate the TR binding by traditional ChIP assay, all were confirmed to be bound by TR in the intestine. Second, most of these 8 genes (6 out 8) were indeed found to be regulated in the intestine when premetamorphic tadpoles were treated with T3 with the expression of the remaining two too low to be determined. Third, by comparing to the previously published developmental tissue-specific microarray, we found that 40% of the genes identified here were regulated in either the epithelium, non-epithelium, or both in the intestine during T3-dependent metamorphosis, indicating that these genes are indeed regulated by T3 during natural metamorphosis. Considering the limited stages (only 3 of the 10 between stages 56–66) used in the intestinal expression microarray study43, it is possible that many other genes are also regulated in the intestine at some stages of metamorphosis. Finally while the ChIP-on-chip data is being analyzed, we characterized the promoter of one of the newly discovered TR-bound genes, the Dot1L gene, and indeed found it to contain a functional TRE near the transcription start site and mutating the functional TRE in the Dot1L promoter abolished T3-activation on the promoter53. Furthermore, replacing the functional TRE with the putative TREs in several other genes discovered from our ChIP-on-chip assay also enabled the promoter to respond to T3 in vivo (Fig. 5). It is worth pointing out that such an assay does not exactly test if the TRE is functional in the context of its own in situ location in the genome. Such a test would require future studies such as mutagenizing the endogenous TRE in the genome and studying the effect in vivo. On the other hand, a promoter fragment containing a TRE similarly identified and characterized for another candidate gene discovered here, histidine ammonia-lyase 2, could drive T3-inducible, transgenic GFP expression in the intestinal stem cells during metamorphosis, mimicking the endogenous promoter55, 78. Regardless, all these together suggest that the genomic regions bound by TR as identified from our ChIP-on-chip assay are likely involved in regulating the corresponding genes during development.
Recent advancements in genome-wide chromatin immunoprecipitation analyses have led to identification of genes bound by TR in vivo in mouse cell cultures or organs. A few studies used ChIP-seq for unbiased analyses of genome-wide TR binding sites in mouse cell cultures82 and adult liver83, 84. These studies led to the identification of thousands of TR binding sites. Many of the identified binding sites are located far away for the nearest genes and thus their roles in T3-dependent gene regulations are unknown and difficult to investigate. Another study employed ChIP-on-chip to analyze TR binding sites in the developing mouse cerebellum85. Here, the authors used a genomic chip containing −8 kb to +2 kb around the transcription start site of 5000 mouse genes and identified 91 genes with TR bindings sites. They confirmed 10 out of 13 binding regions. However, the regulation of these genes by T3 was largely unclear as the authors only analyzed the expression of 4 of the 10 validated genes. Our studies here thus represent the first comprehensive genome-wide analysis of TR target genes during development in vivo where most of the newly identified TR target sites were confirmed and/or found to be regulated by T3 and/or T3-dependent intestinal remodeling. Although the use of genomic chips for microarray left out many genomic regions, especially the large intergenic regions, leaving many other potential TR binding sites undiscovered, the close-proximity of the sites that we have discovered to the transcription start site suggest that the TR bindings sites identified here are indeed responsible for the regulation of the genes by T3 during development. This is indeed supported by the analysis of the promoter of one of the genes, the Dot1L gene, as indicated above. In addition, even with the recent progresses in genome-annotation in Xenopus troplicalis 86, the poor status of the genome assembly and, annotation in Xenopus troplicalis makes it very difficult to carry out genome-wide ChIP-seq analysis as recently discussed by Grimaldi et al.60. In any case, the identification of the direct target genes here should facilitate future studies on the mechanisms of tissue-specific gene regulation by TR since many genes, like the HAL255, 78, are regulated in a tissue-specific manner even within a single organ, and more importantly, on how T3 regulates the formation of adult intestinal stem cells during metamorphosis.
Electronic supplementary material
Acknowledgements
We would like to thank Dr. Sudhir Varma for his help in the bioinformatics analysis. This work was supported by the Intramural Research Program of NICHD, NIH.
Author Contributions
L.F., B.D., K.M., K.F. and R.A.H. designed and carried out experiments and interpreted the findings. L.F. and Y.B.S. prepared the manuscript. All authors approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06679-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 2.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 3.Tata JR. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays. 1993;15:239–248. doi: 10.1002/bies.950150404. [DOI] [PubMed] [Google Scholar]
- 4.Shi, Y.-B. Amphibian Metamorphosis: From morphology to molecular biology. (John Wiley & Sons, Inc., 1999).
- 5.Hetzel, B. S. The story of iodine deficiency: An international challenge in nutrition. (Oxford University Press, 1989).
