Abstract
Objective
To evaluate the influence of age at diagnosis on the frequency of structural incomplete response (SIR) according to the modified risk of recurrence (RR) staging system from the American Thyroid Association guidelines.
Patients and Methods
We performed a retrospective analysis of 268 patients with differentiated thyroid cancer (DTC) followed up for at least 3 years after initial treatment (total thyroidectomy and remnant ablation). The median follow-up in the whole cohort was 74.3 months (range: 36.1-317.9) and the median age at diagnosis was 45.9 years (range: 18-87). The association between age at diagnosis and the initial and final response to treatment was assessed with analysis of variance (ANOVA). Patients were also divided into several groups considering age younger and older than 40, 50, and 60 years.
Results
Age at diagnosis was not associated with either an initial or final statistically significant different SIR to treatment (p = 0.14 and p = 0.58, respectively). Additionally, we did not find any statistically significant differences when the percentages of SIR considering the classification of RR were compared between different groups of patients by using several age cutoffs.
Conclusions
When patients are correctly risk stratified, it seems that age at diagnosis is not involved in the frequency of having a SIR at the initial evaluation or at the final follow-up, so it should not be included as an additional variable to be considered in the RR classifications.
Keywords: Age, Risk of recurrence, Structural incomplete response, Response to treatment, Differentiated thyroid cancer
Introduction
Various staging systems have historically been used to assess the risk of mortality in patients with differentiated thyroid cancer (DTC) [1,2,3,4,5]. All of them are strongly dependent on patient age since age at diagnosis has been demonstrated to be an important predictor of disease-specific survival in patients with DTC [6,7,8,9]. Although the TNM system from the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) proposes the age of 45 years as a threshold to divide patients, this static variable, which probably defines the worst outcome, is nowadays questioned [10,11,12]. Recently, in a multi-institutional study it was proposed that a cutoff at 55 years was more suitable than the traditional 45 years [13]. Such a change resulted in 12% of patients being downstaged. The vast majority of the downstaged patients (97%) were then considered as having stage I disease, and the disease-specific survival rate of this newly defined group of patients was 98% at 10 years of follow-up [13]. This analysis suggested that using an age cutoff of 45 years would lead to a significant number of patients who are at very low risk of death being inappropriately assigned an advanced disease stage [14]. The American Thyroid Association (ATA), the Latin American Thyroid Society (LATS), and the European Thyroid Association (ETA) guidelines for the follow-up and treatment of DTC have recommended the use of the TNM staging system mainly to predict the probability of DTC-related death [15,16,17]. Despite the undoubtable contribution of this system in facilitating the survival prognosis of patients with DTC, it has been demonstrated that the TNM staging system is insufficient to establish the probability of a structural incomplete response (SIR) [18], which made it necessary to create a risk of recurrence (RR) stratification system that was validated in several cohorts of patients around the world [18,19,20,21,22]. This risk-adapted approach, which relies on specific clinical and pathological features, effectively predicts the risk of recurrent/persistent disease in DTC [18,19,20,21,22]. However, these risks of recurrence in thyroid cancer were created independently of the age at diagnosis of the DTC, and literature that assesses the impact of different age cutoffs on the probability of a SIR when the RR classification is considered together is scarce. Therefore, we performed a retrospective analysis of 268 patients with DTC followed up for at least 3 years after initial treatment to evaluate the impact of age on the rates of SIR considering the outcome at the end of follow-up according to the modified RR classification system from the ATA [15].
Patients and Methods
The file records of 610 patients with DTC from our database were reviewed for the period January 2001 to March 2016. The inclusion criteria were: (1) 18 years old or older, (2) adequate clinical and pathological data to allow an accurate determination of the initial RR, (3) a defined initial response to treatment during the first 2 years of follow-up, (4) follow-up for at least 3 years, and (5) received a total thyroidectomy with or without lymph node resection and remnant ablation with radioiodine after thyroid hormone withdrawal or recombinant human thyrotropin (TSH). The data of 268 patients fulfilled the inclusion criteria and were used for the analysis.
Patients were stratified according to the modified 2009 Risk Stratification System proposed by the ATA [15]. We have previously validated this new stratification system in our cohort of patients with DTC, including patients with minor extrathyroidal extension in the low RR group, so these patients were included in the low-risk group for analysis [23].
