Abstract
Messenger RNA (mRNA)-transfected dendritic cell (DC) vaccines have been shown to be a powerful modality for eliciting antitumor immune responses in mice and humans; however, their application has not been fully optimized since many of the factors that contribute to their efficacy remain poorly understood. Work stemming from our laboratory has recently demonstrated that preconditioning the vaccine site with a recall antigen prior to the administration of a dendritic cell vaccine creates systemic recall responses and resultantly enhances dendritic cell migration to the lymph nodes with improved antitumor efficacy. This chapter describes the generation of murine mRNA-transfected DC vaccines, as well as a method for vaccine site preconditioning with protein antigen formulations that create potent recall responses.
Keywords: Dendritic cells, Bone marrow, Transcription, mRNA, Electroporation, Preconditioning, Protein antigen formulation, Intradermal
1 Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that play a pivotal role in the induction of T and B cell immunity [1]. DCs have the exceptional ability to activate and educate naive CD4+ and CD8+ T cells, a critical priming event that occurs predominantly in secondary lymphoid organs such as the spleen and draining lymph nodes. DCs prime cytotoxic T cell responses when they capture antigen, migrate to secondary lymphoid organs, and present antigen complexed with major histo-compatibility complex (MHC) class I and II molecules, which is required for T cell receptor activation and antigen-specific T cell responses [2]. Utilizing this defined cascade of events, DCs can be pulsed or electroporated with antigen and will subsequently assemble antigen-derived peptides for presentation to T cells. Antigen thus can be delivered to DCs ex vivo via (1) peptide-pulsed DCs [3, 4], (2) co-incubation with whole tumor homogenate [5, 6], or (3) electroporation with tumor antigen-derived messenger RNA (mRNA) [7–9]. Both preclinical and clinical studies have demonstrated that immunization with antigen-specific electroporated DCs can prime a cytotoxic cell response that is tumor specific and provides protective immunity [10, 11]. Thus, DC-based therapy is currently being evaluated in clinical trials for a variety of tumors.
DCs transfected with mRNA offer a powerful and tractable vaccine platform for eliciting immune responses against tumor antigens. A common strategy begins with the in vitro transcription of mRNAs encoding a tumor antigen. DCs transfected with these in vitro-transcribed (IVT) mRNAs generate short tumor antigen-specific peptides, some of which are able to complex with MHC molecules. These MHC-peptide complexes are then presented on the surface of DCs, where they can interact with and activate tumor antigen-cognate T cells.
1.1 Generation of In Vitro-Transcribed mRNA
IVT mRNA is a particularly appealing means of delivering antigen to DCs due to its short half-life, ease of production, and inability to integrate into the genome. Tumor epitopes derived from mRNA can be loaded onto MHC I or MHC II by incorporating molecular tags that traffic antigen to these loading compartments, such as ubiquitin or lysosome-associated membrane protein 1 (LAMP1) [12], respectively. Consequently, mRNA-transfected DCs can be used to engender CD4+ and CD8+ T cell immune responses. An oft-used alternative to delivering tumor epitopes onto surface MHC molecules of DCs is by pulsing with MHC-restricted peptides. These peptides compete with endogenously processed epitopes on the cell surface for binding to MHC molecules. A drawback of pulsing with MHC-restricted peptides is that it generally requires knowledge of the underlying MHC haplotypes expressed in an individual, considering that diverse MHC polymorphisms exhibit varying peptide-binding characteristics. However, because tumor antigen encoded by IVT mRNA is processed by endogenous MHC-peptide-loading mechanisms, mRNA-derived tumor antigen is not MHC haplotype restricted.
1.2 Bone Marrow-Derived DC Vaccine Preparation and Electroporation
DCs of both myeloid and lymphoid lineages are derived from the bone marrow and have been characterized in both mice and humans. As such, DC precursors can be harvested and differentiated in vitro using a series of growth factors and cytokines conducive to the mature DC of interest. Myeloid DCs can be generated in vitro either (1) by harvest of early common progenitor cells in the bone marrow (predominantly murine protocols) [13] or (2) by isolation of monocytes from peripheral blood (human cancer vaccine protocols) [14]. Ultimately, such precursors are differentiated into mature DCs with provision of granulocyte macrophage colony-stimulating factor (GM-CSF) as a key stimulus [15] and interleukin-4 (IL-4) as a potent activator of IL-12 production [16, 17].
1.3 Immunophenotyping of DC Vaccine Prior to Injection
Although there is currently no consensus on the optimal DC vaccine, it is well accepted that DCs used in vaccines must be in a mature state, as immature DCs can lead to immunological tolerance toward an antigen [18]. DC maturation can be defined phenotypically and includes expression of costimulatory molecules (e.g., CD80, CD86) and activation markers (e.g., MHC II) [19]. This phenotype is typically induced by a prolonged incubation period in maturation cocktails that include cytokines and toll-like receptor (TLR) ligands [20]. However, many of these treatments have been shown to inhibit IVT mRNA expression [21], and, in our experience, this step is not required to elicit DC vaccine-mediated antitumor immune responses in mice. This may be explained by the fact that IVT mRNA behaves as a TLR3 agonist [22].
1.4 Vaccine Site Preconditioning and Intradermal DC Vaccination
Aside from the activation status of DCs used in vaccines, there are several other factors that influence their effectiveness, such as their ability to effectively migrate to draining lymph nodes upon antigen encounter in the periphery. Effective homing of dendritic cells to local draining lymph nodes is a key event for the priming of naïve T cells and induction of antigen-specific immune response s. Clinical trials with corroborating preclinical studies have demonstrated that generally less than 5 % of injected DCs actually reach the vaccine site-draining lymph nodes (VDLNs) [23–25]. To address this limitation, preclinical investigations by Martin-Fontecha et al. revealed that preconditioning the vaccine site with inflammatory cytokines or mature, unpulsed DCs could significantly increase the migration of a subsequent DC vaccine to VDLNs [26].