- 6.Freake HC, Oppenheimer JH. Thermogenesis and thyroid function. Annu Rev Nutr. 1995;15:263–291. doi: 10.1146/annurev.nu.15.070195.001403. [DOI] [PubMed] [Google Scholar]
- 7.Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y-B. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid. 2009;19:987–999. doi: 10.1089/thy.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development–current perspectives. Endocr Rev. 1993;14:94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- 10.Hsu JH, Brent GA. Thyroid hormone receptor gene knockouts. TEM. 1998;9:103–112. doi: 10.1016/s1043-2760(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 11.Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110:337–348. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Escobar GM, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.de Escobar GM, Obregon MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutrition. 2007;10:1554–1570. doi: 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- 14.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292:691–695. doi: 10.1001/jama.292.6.691. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, L. I., Tata, J. R. & Atkinson, B. G. Metamorphosis: Post-embryonic reprogramming of gene expression in amphibian and insect cells. (Academic Press, 1996).
- 16.Wang F, et al. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 2015;5:15. doi: 10.1186/s13578-015-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei Y, Guo X, Deng Y, Chen Y, Zhao H. Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci. 2013;3:21. doi: 10.1186/2045-3701-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Z, et al. Heritable CRISPR/Cas9-mediated targeted integration in Xenopus tropicalis. FASEB J. 2015;29:4914–4923. doi: 10.1096/fj.15-273425. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama T, et al. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis. 2013;51:835–843. doi: 10.1002/dvg.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blitz IL, Biesinger J, Xie X, Cho KW. Biallelic genome modification in F(0) Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis. 2013;51:827–834. doi: 10.1002/dvg.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DD, Cai L. Amphibian metamorphosis. Dev Biol. 2007;306:20–33. doi: 10.1016/j.ydbio.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchholz DR, Tomita A, Fu L, Paul BD, Shi Y-B. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol. Cell. Biol. 2004;24:9026–9037. doi: 10.1128/MCB.24.20.9026-9037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchholz DR, Paul BD, Fu L, Shi YB. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol. 2006;145:1–19. doi: 10.1016/j.ygcen.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Matsuda H, Shi Y-B. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology. 2008;149:5610–5618. doi: 10.1210/en.2008-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima K, Yaoita Y. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn. 2003;227:246–255. doi: 10.1002/dvdy.10300. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. PNAS. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchholz DR, Hsia VS-C, Fu L, Shi Y-B. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol. Cell. Biol. 2003;23:6750–6758. doi: 10.1128/MCB.23.19.6750-6758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda H, Paul BD, Choi CY, Hasebe T, Shi Y-B. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol. Cell. Biol. 2009;29:745–757. doi: 10.1128/MCB.00827-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato Y, Buchholz DR, Paul BD, Shi Y-B. A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mechanisms of Development. 2007;124:476–488. doi: 10.1016/j.mod.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul BD, Shi Y-B. Distinct expression profiles of transcriptional coactivators for thyroid hormone receptors during Xenopus laevis metamorphosis. Cell Research. 2003;13:459–464. doi: 10.1038/sj.cr.7290188. [DOI] [PubMed] [Google Scholar]
- 31.Paul BD, Buchholz DR, Fu L, Shi Y-B. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J. Biol. Chem. 2005;280:27165–27172. doi: 10.1074/jbc.M503999200. [DOI] [PubMed] [Google Scholar]
- 32.Havis E, Sachs LM, Demeneix BA. Metamorphic T3-response genes have specific co-regulator requirements. EMBO Reports. 2003;4:883–888. doi: 10.1038/sj.embor.embor908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul BD, Fu L, Buchholz DR, Shi Y-B. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol. Cell. Biol. 2005;25:5712–5724. doi: 10.1128/MCB.25.13.5712-5724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yen PM. Unliganded TRs regulate growth and developmental timing during early embryogenesis: evidence for a dual function mechanism of TR action. Cell Biosci. 2015;5:8. doi: 10.1186/2045-3701-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi YB. Unliganded thyroid hormone receptor regulates metamorphic timing via the recruitment of histone deacetylase complexes. Curr Top Dev Biol. 2013;105:275–297. doi: 10.1016/B978-0-12-396968-2.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J, et al. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinology. 2015;156:735–744. doi: 10.1210/en.2014-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen L, Shi YB. Unliganded thyroid hormone receptor alpha controls developmental timing in Xenopus tropicalis. Endocrinology. 2015;156:721–734. doi: 10.1210/en.2014-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denver RJ, Pavgi S, Shi YB. Thyroid hormone-dependent gene expression program for Xenopus neural development. J Biol Chem. 1997;272:8179–8188. doi: 10.1074/jbc.272.13.8179. [DOI] [PubMed] [Google Scholar]