The comparison of the 2 stratification systems, 2009 and modified 2009 (adding extrathyroidal extension as a low-risk feature) can be seen in Table 1.
Table 1.
ATA 2009 RR classification with proposed modifications for the modified 2009 Risk Stratification System
| ATA 2009 RR classification | Modified ATA 2015 RR classification | |
|---|---|---|
| High risk | Gross ETE Incomplete tumor resection Distant metastases Inappropriate Tg |
Gross ETE Incomplete tumor resection Distant metastases Inappropriate Tg Any LN >3 cm >3 LN with extranodal extension |
| Intermediate risk | N1 Minor ETE Vascular invasion Aggressive histology Uptake outside TB |
PTC vascular invasion N1 (clinical) N1 >5 lymph nodes Aggressive histology |
| Low risk | Any size, intrathyroidal N0 M0 No aggressive histology No vascular invasion No uptake outside TB |
Intrathyroidal tumors, or with minor ETE No aggressive histology No vascular invasion N1 all MTS <2 mm N1 <5 lymph nodes |
ETE, extrathyroidal extension; Tg, thyroglobulin; N0, absence of lymph node metastasis; N1, proven lymph node metastasis; M0, absence of distant metastasis; TB, thyroid bed; LN, lymph node; PTC, papillary thyroid cancer; MTS, metastasis; RR, risk of recurrence.
Ablation Protocol
Patients received radioiodine ablation after stimulation with recombinant human TSH (n = 13) or endogenous TSH (n = 255) and using activities of 3.70 GBq (100 mCi 131I) for low risk (ATA 2009 RR, Table 1) and 5.55 GBq (150 mCi 131I) for intermediate risk (ATA 2009 RR, Table 1). Endogenous TSH stimulation was obtained after thyroid hormone withdrawal for at least 3 weeks. Radioiodine was administered following that interval in all cases with TSH levels above 50 mIU/L.
Thyroglobulin/Thyroidglobulin Antibody Measurement
Samples for thyroglobulin (Tg) and thyroglobulin antibody (TgAb) measurement were obtained on the day of ablative radioiodine administration. Tg and TgAb levels were assessed in 1 of 2 reference laboratories from Argentina using either of 2 commercial immunometric assays; the same laboratory and assay were used throughout a patient's follow-up. Tg assays comprised the Elecsys Tg Electrochemiluminescence Immunoassay (Roche Diagnostics GmbH, Mannheim, Germany), which has a detection limit of 0.2 ng/mL, or the Immulite 2000 Tg Chemiluminiscence Assay (Siemens Corp., Los Angeles, CA, USA), with a functional sensitivity of 0.9 ng/mL. TgAb assays comprised the Elecsys Anti-Tg Electrochemiluminescence Immunoassay (RSR Ltd., Cardiff, UK), or the Immulite 2000 Anti-TG Ab chemiluminescent immunometric assay method (Siemens). For both TgAb assays, values >20 IU/mL were considered to be positive and to render Tg measurements uninterpretable. These patients with positive TgAb were excluded from the study.
Clinical Management during Follow-Up
To assess clinical status in response to the initial therapy, Tg and TgAb measurements were obtained on levothyroxine suppression, and neck ultrasonography was performed every 6 months after initial therapy. A stimulated Tg value was also obtained to define the disease status. When a measurable stimulated or unstimulated Tg and/or suspicious neck ultrasound (US) findings were found during a patient follow-up, morphological or functional imaging was then performed. This included computed tomography or 18-fluorodeoxyglucose positron emission tomography. Ultrasonographically suspicious nodes >10 mm in diameter were biopsied for cytology with Tg measurement in the needle washout fluid. After initial therapy, serum TSH values were kept below 0.1 mUI/L in all patients until January 2008. Since then, the degree of serum TSH suppression has been adapted to the RR (TSH <0.1 mUI/L for high risk, TSH 0.1-0.5 mUI/L for intermediate and low risk). In those patients with initial low-risk stratification who had an excellent response to treatment, serum TSH was maintained between 0.5 and 2 mUI/L.