In the preclinical setting, a variety of adjuvants have been employed to induce local inflammation with the goal of enhancing the immunogenicity of administered tumor-specific DCs or tumor-derived peptides [27, 28]. Protein antigens offer the advantage of inducing robust T cell responses with cytokine activation that can subsequently potentiate the magnitude of innate inflammatory responses [29] and control of infection [30]. Preclinical studies have demonstrated that the induction of memory CD4+-dependent inflammatory cytokines and chemokines in mice did not require conserved pathogen recognition pathways and acted independently of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) [29]. As a result, memory CD4+ T cell activation can be leveraged to enhance non-cognate innate inflammatory responses.
Our previous work has identified a role for CD4+ T cell recall responses to protein antigens in facilitating the migration of DC vaccines to draining lymph nodes. Preconditioning the vaccine site with a potent recall antigen (i.e., an antigen for which the host has prior immunity) such as tetanus-diphtheria (Td) toxoid produced a local and systemic response that facilitated the lymph node homing of tumor antigen-specific DC vaccines and promoted a significant decrease in growth of established tumors [31]. Although the exact mechanism remains to be elucidated, this outcome appears to require activated CD4+ T cells and host production of the chemokine CCL3.
In this chapter, the development and delivery of mRNA-transfected murine DCs are described. Methods outlined include the differentiation of DCs from murine bone marrow precursors, in vitro transcription of mRNA, antigen loading via electroporation, phenotypic validation by flow cytometry, and a vaccination protocol. Additionally, detailed instructions for recall antigen preconditioning are also presented.
2 Materials
The materials used are listed below. Comparable products from other suppliers should also be effective.
2.1 Generation of In Vitro Transcribed mRNA
Modified pGEM-4Z vector (Promega, Madison, WI).
SpeI-HF restriction enzyme + CutSmart® buffer (New England Biolabs, Ipswich, MA).
DNA Clean and Concentrator-25 (Zymo Research, Irvine, CA).
1.5 mL microcentrifuge tubes (Eppendorf, Hamburg, Germany).
DNase/RNase-free ultrapure water (Life Technologies, Carlsbad, CA).
Agarose gel electrophoresis system (Thermo Scientific, Waltham, MA).
mMESSAGE mMACHINE T7 Transcription Kit (Life Technologies, Carlsbad, CA).
RNeasy Mini Kit (Qiagen, Valencia, CA).
NanoDrop-1000 spectrophotometer (Thermo Scientific, Waltham, MA).
Ethanol (Sigma Aldrich, St. Louis, MO).
Agarose (BioExpress, Kaysville, UT).
TAE buffer (BioExpress, Kaysville, UT).
1 kb DNA ladder (New England Biolabs, Ipswich, MA).
250 mL Erlenmeyer flask.
SYBR® safe DNA gel stain (Life Technologies, Carlsbad, CA).
6× gel loading dye (New England Biolabs, Ipswich, MA).
FluorChem FC2 UV transilluminator with camera (Alpha Innotech, San Jose, CA).
37 % formaldehyde solution (Sigma-Aldrich, St. Louis, MO).
MOPS (Sigma-Aldrich, St. Louis, MO).
2× RNA loading dye (New England Biolabs, Ipswich, MA).
Sodium acetate (Sigma-Aldrich, St. Louis, MO).
EDTA (Sigma-Aldrich, St. Louis, MO).
Ultrapure DEPC-treated water (Life Technologies, Carlsbad, CA).
0.5–10 kb RNA ladder (Life Technologies, Carlsbad, CA).
2.2 Bone Marrow-Derived DC Vaccine Preparation and Electroporation
2.2.1 Bone Marrow DC Preparation
Syngeneic mice of desired number.
Sterile scissors, Mayo scissors, sterile chucks, and sterile gauze.
Two pairs of sterile forceps.
70 % ethanol.
Halothane or isoflurane and gas chamber.
50 mL conical tubes (BD, Franklin Lakes, NJ).
RPMI 1640 (Gibco, Grand Island, NY).
Penicillin/streptomycin (P/S) (Gibco, Grand Island, NY) (store in 5.5 mL aliquots at −20 °C).
Heat-inactivated fetal bovine serum (FBS) (Gemini, West Sacramento, CA).
55 mM β-mercaptoethanol (ME) (Gibco, Grand Island, NY).
100 mM sodium pyruvate (Gibco, Grand Island, NY).
10 mM nonessential amino acids (NEAA) (Gibco, Grand Island, NY).
1 M HEPES buffer (Gibco, Grand Island, NY).
200 mM l -glutamine (Gibco, Grand Island, NY) (store in 4 mL aliquots at −20 °C) (may be refrozen).
GM-CSF (Gemini, West Sacramento, CA) (store at −20 °C).
IL-4 (Gemini, West Sacramento, CA) (store at −20 °C).
Cell lysis buffer (10×; BD Pharm Lyse: BD, Franklin Lakes, NJ) diluted 1:10 in sterile H2O (DNase, RNase free).
100 mm tissue culture dishes (BD, Franklin Lakes, NJ).
70 µm cell screens (BD, Franklin Lakes, NJ).
25 g needles (BD, Franklin Lakes, NJ) and 10 mL syringes (BD, Franklin Lakes, NJ).
Trypan blue and hemocytometer.
6-well tissue culture plates (BD, Franklin Lakes, NJ).
Pipet-Aid with 5, 10, and 25 mL pipettes.
250 mL conical tubes (Corning, NY).
- Complete DC media (CDCM)—To a 500 mL bottle of RPMI 1640, add the following:27.5 (5 %) FCS, 5.5 mL penicillin/streptomycin (stored frozen), 2 mL l-glutamine (stored frozen), 550 µl β-ME, 5.5 mL sodium pyruvate, 5.5 mL NEAA, 5.5 mL HEPES. Thaw 10 µg GM-CSF (should be stored in frozen aliquots of 200 µL) and 10 µg IL-4 (also stored frozen in aliquots of 200 µL). Pour entire bottle of media over a 0.2 µm, 500 mL sterile vacuum filtration unit (see Note 1).
2.2.2 mRNA Electroporation of DCs
50 mL conical tubes (BD, Franklin Lakes, NJ).
60 mm tissue culture dishes (BD, Franklin Lakes, NJ).
Opti-MEM media (Gibco, Grand Island, NY).
2 mm gene pulser cuvettes with sterile disposable fine-tipped transfer pipette (BTX Harvard Apparatus, Holliston, MA).
Electroporator (BTX model ECM 830).