- 39.Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev. Biol. 2007;303:576–590. doi: 10.1016/j.ydbio.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Das B, et al. Gene expression changes at metamorphosis induce by thyroid hormone in Xenopus laevis tadpoles. Dev. Biol. 2006;291:342–355. doi: 10.1016/j.ydbio.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 41.Cai L, Das B, Brown DD. Changing a limb muscle growth program into a resorption program. Dev Biol. 2007;304:260–271. doi: 10.1016/j.ydbio.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helbing CC, et al. Expression profiles of novel thyroid hormone-responsive genes and proteins in the tail of Xenopus laevis tadpoles undergoing precocious metamorphosis. Mol Endocrinol. 2003;17:1395–1409. doi: 10.1210/me.2002-0274. [DOI] [PubMed] [Google Scholar]
- 43.Sun G, et al. Expression Profiling of Intestinal Tissues Implicates Tissue-Specific Genes and Pathways Essential for Thyroid Hormone-Induced Adult Stem Cell Development. Endocrinology. 2013;154:4396–4407. doi: 10.1210/en.2013-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi YB. Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol. 2010;11:R55. doi: 10.1186/gb-2010-11-5-r55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amano T, Yoshizato K. Isolation of genes involved in intestinal remodeling during anuran metamorphosis. Wound Repair Regen. 1998;6:302–313. doi: 10.1046/j.1524-475X.1998.60406.x. [DOI] [PubMed] [Google Scholar]
- 46.Ranjan M, Wong J, Shi YB. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269:24699–24705. [PubMed] [Google Scholar]
- 47.Furlow JD, Kanamori A. The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinol. 2002;143:3295–3305. doi: 10.1210/en.2002-220126. [DOI] [PubMed] [Google Scholar]
- 48.Fu L, Tomita A, Wang H, Buchholz DR, Shi Y-B. Transcriptional regulation of the Xenopus laevis stromelysin-3 gene by thyroid hormone is mediated by a DNA element in the first intron. J. Biol. Chem. 2006;281:16870–16878. doi: 10.1074/jbc.M603041200. [DOI] [PubMed] [Google Scholar]
- 49.Machuca I, Esslemont G, Fairclough L, Tata JR. Analysis of structure and expression of the Xenopus thyroid hormone receptor b gene to explain its autoregulation. Mol. Endocrinol. 1995;9:96–107. doi: 10.1210/mend.9.1.7760854. [DOI] [PubMed] [Google Scholar]
- 50.Furlow JD, Brown DD. In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol Endocrinol. 1999;13:2076–2089. doi: 10.1210/mend.13.12.0383. [DOI] [PubMed] [Google Scholar]
- 51.Nieuwkoop, P. D. & Faber, J. Normal table of Xenopus laevis. North Holland Publishing, Amsterdam (1965).
- 52.Leloup J, Buscaglia M. La triiodothyronine: hormone de la métamorphose des amphibiens. C.R. Acad. Sci. 1977;284:2261–2263. [Google Scholar]
- 53.Matsuura K, et al. Histone H3K79 methyltransferase Dot1L is directly activated by thyroid hormone receptor during Xenopus metamorphosis. Cell Biosci. 2012;2:25. doi: 10.1186/2045-3701-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada M, Miller TC, Fu L, Shi YB. Direct activation of amidohydrolase domain-containing 1 gene by thyroid hormone implicates a role in the formation of adult intestinal stem cells during Xenopus metamorphosis. Endocrinology. 2015;156:3381–3393. doi: 10.1210/en.2015-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luu N, Fu L, Fujimoto K, Shi Y-B. Direct regulation of histidine ammonia-lyase 2 gene by thyroid hormone in the developing adult intestinal stem cells. Endocrinology. 2017;158:1022–1033. doi: 10.1210/en.2016-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das B, Heimeier RA, Buchholz DR, Shi YB. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem. 2009;284:34167–34178. doi: 10.1074/jbc.M109.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buchholz DR, Paul BD, Shi YB. Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. Implications for developmental gene regulation. J Biol Chem. 2005;280:41222–41228. doi: 10.1074/jbc.M509593200. [DOI] [PubMed] [Google Scholar]
- 58.Wong J, Shi Y-B. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem. 1995;270:18479–18483. doi: 10.1074/jbc.270.31.18479. [DOI] [PubMed] [Google Scholar]
- 59.Wong J, Shi Y-B, Wolffe AP. Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptinal activation. EMBO J. 1997;16:3158–3171. doi: 10.1093/emboj/16.11.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grimaldi AG, Buisine N, Bilesimo P, Sachs LM. High-throughput sequencing will metamorphose the analysis of thyroid hormone receptor function during amphibian development. Curr Top Dev Biol. 2013;103:277–303. doi: 10.1016/B978-0-12-385979-2.00010-1. [DOI] [PubMed] [Google Scholar]