The initial response to therapy were assessed at the 15 (±5)- month follow-up visit based on suppressed and/or stimulated Tg values, neck US, diagnostic whole body scans, and additional morphological/functional images when considered clinically appropriate. Patients were considered to have an excellent response to therapy if they had undetectable suppressed Tg values or a stimulated Tg level below 1 ng/mL, absence of TgAb, no uptake outside thyroid bed on dxWBS (if done), and no suspicious lymph or thyroid bed nodules on neck US. Patients with stimulated serum Tg between 1 and 10 ng/mL or unstimulated Tg values between 0.2 and 1 ng/mL and no structural evidence of disease or nonspecific findings in the neck US or other cross-sectional images, or with persistent measurable TgAb, were considered as having an indeterminate response. Patients with stimulated serum Tg >10 ng/mL or unstimulated Tg levels >1 ng/mL, or those with increasing TgAb without structural evidence of disease, were classified as having a biochemical incomplete response. Patients with SIR were those showing positive cytology or histology, highly suspicious findings on neck US or findings on dxWBS, and functional or morphological imaging suggesting DTC metastasis.
According to the clinical status at time of the final follow-up, patients were classified as having no evidence of disease (NED) if they had undetectable suppressed Tg values or a stimulated Tg level below 1 ng/mL, undetectable TgAb, nonsuspicious findings on neck US, and no evidence of structural disease on any other imaging studies. The remaining group of patients was classified as having an indeterminate, biochemical, or structural incomplete response by using the same definitions that were described above. Recurrent disease was defined as having structural or biochemical evidence of disease following a period of NED.
Statistical Analysis
Quantitative variables were expressed as the mean ± standard error mean and median and range, while qualitative variables were shown as percentages. To evaluate significant differences in categorical data, we analyzed 2-way contingency tables by the Fisher exact test or 2 × 2 contingency tables by the χ2 test. Continuous data were analyzed by analysis of variance (ANOVA). A regression logistic analysis was also performed assessing the age at diagnosis as an explanatory variable of SIR at the initial and final follow-up. Statistical analysis was developed using the SPSS statistical software (SPSS version 20.0, Inc., Chicago, IL, USA). We considered p < 0.05 to be statistically significant for all analyses.
Results
The demographic and clinical features for each of the 268 patients included in this study can be observed in Table 2. The majority of patients had the classic variant of papillary thyroid carcinoma (70.1%), were female (87.3%), and were AJCC stage I (59.0%). The median follow-up in the whole cohort was 74.3 months (range: 36.1-317.9; mean 92.8 ± 3.3). Patients were classified as having low (54.5%), intermediate (23.9%), or high RR (21.6%). For patients classified as having low RR, 55.5% had an excellent response after the initial therapy and 74.7% achieved the state of NED at the end of follow-up. A minority of the low-risk patients (8.9%) had SIR at the initial evaluation, and only 2% showed SIR at the final follow-up. Patients classified as having intermediate RR were less likely to have an excellent initial response to treatment. However, 45.3% of these patients had NED as their final outcome. As expected, only 3.4% of patients with an initial high RR had an excellent response to treatment, whereas 10.3% achieved the state of NED at the end of follow-up (Table 3). Men had higher percentages of SIR at the initial response to treatment (47.1 vs. 23.9%, p = 0.008, OR 2.8 [1.3-6.3]) and at the final outcome (47.1% vs. 12.8, p < 0.0001, OR 4.3 [2.5-7.4]).
Table 2.