- Fresh complete DC media (CDCM)—To a 500 mL bottle of RPMI 1640, add the following:27.5 (5 %) FCS, 5.5 mL penicillin/streptomycin (stored frozen), 2 mL l-glutamine (stored frozen), 550 µl β-ME, 5.5 mL sodium pyruvate, 5.5 mL NEAA, 5.5 mL HEPES. Thaw 10 µg GM-CSF (should be stored in frozen aliquots of 200 µL) and 10 µg IL-4 (also stored frozen in aliquots of 200 µL).
2.3 Immunophenotyping of DC Vaccine Prior to Injection
FACSCalibur™ cell analyzer (Becton Dickinson, Franklin Lakes, NJ).
12 × 75 mm polystyrene tubes (VWR, Radnor, PA).
Phosphate-buffered saline (Life Technologies, Carlsbad, CA).
Fetal bovine serum (Gemini, West Sacramento, CA).
PE mouse anti-mouse I-Ab antibody (Becton Dickinson, Franklin Lakes, NJ).
PE hamster anti-mouse CD11c antibody (Becton Dickinson, Franklin Lakes, NJ).
PE hamster anti-mouse CD80 antibody (Becton Dickinson, Franklin Lakes, NJ).
PE rat anti-mouse CD86 antibody (Becton Dickinson, Franklin Lakes, NJ).
PE rat anti-mouse Ly-6G antibody (Becton Dickinson, Franklin Lakes, NJ).
2.4 Vaccine Site Preconditioning and Intradermal DC Vaccination
FACS tubes with caps (BD, Franklin Lakes, NJ).
0.5 mL monoject insulin syringes (Covidien, Dublin, Ireland).
1.5 mL microcentrifuge tubes (Eppendorf, Hamburg, Germany).
70 % ethanol.
Sterile gauze pads, 2 × 2.
Td toxoid vaccine in prefilled syringes (or other protein antigen vaccine formulation in alum) (see Note 2).
3 Methods
The following is a procedure for generating and implementing an mRNA-transfected murine DC vaccine in combination with vaccine site preconditioning with a recall antigen. This process begins with the development of mRNA-transfected murine DCs (see Subheadings 3.1 and 3.2). The next steps involve immunophenotyping of the DC vaccine (see Subheading 3.3) followed by vaccine site preconditioning and intradermal DC vaccination (see Subheading 3.4).
3.1 Generation of In Vitro Transcribed mRNA
3.1.1 Preparation of Plasmid Template for In Vitro Transcription
Several commercial plasmid vectors exist for IVT. At a minimum, IVT templates must include a promoter and the coding sequence for the antigen of interest, which should be derived from cDNA. Poly(A) sequences may be added directly into the template sequence or can be added following IVT using poly(A) tailing kits. The promoters for IVT are generally those that are recognized by phage RNA polymerase (e.g., T7, T3, SP6), which are compatible with numerous commercial IVT kits. Several IVT plasmid templates also incorporate untranslated regions (UTRs) flanking the antigen coding sequence in order to help stabilize IVT mRNA. UTRs from globins are a common choice due to the inherent stability of globin mRNAs [32]. Finally, a unique restriction enzyme cleavage site should be placed at the intended transcriptional termination site to allow for plasmid linearization (see Note 3).
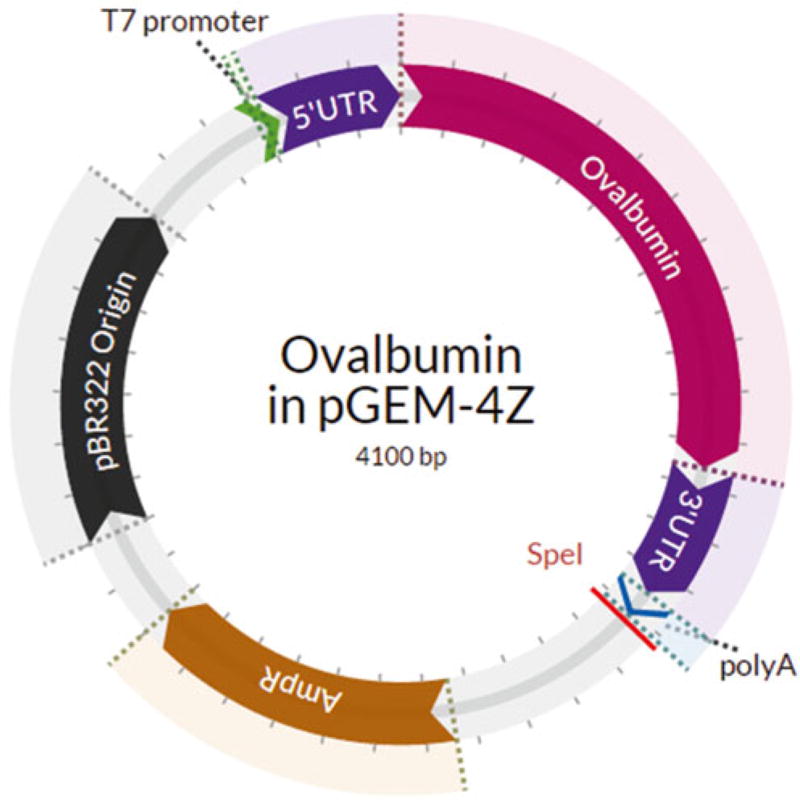
The IVT templates used in our lab are cloned into the pGEM-4Z backbone under the T7 promoter. The start codon for the antigen coding sequence is preceded by the minimal kozak sequence GCCACC. Constructs also contain a 5′ and 3′ UTR from Xenopus laevis β-globin mRNA to enhance mRNA stabilization. The 3′ UTR is followed by poly(A) sequence 62 base pairs in length, which terminates with a SpeI restriction enzyme cleavage site (Fig. 1).
Fig. 1.
Plasmid map of sample T7 polymerase-transcribed IVT vector used in our lab. The cDNA-derived sequence for chicken ovalbumin was cloned between UTRs obtained from Xenopus laevis β-globin cDNA. The poly(A) tail is encoded by a 62 base pair sequence, which is immediately followed by a SpeI restriction enzyme cleavage site to allow for plasmid linearization
Prior to performing IVT, plasmid templates are linearized via a restriction enzyme cut site immediately following the poly(A) sequence:
In a DNase-free 1.5 mL microcentrifuge tube, add 30 µg of plasmid DNA (resuspended in Tris-Cl) and bring volume up to 43 µL using ultrapure water. Add 5 µL 10× CutSmart® buffer and 2 µL (40 U) of restriction enzyme (see Note 4 : restriction enzyme may be at different concentration than 20 U/µL. Adjust accordingly. Keep in mind that RE are in glycerol, and keep glycerol content to <5 %).