- 61.Bilesimo P, et al. Specific Histone Lysine 4 Methylation Patterns Define TR-Binding Capacity and Differentiate Direct T3 Responses. Mol Endocrinol. 2011;25:225–237. doi: 10.1210/me.2010-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sterling J, Fu L, Matsuura K, Shi Y-B. Cytological and morphological analyses reveal distinct features of intestinal development during Xenopus tropicalis metamorphosis. PLoS One. 2012;7:e47407. doi: 10.1371/journal.pone.0047407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuura K, Fujimoto K, Fu L, Shi Y-B. Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development. Endocrinology. 2012;153:961–972. doi: 10.1210/en.2011-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 66.van der Flier LG, Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 67.Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: Embryogensis and remodeling during metamorphosis. Current Topics in Develop. Biol. 1996;32:205–235. doi: 10.1016/S0070-2153(08)60429-9. [DOI] [PubMed] [Google Scholar]
- 68.Ishizuya-Oka A, Shi YB. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci. 2011;1:37. doi: 10.1186/2045-3701-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muncan V, et al. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun. 2011;2:452. doi: 10.1038/ncomms1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci USA. 2011;108:10585–10590. doi: 10.1073/pnas.1105852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun G, Shi Y-B. Thyroid hormone regulation of adult intestinal stem cell development: Mechanisms and evolutionary conservations. Int J Biol Sci. 2012;8:1217–1224. doi: 10.7150/ijbs.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishizuya-Oka A, et al. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. Faseb J. 2009;23:2568–2575. doi: 10.1096/fj.08-128124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schreiber AM, Cai L, Brown DD. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci USA. 2005;102:3720–3725. doi: 10.1073/pnas.0409868102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells. 2011;29:154–161. doi: 10.1002/stem.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuda H, Shi YB. An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development. Stem Cells. 2010;28:2073–2083. doi: 10.1002/stem.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun G, et al. Spatio-temporal expression profile of stem cell-associated gene LGR5 in the intestine during thyroid hormone-dependent metamorphosis in Xenopus laevis. PLoS One. 2010;5:e13605. doi: 10.1371/journal.pone.0013605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. The development of the adult intestinal stem cells: Insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci. 2011;1:30. doi: 10.1186/2045-3701-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luu N, et al. Differential regulation of two histidine ammonia-lyase genes during Xenopus development implicates distinct functions during thyroid hormone-induced formation of adult stem cells. Cell Biosci. 2013;3:43. doi: 10.1186/2045-3701-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun G, Fu L, Shi Y-B. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci. 2014;4:73. doi: 10.1186/2045-3701-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hasebe T, et al. Thyroid hormone-induced cell-cell interactions are required for the development of adult intestinal stem cells. Cell Biosci. 2013;3:18. doi: 10.1186/2045-3701-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okada M, Wen L, Miller TC, Su D, Shi YB. Molecular and cytological analyses reveal distinct transformations of intestinal epithelial cells during Xenopus metamorphosis. Cell Biosci. 2015;5:74. doi: 10.1186/s13578-015-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chatonnet F, Guyot R, Benoit G, Flamant F. Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proc Natl Acad Sci USA. 2013;110:E766–775. doi: 10.1073/pnas.1210626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramadoss P, et al. Novel mechanism of positive versus negative regulation by thyroid hormone receptor beta1 (TRbeta1) identified by genome-wide profiling of binding sites in mouse liver. J Biol Chem. 2014;289:1313–1328. doi: 10.1074/jbc.M113.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grontved L, et al. Transcriptional activation by the thyroid hormone receptor through ligand-dependent receptor recruitment and chromatin remodelling. Nat Commun. 2015;6:7048. doi: 10.1038/ncomms8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong H, et al. Identification of thyroid hormone receptor binding sites and target genes using ChIP-on-chip in developing mouse cerebellum. PLoS One. 2009;4:e4610. doi: 10.1371/journal.pone.0004610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buisine N, et al. Xenopus tropicalis Genome Re-Scaffolding and Re-Annotation Reach the Resolution Required for In Vivo ChIA-PET Analysis. PLoS One. 2015;10:e0137526. doi: 10.1371/journal.pone.0137526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