Baseline characteristics of 268 patients with differentiated thyroid cancer included in the study
| Age at diagnosis, years (n = 268) | |
| Mean ± SEM | 45.5 ± 15.7 |
| Median (range) | 45.9 (18–87) |
| Female gender (n = 234) | 87.3% |
| Histology | |
| Papillary classic variant (n = 188) | 70.1% |
| Papillary follicular variant (n = 44) | 16.4% |
| Papillary oncocytic variant (n = 1) | 0.4% |
| Insular variant (n = 1) | 0.4% |
| Poorly differentiated variant (n = 1) | 0.4% |
| Diffuse sclerosing variant (n = 6) | 2.2% |
| Tall cell variant (n = 6) | 2.2% |
| Follicular (n = 15) | 5.6% |
| Hürthle cell (n = 6) | 2.2% |
| T stage | |
| T1a (n = 51) | 19.0% |
| T1b (n = 62) | 23.1% |
| T2 (n = 40) | 14.9% |
| T3 (n = 76) | 28.4% |
| T4 (n = 33) | 12.3% |
| Tx (n = 6) | 2.2% |
| AJCC stage | |
| I (n = 158) | 59.0% |
| II (n = 30) | 11.2% |
| III (n = 33) | 12.3% |
| IV (n = 47) | 17.5% |
| Follow-up duration, months (n = 268) | |
| Mean ± SEM | 92.8 ± 3.3 |
| Median, range | 74.3 (36.1–317.9) |
| 131I cumulative dose, mCi (n = 207) | |
| Mean ± SEM | 367.6 ± 18.7 |
| Median, range | 250 (30–980) |
| Risk of recurrence (modified 2009 RRS) | |
| Low risk (n = 146) | 54.5% |
| Intermediate risk (n = 64) | 23.9% |
| High risk (n = 58) | 21.6% |
SEM, standard error mean; AJCC, American Joint Cancer Committee.
Table 3.
Clinical outcomes after initial therapy and at final follow-up for each risk of recurrence categories in the whole cohort
| Number | Percentage | |
|---|---|---|
| Initial response to treatment | ||
| Low risk | 146 | |
| Excellent | 81 | 55.5 |
| Indeterminate | 37 | 25.3 |
| BIR | 15 | 10.3 |
| SIR | 13 | 8.9 |
| Intermediate risk | 64 | |
| Excellent | 17 | 26.6 |
| Indeterminate | 20 | 31.2 |
| BIR | 13 | 20.3 |
| SIR | 14 | 21.9 |
| High risk | 58 | |
| Excellent | 2 | 3.4 |
| Indeterminate | 4 | 6.8 |
| BIR | 7 | 12.2 |
| SIR | 45 | 77.6 |
| Status at final follow-up | ||
| Low risk | 146 | |
| NED | 109 | 74.7 |
| Indeterminate | 26 | 17.8 |
| BIR | 8 | 5.5 |
| SIR | 3 | 2.0 |
| Intermediate risk | 64 | |
| NED | 29 | 45.3 |
| Indeterminate | 20 | 31.2 |
| BIR | 5 | 7.8 |
| SIR | 10 | 15.6 |
| High risk | 58 | |
| NED | 6 | 10.3 |
| Indeterminate | 8 | 13.8 |
| BIR | 11 | 18.9 |
| SIR | 33 | 56.9 |
NED, no evidence of disease; BIR, biochemical incomplete response; SIR, structural incomplete response.
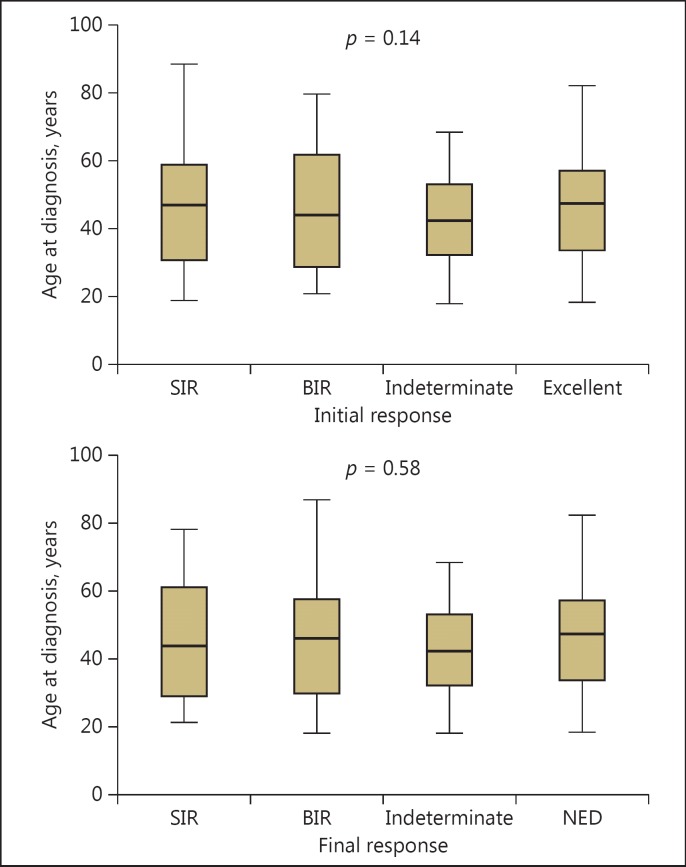
Age at diagnosis was not associated with the disease status of the patient, considering any of the responses to treatment, neither at the initial response nor at the final follow-up (p = 0.14 and p = 0.58, respectively, ANOVA) (Table 4; Fig. 1). To evaluate if older patients did not have a greater chance of being considered into the high RR group, we analyzed the OR by using the 40-, 50-, and 60-years-of-age cutoffs. The ORs for this were 0.85 (0.47-1.52), 0.92 (0.53-1.75), and 1.18 (0.57-2.44), respectively. Therefore, the presence of a high RR was not a variable that might have acted as a confounding factor for the analysis.