Gently, pipette up and down several times to mix the reaction, followed by brief pulse centrifugation.
Incubate for 3 hr in a 37 °C water bath to ensure complete digestion of plasmid (see Note 5).
To the completed reaction, add 100 µL DNA-binding buffer from Zymo Research DNA Clean and Concentrator-25 kit, and add entire volume to supplied spin column.
Centrifuge at ~10,000 × g for 30 s at RT. Discard flow through.
Wash column two times using 200 µL DNA wash buffer, spinning at ~10,000 × g for 30 s at RT. Discard flow through.
Place spin column in new DNase-free microcentrifuge tube and elute in 25 µL elution buffer.
Determine DNA concentration using NanoDrop spectrophotometer.
Store plasmid sample at −20 °C until further use.
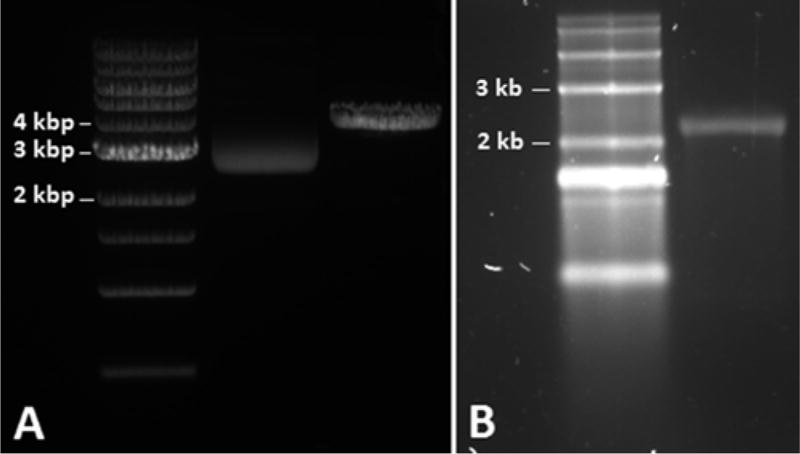
Optional: Confirm plasmid linearization by running 1 µg digested and undigested DNA on a 1 % agarose/TAE gel (Fig. 2a). Combine 100 mL 1× TAE buffer and 1 mg agarose in a 250 mL Erlenmeyer flask; mix by swirling. Microwave for 2 min, swirling mixture every 30 s. Mix 10 µL SYBR® safe stain into molten agarose, pour into agarose gel mold, insert appropriate lane comb, and allow mixture to congeal. Immerse solidified gel in 1× TAE and remove comb. Prepare 1 µg digested and undigested plasmids in water and 1× gel loading dye. Load lanes with 5 µL 1 kb DNA ladder and plasmid samples. Run gel at 4–10 V/cm for approximately 45 min. Visualize/image using UV transilluminator.
Fig. 2.
Confirmation of plasmid linearization and single IVT mRNA product using gel electrophoresis. (a) Verification of plasmid linearization using 1 % agarose/TAE gel electrophoresis. From left to right: NEB 1 kb DNA ladder, 1 µg undigested ovalbumin in pGEM (see Fig. 1), and 1 µg Spe1-digested ovalbumin in pGEM (expected MW = 4100 bp). (b) Verification of single IVT mRNA product using 1.5 % denaturing agarose gel electrophoresis. From left to right: Invitrogen 0.5–10 kb RNA ladder and 1 µg IVT ovalbumin mRNA (expected MW = 2054 bases)
3.1.2 In Vitro Transcription
Several commercial in vitro transcription kits are available that produce high-yield, high-purity capped mRNA from phage promoters. Many kits contain a standard m7 G(5′)ppp(5′)G cap analog that can incorporate onto mRNA in the reverse orientation, leading to translational inefficiencies. Alternatively, some kits contain a modified m7 (3′-O-methyl)-(5′)ppp(5′)G ARCA cap analog that can only be incorporated onto mRNA in the correct orientation, generating 100 % functional mRNA. Our lab uses Life Technologies mMESSAGE mMACHINE T7 Transcription Kit, which yields a 1:20 DNA template input-mRNA output ratio:
Thaw frozen IVT reagents, leaving RNA polymerase enzyme mix on ice.
In a DNase/RNase-free 1.5 mL microcentrifuge tube, add 5 µg linearized plasmid template and bring volume to 30 µL with nuclease-free water.
Assembling reaction at RT, add 50 µL 2× NTP/CAP, 10 µL 10× reaction buffer, and 10 µL enzyme mix.
Gently, pipette up and down several times to mix the reaction, followed by brief pulse centrifugation.
Incubate in a 37 °C water bath for 2 hr.
Add 5 µL TURBO DNase, mix by gently pipetting up and down.
Incubate in a 37 °C water bath for 15 min.
Add 350 µL RLT buffer from RNeasy kit and 250 µL 100 % ethanol, gently mix by pipetting.
Apply entire mixture to RNeasy spin column.
Centrifuge at 8000 × g for 30 s at RT. Discard flow through.
Wash column two times with 500 µL RPE buffer, spinning at 8000 × g for 1 min at RT. Discard flow through each time.
Place column in new nuclease-free microcentrifuge tube and spin at max speed to remove residual RPE buffer.
Place column in new nuclease-free microcentrifuge tube, add 40 µL nuclease-free water to the center of the column membrane, and elute by spinning at 8000 × g for 1 min at RT (see Note 6).
Determine mRNA concentration using NanoDrop spectrophotometer.
Bring mRNA concentration to 1 µg/µL in RNase-free water, prepare 10 µL aliquots, and freeze at −80 °C until further use.
Optional: Confirm presence of single mRNA product by running 1 µg IVT mRNA on 1.5 % denaturing agarose gel (Fig. 2b). Prepare 10× MOPS running buffer by combining 400 mM MOPS (pH 7.0), 100 mM sodium acetate, and 10 mM EDTA. Combine 72 mL DEPC-treated water and 1.5 mg agarose in 250 mL RNase-free Erlenmeyer flask (see Note 7). Microwave for 2 min, swirling mixture every 30 s. Let gel cool to ~60 °C then add 10 mL 10× MOPS running buffer, 18 mL 37 % formaldehyde solution, and 10 µL SYBR® gold nucleic acid stain into molten agarose (see Note 8); mix by swirling. Pour mixture into agarose gel mold, insert appropriate lane comb, and allow mixture to congeal. Prepare RNA samples in RNA loading dye, followed by heat denaturation at 65 °C for 10 min. Load lanes with 5 µL RNA ladder and IVT mRNA sample. Run gel at 4–10 V/cm for approximately 45 min. Visualize/image using UV transilluminator.
3.2 Bone Marrow-Derived DC Vaccine Preparation and Electroporation
3.2.1 Bone Marrow DC Preparation
Day 0
Sacrifice mouse in gas chamber. Perform cervical displacement on mice. Wash mouse by submersion in 70 % ethanol.
Place mouse on sterile gauze on sterile chuck. Cut the achilles tendon on one hind leg. Then, beginning at this site, use scissors to extend skin opening up the entire rear length of the leg. Peel the skin from the ankle up the entire length of the leg, exposing the musculature. Cut the patellar tendon, found on the anterior surface. Turning the leg over again, bend the leg backward at the knee, exposing the proximal tibia. Continue to push, exposing the tibia to the ankle. Holding the foot and tibia firmly in opposing hands, loosen the joint by gentle twisting until the tibia can be pulled in one swift motion from the foot. Clean the bone with gauze and place on ice in conical containing cold RPMI-P/S. Returning to the same leg, cut the remaining foot and lower leg muscles off at the knee, leaving only the upper leg. Slide the scissors in between the muscle and anterior surface of the femur. Spread the scissors to bluntly dissect the muscle off the bone. Repeat for the posterior surface of the bone. This will leave the distal femur and attached patella exposed. Grasp the exposed end of the femur and pull outwards from the body at a 45° angle to dislocate the hip. At this point, the scissors can be used to feel between the dislocated head of the femur and pelvis and to cut the femur away. Once the bone is removed, the patella may be grasped with the finger and folded back over the femur to remove it. Much of the musculature will come with the patella, and the femur may then be easily cleaned with gauze and placed in RPMI-Penicillin/Streptomycin (P/S) with 10 % FBS. Repeat this procedure for each leg of each mouse.
Once both legs have been removed, the sternum may be removed. Make a horizontal incision in the skin, over the area of the xiphoid process. The proximal flap may then be grasped and pulled rostrally, exposing the rib cage and shoulders. Pectoral muscles should be detached from the chest wall. Then, make a horizontal incision in the peritoneum, over the xiphoid process. Sliding the scissors rostrally under the sternum, bluntly dissect the organs away from the sternum. Scissors may then be used to cut along each side of the sternum, toward the head. Cut horizontally proximal to the manubrium to detach the sternum. Place it on ice in the same conical tube containing the long bones in RPMI-P/S. Repeat steps 1 through 3 for each mouse.
Following bone harvest, transport bones to tissue culture hood.
Decant off RPMI-P/S + 10 % FBS and pour long bones and sternums separately into 100 mm dishes. Add enough fresh RPMI-P/S + 10 % FBS to submerge bones.
For long bones, use scissors or Mayos to cut off the distal (white) ends of the tibias and proximal (head) ends of the femurs. Place bones in fresh dish containing RPMI-P/S + 10 % FBS.
Fill a 10 mL syringe with RPMI-P/S + 10 % FBS from dish containing bones, and place a 25 g needle on the end. The needle can be inserted into the proximal (uncut) end of tibia or distal (uncut) end of femur (patella must have been removed) and used to squirt marrow out the cut end. Bones should be white when emptied. Subsequently insert the needle into the opposite (cut) end of the bone to ensure efficient marrow harvest. The marrow will appear as long red strings in dish. If all RPMI mixture is expelled from the syringe, remove the needle, refill from the same dish, and attach a fresh needle.
Once all long bones are emptied and discarded, use just the 10 cc syringe to draw the marrow and RPMI mixture up and down in the dish until marrow is dispersed and dissociated.
Transfer marrow through a 70 µm cell screen fitted on a 45° angle into a 50 mL conical tube. Let sit until sternum marrow is harvested.
For sternums, cut away xiphoid and loose muscle still attached. Transfer to fresh dish containing RMPI-P/S + 10 % FBS.
Holding sternums at one end with forceps (keep submerged), crush the sternum from one end to the other with Mayos, squeezing out the marrow (as if from a tube of toothpaste). Repeat until sternum is white. Discard sternums.
Repeat steps 10 and 11 for sternums. If space allows, marrow may be added to same conical and cell screen as long bones.
Spin the cell suspension at 500 ×g×5 min ×4 °C.
Resuspend pellet (combine pellets if there are more than one) in 5 mL lysis buffer per five mice harvested. Incubate in 37 °C bath for 3–4 min.
Stop reaction by adding 25–30 mL cold RPMI + 10 % FBS. Spin as in step 13.
Resuspend pellet in 10 mL freshly made CDCM. Run cells over another 70 µm cell screen into a new 50 mL conical on ice. This will remove any lysed cell aggregates.
Count cell numbers via trypan blue exclusion (using a 1:20 dilution of cell suspension: trypan blue).
Adjust cell concentration to 1 × 106 /mL and plate in 6-well plates at 3 mL per well. Incubate at 37 °C, 5 % CO2, humidified (see Note 9).
Day 3 wash: Removal of nonadherents
Harvest media from wells, three at a time, using a Pipet-aid with 10 mL pipette. (Place tip down the side of each well to draw up media—do not scrape the bottom of the well with the pipette.)
With the Pipet-Aid on “slow,” expel media in circle down inside wall of well and draw back up. Do this once per well for all three wells.
Discard media and replace with 3 mL fresh CDCM per well. Repeat these steps for all wells and reincubate in Day 0 Step 18 conditions.
Day 7: Harvesting of nonadherents prior to electroporation
With Pipet-Aid on “fast” and a 10 mL pipette, harvest media from wells three at a time.