Table 4.
Association between age at diagnosis and initial and final outcomes using analysis of the variance
| n | Age at diagnosis, years |
p value | ||
|---|---|---|---|---|
| mean ± SEM | median (range) | |||
| Initial response | ||||
| Excellent | 100 | 47.2 ± 1.5 | 48.5 (19.6–82.3) | 0.14 |
| Indeterminate | 61 | 47.5 ± 1.9 | 47.3 (18.5–80.6) | |
| BIR | 35 | 42.2 ± 2.9 | 38.5 (19.0–78.4) | |
| SIR | 72 | 43.0 ± 1.9 | 44.1 (18.0–87.0) | |
| Final response | ||||
| NED | 144 | 45.2 ± 1.3 | 47.5 (18.5–82.3) | 0.58 |
| Indeterminate | 54 | 42.5 ± 1.8 | 42.4 (18.0–68.5) | |
| BIR | 24 | 45.7 ± 3.8 | 43.9 (21.3–78.4) | |
| SIR | 46 | 46.7 ± 2.5 | 45.8 (18.0–87.0) | |
NED, no evidence of disease; BIR, biochemical incomplete response; SIR, structural incomplete response; SEM, standard error mean.
Fig. 1.
Box plot showing the lack of association between age at diagnosis and responses to treatment at initial and final follow-up (analysis of variance). SIR, structural incomplete response; BIR, biochemical incomplete response; NED, no evidence of disease.
When patients were stratified according to the initial RR and then divided by using different age cutoffs (older and younger than 40, 50, and 60 years), there were no statistically significant differences between the percentages of SIR at the initial response to treatment (Table 5) or at final follow-up (Table 6).
Table 5.
Comparison of the frequency of initial structural incomplete response to treatment according to the risk of recurrence between younger and older patients by using different age cutoffs
| Age cutoff, years | SIR as initial response, n (%) |
p value | |
|---|---|---|---|
| 7lt; age cutoff | ≥ age cutoff | ||
| Low risk (n = 146) | |||
| 40 | 5/49 (10.2) | 8/97 (8.2) | 0.93 |
| 50 | 7/82 (8.5) | 6/64 (9.4) | 0.91 |
| 60 | 11/118 (9.3) | 2/28 (7.1) | 0.99 |
| Intermediate risk (n = 64) | |||
| 40 | 8/33 (24.2) | 6/31 (19.3) | 0.86 |
| 50 | 10/43 (23.2) | 4/21 (19.0) | 0.95 |
| 60 | 12/54 (22.2) | 2/10 (20) | 0.79 |
| High risk (n = 58) | |||
| 40 | 20/25 (80.0) | 25/33 (75.8) | 0.95 |
| 50 | 29/35 (82.8) | 16/23 (69.6) | 0.39 |
| 60 | 37/46 (80.4) | 8/12 (66.7) | 0.26 |
SIR, structural incomplete response.
Table 6.