For each well, expel media in circular fashion down sides of well and draw back up. Perform this wash three times for each well in the three-well set, one after the other.
Save the collected media (see Note 10) and cells by transferring to a 250 mL conical on ice. Discard 6-well plates. Repeat for all wells, and pool cells and media in 250 mL conicals.
Spin cells 500 × g × 5 min × 4 °C. Following spin, save the decanted media (will be used later in Subheading 3.2.2).
Resuspend pellet in 10 mL cold Opti-MEM media, and count via trypan blue.
3.2.2 mRNA Electroporation of DCs
Once total cell count is calculated on Day 7 harvest, wash cell suspension twice in cold, fresh Opti-MEM media at 500 × g × 5 min × 4 °C.
Adjust cell concentration to 2.5 × 107 /mL in Opti-MEM media (will need 5 × 106 /200 µL in Opti-MEM for this electroporation step).
Obtain IVT mRNA to be electroporated (see Subheading 3.1). Keep on ice when not using.
First mix cell suspension thoroughly and transfer 200 µL cell suspension to bottom corner of a 2 mm fresh electroporator cuvette.
Mix mRNA (10 µg, 10 µL) with a single aliquot of cells by pipetting up and down 8–10 times (do not allow bubbles to form) using a P-10.
Using a P-200, mix the mRNA/cell suspension immediately in the gene pulser cuvette up and down 8–10 times (without creating bubbles).
Place cuvette in electroporator with plastic notch on cuvette facing outward and pulse at 300 V for 500 µs (ECM 830 electroporator; BTX, San Diego, CA) (see Note 11).
Using sterile disposable dropper from cuvette package, transfer electroporated cells and RNA immediately into 5 mL 50 % conditioned media (2.5 mL fresh CDCM + 2.5 mL saved media (see Subheading 3.2.1) in a 60 mm tissue culture dish. Repeat steps 4–8 for each aliquot. Reincubate cells at 37 °C, 5 % CO2, humidified for 6 to 24 h.
3.3 Immunophenotyping of DC Vaccine Prior to Injection
3.3.1 Immunophenotyping
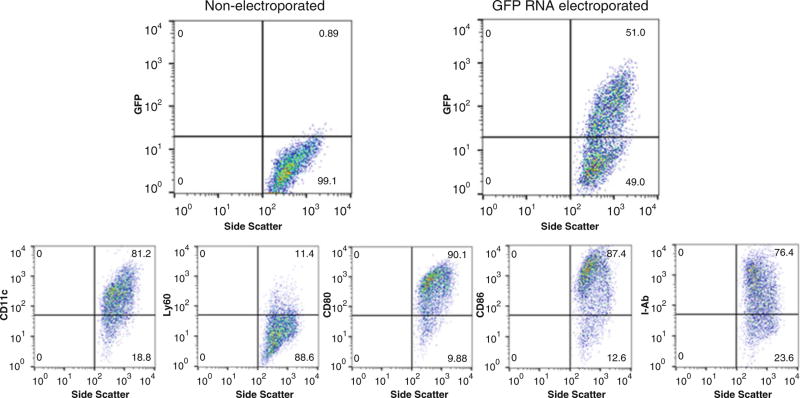
At this point, 2–3 × 106 cells from each group may be set aside on ice for phenotyping (using murine antibodies for CD11c, Ly6G, CD80, and CD86, and MHC II (I-Ab)). Alternatively, cells leftover after vaccination may be brought back for phenotyping (Fig. 3).
Fig. 3.
Representative panel of RNA-transfected DC maturation markers prior to injection. Top: DCs electroporated with green fluorescent protein (GFP) RNA (transcribed as above) were analyzed for effective RNA uptake and protein expression by flow cytometry 24 h following electroporation. Bottom: DCs were stained for phenotypic and costimulatory markers on Day 7 prior to vaccination. CD11c, integrin with high expression on myeloid or conventional DC; Ly6G, myeloid differentiation antigen that remains highly expressed on bone marrow granulocytes and peripheral; CD80 (B7-1) and CD86 (B7-2), costimulatory molecules expressed on DCs required for T cell activation and survival; I-Ab, MHC class II haplotype in C57BL/6 mouse background
3.3.2 Intradermal Vaccination
Harvest cells from 60 mm culture dishes 6–24 h after mRNA electroporation using a 10 mL sterile pipette and Pipet-Aid on “fast.”
Pool cells that were electroporated with the same RNA species in 50 mL conicals on ice. Spin 500 × g × 5 min × 4 °C.
Resuspend pellet in 5 mL 1× PBS and count. Respin cells, washing two more times in 1× PBS, and resuspend in PBS at desired concentration for vaccination (usually 10 × 106 DC/mL). Transfer to capped FACS tubes and place on ice. Be sure to agitate cells prior to drawing into syringe for vaccination (see Subheading 3.4.2).
3.4 Vaccine Site Preconditioning and Intradermal DC Vaccination
3.4.1 Vaccine Site Preconditioning
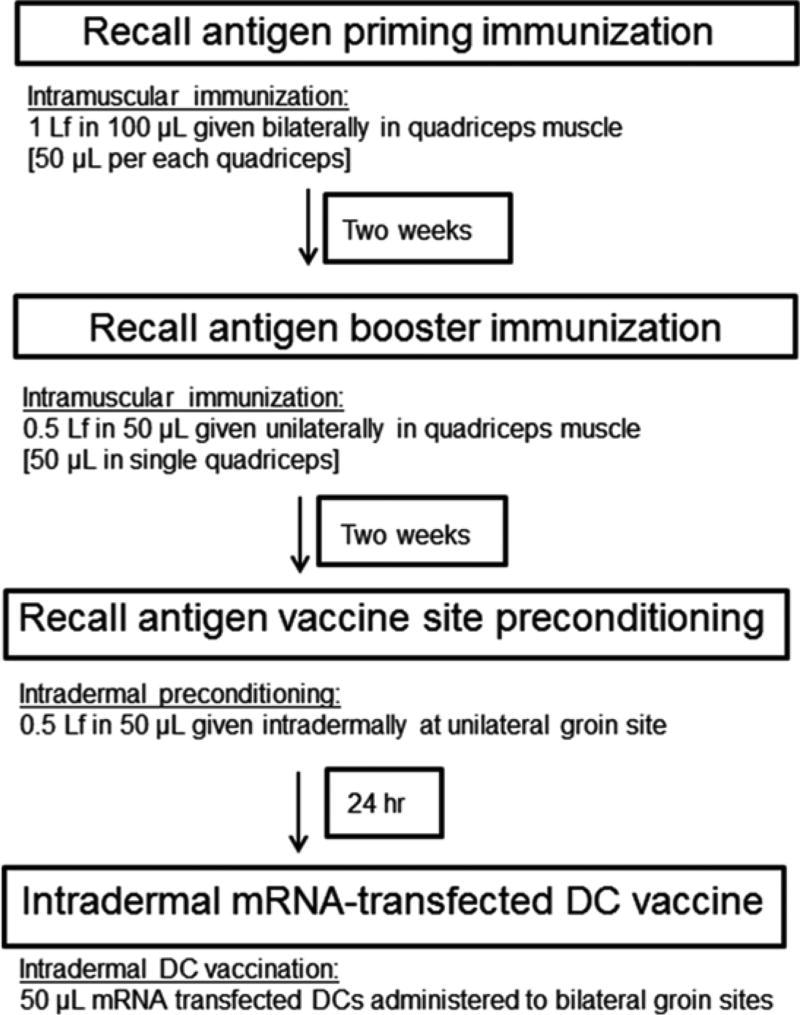
Primary immunization: To be administered 2 weeks prior to vaccine site preconditioning for adequate induction of immunity to protein antigen (Fig. 4).
Expel 500 µL (5 Lf) of the prefilled Tenivac® into a sterile 1.5 mL Eppendorf tube.
Take 100 µL (1 Lf) of the Tenivac® vaccine into a 0.5 mL insulin syringe.
Holding the mouse supine in one hand, with a firm grasp on the back of the neck/shoulders and tail tucked tightly under the pinky finger, extend one leg straight out and tuck the foot under the pinky, exposing the quadriceps muscle above the femur.
Position the insulin needle (bevel facing upwards) completely parallel to the mouse femur and gently guide the needle tip under the skin into the quadriceps muscle. You should feel considerable resistance against the needle tip. This means that the needle is appropriately inserted in dense muscle.
Inject 50 µL Tenivac® into the first quadriceps muscle at a slow rate. Let the needle sit in the muscle for 3 s and slowly withdraw the needle out of the leg in 1 s increments. This helps prevent backflow of the vaccine out of the injection path.
Uncover the first leg and extend the other leg as described above, tucking the foot again under the pinky.
Insert the needle completely parallel as described in Step 4 and inject the remaining 50 µL Tenivac® as in Step 5.
Two weeks later, repeat 50 µL as a booster immunization in a single quadriceps muscle (Step 5).
Fig. 4.
Immunization schema for preconditioning DC vaccine site with protein antigen vaccine formulations. The following recall antigen preconditioning protocol requires a primary intramuscular immunization and booster immunization separated by two weeks followed by an intradermal single preconditioning 2 weeks thereafter
Recall stimulus with preconditioning: To be administered at a single site intradermally in the groin 2 weeks following booster intramuscular immunization. This step requires the mouse to be anesthetized in an isoflurane chamber with oxygen flow:
Ensure the mouse is adequately anesthetized with slowed breathing rate for 30 s. Position the mouse in a supine position.
Wet a piece of sterile gauze with 70 % ethanol and fold the gauze. Gently press on a single groin site and wipe the hair and skin to clean the area.
Take 50 µL (0.5 Lf) of the protein antigen formulation (i.e., Tenivac®) into a 0.5 mL insulin syringe.
Again positioning the needle completely parallel to the groin skin (bevel facing upwards), slowly insert the needle tip under the epidermal skin layer. As this is an intradermal preconditioning administration, one should very clearly see the needle bevel under the skin, almost as if the skin layer were transparent to expose the needle tip.
Once the needle can be appreciated in the intradermal compartment, slowly release 50 µL of Tenivac® under the skin.
Leave the needle under the skin for at least 3 s after injecting all 50 µL and slowly withdraw the needle tip, in 1 s increments. The slow withdrawal of the needle during this step is absolutely critical, because the high pressures in the intradermal vaccine bleb have a tendency to expel the formulation out of the injection tract.
Return the mouse to normal arousal state and wait 24 h for intradermal DC vaccination.
3.4.2 Intradermal DC Vaccination
Intradermal vaccination in bilateral groin sites with mRNA-transfected DCs occurs 24 h following vaccine site preconditioning of a single groin site. This step requires the mouse to be anesthetized in an isoflurane chamber with oxygen flow:
Take cell suspension from Subheading 3.3.2 and mix well. Draw up 100 µL per mouse to be vaccinated (50 µL per each groin side).
Again ensure the mouse is adequately anesthetized with slowed breathing rate for 30 s. Position the mouse in a supine position.
Wet a piece of sterile gauze with 70 % ethanol and fold the gauze. Gently press on both left and right groin sites, wiping the hair and skin to clean the area.
For each groin site, position the needle completely parallel to the groin skin (bevel facing upwards), slowly insert the needle tip under the epidermal skin layer. As this is another intradermal administration, one should very clearly see the needle bevel under the skin, almost as if the skin layer were transparent to expose the needle tip.
Once the needle can be appreciated in the intradermal compartment, slowly release 50 µL of the DCs in 1× PBS under the skin.
Leave the needle under the skin for at least 3 s after injecting all 50 µL and slowly withdraw the needle tip, in 1 s increments. The slow withdrawal of the needle during this step is required to control for high pressures within the intradermal bleb that may to expel the formulation out of the injection tract.
Return the mouse to normal arousal state.
Footnotes
Complete DC media (CDCM) should be made on Day 0 and used only for that DC prep (i.e., on Days 0–9). It should not be stored for multiple uses. Occasionally, bone marrow culture yields will be high enough to warrant more than one 500 mL bottle per prep.
Alum-containing protein antigen formulation s can be used at this step for preconditioning of vaccine sites prior to DC injection. We have previously used the Td toxoid vaccine (Sanofi Aventis, Tenivac®), which produced inflammation at the preconditioning site (erythema and induration) and activated CD4+ T cells. Other protein antigen formulations similar to Td toxoid that activate the CD4+ T cell compartment may show similar efficacy in preconditioning the DC vaccine site.