Comparison of the frequency of structural incomplete response at final follow-up according to the initial risk of recurrence between younger and older patients by using different age cutoffs
| Age cutoff, years | SIR as final response, n (%) |
p value | |
|---|---|---|---|
| < age cutoff | ≥ age cutoff | ||
| Low risk (n = 146) | |||
| 40 | 1/49 (2.0) | 2/97 (1.1) | 0.74 |
| 50 | 2/82 (2.4) | 1/64 (1.6) | 0.59 |
| 60 | 2/118 (1.7) | 1/28 (3.6) | 0.47 |
| Intermediate risk (n = 64) | |||
| 40 | 3/33 (9.1) | 7/31 (22.6) | 0.18 |
| 50 | 6/43 (13.9) | 4/21 (19.0) | 0.72 |
| 60 | 8/54 (14.8) | 2/10 (20) | 0.65 |
| High risk (n = 58) | |||
| 40 | 11/25 (44) | 22/33 (66.7) | 0.14 |
| 50 | 20/35 (57.1) | 13/23 (56.5) | 0.82 |
| 60 | 26/46 (56.5) | 7/12 (58.3) | 0.83 |
SIR, structural incomplete response.
Discussion
In this retrospective analysis of 268 patients with DTC aged 18-87 years, risk stratified according to the modified 2009 RSS, and followed up for at least 3 years after initial treatment, we did not find any age threshold that could distinguish an older group of patients with a higher risk for SIR at the initial response to treatment or at the end of follow-up.
The idea that advanced age would be a disadvantage in the frequency of SIR in patients with DTC was proposed by Mazzaferri and Jhiang [24], who showed that recurrence in papillary and follicular carcinoma was more frequent at the extremes of life, in patients younger than 20 and older than 59 years. However, for mortality, rates were increased in patients over 40 years of age [24]. In fact, most of the mortality staging systems include age at the diagnosis as a significant prognostic factor [1,2,3,4,5]. On the other side, in a study performed by Ito et al. [25], it was also shown that the outcome of patients with DTC has a bimodal curve where the risk of lymph node recurrence is higher in patients younger than 20 and for those older than 60 years, and that distant metastasis is markedly increased in the latter group. Sugino et al. [26] also reported that tumor size had no effect on the rate of lymph node recurrence and noted a significantly higher rate of recurrence among both younger (<30 years) and older (>50 years) patients.
In a previous publication analyzing the recurrence rates in patients older than 65 years, Hollenbeak et al. [27] suggested that age at diagnosis could be a risk factor, showing that older age significantly influences the outcome in DTC patients. The limitation of this study is that they considered that a patient had a recurrence if they received additional radioiodine therapy, if they had thyroid bed uptake on dxWBS, imaging suggesting DTC metastasis, completion of thyroidectomy, or lymphadenectomy 6 months after the initial procedure. This definition does not necessarily match the actual SIR classification proposed by the ATA guidelines [15]. On the other hand, Orlov et al. [28] found no differences in age or primary tumor size among patients who had long-term detectable stimulated Tg levels (≥2 ng/ml) on a mean follow-up of 5.8 years. This was also consistent with other publications such as the one from Jukkola et al. [29], who demonstrated that age at diagnosis did not predict recurrence. Once more, one of the caveats of these previous studies [24,25,26,27,28,29] might be that they did not consider the classification of the initial RR as we did in our investigation. By classifying patients according to the well-validated RR classifications, one can have not only the scope regarding the probability of predicting SIR, but also keep in mind the possibility that a patient will have an excellent response to treatment [18,19,20,21,22]. A biochemical incomplete response and indeterminate response to treatment are usually associated with a low rate of SIR in the long-term follow-up that is not usually higher than 10-15%. Almost 70% of these patients will present an excellent response to treatment in the long-term follow-up with no other action more than the mere observation [30,31,32].
There are several limitations to our study, such as the low number of patients in each of the individual groups when divided by age and RR, which might have had an impact on the statistical results. Another limitation is that our population was mainly comprised of females (87.3%). Several studies have shown that male patients with DTC have a worse outcome, mainly those older than 55 years [26]. As such, the results of this study remain to be validated by larger multicenter studies that can pool data in order to minimize such confounders. In our cohort, on the other hand, every patient (even the ones classified as low risk) were treated with total thyroidectomy and remnant ablation, a common practice in the past in most countries of Latin America [17]. This practice of care may not apply to many institutions where low risk patients currently do not receive RAI; therefore, it raises concerns about the generalizability of these results.