RNA polymerases are very processive enzymes. Therefore, circular plasmids should be linearized prior to in vitro transcription to avoid the production of RNAs that exceed the region of interest, as this may affect downstream applications.
SpeI-HF used by our lab is supplied at 20 U/µL. If using a restriction enzyme that is at a different concentration, adjust the volume accordingly to achieve 40 U. Restriction enzymes are typically suspended in glycerol, which can inhibit enzymatic reactions. Maintain final glycerol concentration to <5 %.
Shorter incubation times may suffice. Refer to the enzymatic activity specifications for each enzyme. If incubating for longer periods of time (e.g., overnight), use a restriction enzyme with reduced nonspecific activity (e.g., high-fidelity enzymes from NEB).
Our lab has experienced increased yields by returning eluate to spin column membrane and re-eluting.
To avoid RNase-mediated degradation of samples, all glassware should be baked at >180 °C or soaked in 0.1 % DEPC-treated water for several hours, followed by autoclaving. Plastics can be soaked in 0.1 % DEPC-treated water for several hours then rinsed or sprayed with RNase cleaning product.
Formaldehyde is a carcinogen. Therefore, it is strongly recommended that a dedicated chemical fume hood be used for all formaldehyde work.
Each DC bone marrow preparation is outlined to use five mice for each harvest and DC vaccination experiment. Therefore, one can expect a generation of 350 × 106 total marrow cells to be extracted from a total of five mice on Day 0. Upon Day 7, the usual number of DCs cultivated should reach 50 × 106 cells total. These numbers are the average numbers generated for Days 0 and 7 for a 5-mouse bone marrow procedure.
The difference in the technique for Day 3 and Day 7 washes in the DC bone marrow procedure reflects the morphologic changes in cultured DCs with time. On Day 3, it is absolutely critical that cells are washed with the Pipet-Aid on “slow.” However, on Day 7, the DCs alter their adherent properties and can be found in the supernatant of the wells. This is why on Day 7, the nonadherent cell mixture is saved and replated. For Day 7 washes, the Pipet-Aid should be operated on the “fast” setting.
For the RNA electroporation step, the highest number of viable electroporated DCs will be achieved if the operator moves as quickly as possible. The quickest and most effective procedure for electroporation is to first remove the 200 µl aliquot of DCs into the electroporator cuvette with a P-200 pipette. Then, take a P-10 pipette and mix thoroughly (3–5 times) 10 µL of the RNA in its microcentrifuge tube. Pipette 10 µL RNA and inject into the deep corner of the floor of the cuvette with the DCs. Quickly transfer to the P-200 and mix this RNADC suspension (eight times is sufficient) without generating bubbles in the cuvette. This entire process (from the insertion of the cells to the implantation of the cuvette into the electroporator) should not exceed 15 s.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 4.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamanaka R, Abe T, Yajima N, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho DY, Yang WK, Lee HC, et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: a phase II clinical trial. World Neurosurg. 2012;77:736–744. doi: 10.1016/j.wneu.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–1034. [PubMed] [Google Scholar]
- 8.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair SK, Morse M, Boczkowski D, et al. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg. 2002;235:540–549. doi: 10.1097/00000658-200204000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair SK, De Leon G, Boczkowski D, et al. Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus pp 65-specifi c cytotoxic T cells. Clin Cancer Res. 2014;20:2684–2694. doi: 10.1158/1078-0432.CCR-13-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair SK, Boczkowski D, Morse M, et al. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 12.Bonehill A, Heirman C, Tuyaerts S, et al. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol. 2004;172:6649–6657. doi: 10.4049/jimmunol.172.11.6649. [DOI] [PubMed] [Google Scholar]
- 13.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulolcyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair S, Archer GE, Tedder TF. Isolation and generation of human dendritic cells. Curr Protoc Immunol. 2012;7(Unit 7.32):1–23. doi: 10.1002/0471142735.im0732s99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inaba K, Swiggard WJ, Steinman RM, et al. Isolation of dendritic cells. Curr Protoc Immunol. 2009;86(3.7):3.7.1–3.7.19. doi: 10.1002/0471142735.im0307s86. [DOI] [PubMed] [Google Scholar]
- 16.Lutz MB, Schnare M, Menges M, et al. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574–3580. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 17.Hochrein H, O'Keeffe M, Luft T, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–833. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 19.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 20.Van Brussel I, Berneman ZN, Cools N. Optimizing dendritic cell-based immunotherapy: tackling the complexity of different arms of the immune system. Mediators Inflamm. 2012;2012:690643. doi: 10.1155/2012/690643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuurhuis DH, Lesterhuis WJ, Kramer M, et al. Polyinosinic polycytidylic acid prevents efficient antigen expression after mRNA electroporation of clinical grade dendritic cells. Cancer Immunol Immunother. 2009;58:1109–1115. doi: 10.1007/s00262-008-0626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 23.De Vries I, Krooshoop D, Scharenborg N, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:7–12. [PubMed] [Google Scholar]
- 24.Eggert A, Schreurs M, Boerman O, et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59:3340–3345. [PubMed] [Google Scholar]
- 25.Eggert A, van der Voort R, Torensma R, et al. Analysis of dendritic cell trafficking using EGFP-transgenic mice. Immunol Lett. 2003;89:17–24. doi: 10.1016/s0165-2478(03)00105-6. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Fontecha A, Sebastiani S, Hopken UE, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prins RM, Craft N, Bruhn KW, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 28.Chagnon F, Tanguay S, Ozdal OL, et al. Potentiation of a dendritic cell vaccine for murine renal cell carcinoma by CpG oligonucleotides. Clin Cancer Res. 2005;11:1302–1311. [PubMed] [Google Scholar]
- 29.Strutt TM, McKinstry KK, Dibble JP, et al. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16:558–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narni-Mancinelli E, Campisi L, Bassand D, et al. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell JE, Liebhaber SA. The stability of human beta-globin mRNA is dependent on structural determinants positioned within its 3′ untranslated region. Blood. 1996;87:5314–5323. [PubMed] [Google Scholar]