In conclusion, when patients are correctly risk stratified, it seems that age at diagnosis would not be involved in the frequency of having a SIR at the initial evaluation or at the final follow-up. Therefore, it should not be included as an additional variable to consider in the RR classifications.
Disclosure Statement
Fabián Pitoia is a consultant for Genzyme-Sanofy and Bayer. The remaining authors declare that they have no conflicts of interest.
Acknowledgement
We thank Marta Alarcon Beretta for assistance with the statistical analysis.
References
- 1.Greene FL, Page DL, Fleming ID, editors. AJCC/UICC Cancer Staging Handbook: TNM Classification of Malignant Tumors. ed 7. New York: Springer; 2009. [Google Scholar]
- 2.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;6:1050–1058. [PubMed] [Google Scholar]
- 3.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 4.Shaha AR, Loree TR, Shah JP. Intermediate-risk group for differentiated carcinoma of the thyroid. Surgery. 1994;116:1036–1041. [PubMed] [Google Scholar]
- 5.Yang L, Shen W, Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol. 2013;31:468–474. doi: 10.1200/JCO.2012.42.4457. [DOI] [PubMed] [Google Scholar]
- 6.Wong RM, Bresee C, Braunstein GD. Comparison with published systems of a new staging system for papillary and follicular thyroid carcinoma. Thyroid. 2013;23:566–574. doi: 10.1089/thy.2012.0181. [DOI] [PubMed] [Google Scholar]
- 7.Oyer SL, Lentsch EJ. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg. 2012;147:221–226. doi: 10.1177/0194599812441587. [DOI] [PubMed] [Google Scholar]
- 8.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg. 2007;245:366–378. doi: 10.1097/01.sla.0000250445.92336.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid. 2015;25:125–132. doi: 10.1089/thy.2014.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. 2012;97:878–E887. doi: 10.1210/jc.2011-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitoia F, Califano I, Vázquez A, Faure E, Gauna A, Orlandi A, Vanelli A, Novelli JL, Mollerach A, Cabezón C, Fadel A, San Martín A, Figari M. Consenso intersocietario sobre tratamiento y seguimiento de pacientes con cáncer diferenciado de tiroides. Rev Argent Endocrinol Metab. 2014;51:85–118. [Google Scholar]
- 12.Hendrickson-Rebizant J, Sigvaldason H, Nason RW, Pathak KA. Identifying the most appropriate age threshold for TNM stage grouping of well-differentiated thyroid cancer. Eur J Surg Oncol. 2015;41:1028–1032. doi: 10.1016/j.ejso.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Shah JP. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol. 2016;23:410–415. doi: 10.1245/s10434-015-4762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D, Vaisman M, Carvalho A, Learoyd D, Leslie WD, Nason RW, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Pathak KA, Shen WT, Sywak M, Kowalski L, Freeman J, Perrier N, Shah JP. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016;26:373–380. doi: 10.1089/thy.2015.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W, European Thyroid Cancer Taskforce European consensus for the management of patients with differentiated thyroid cancer of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 17.Pitoia F, Ward L, Wohllk N, Friguglietti C, Tomimori E, Gauna A, Camargo R, Vaisman M, Harach R, Munizaga F, Corigliano S, Pretell E, Niepomniszcze H. Recommendations of the Latin American Thyroid Society on diagnosis and management of differentiated thyroid cancer. Arq Bras Endocrinol Metabol. 2009;53:884–887. doi: 10.1590/s0004-27302009000700014. [DOI] [PubMed] [Google Scholar]
- 18.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 20.Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165:441–446. doi: 10.1530/EJE-11-0466. [DOI] [PubMed] [Google Scholar]
- 21.Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, Vaisman M, Tuttle RM. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf) 2012;77:132–138. doi: 10.1111/j.1365-2265.2012.04342.x. [DOI] [PubMed] [Google Scholar]
- 22.Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. Outcomes of patients with differentiated thyroid cancer risk-stratified according to the American Thyroid Association and Latin American Thyroid Society risk of recurrence classification systems. Thyroid. 2013;23:1401–1407. doi: 10.1089/thy.2013.0011. [DOI] [PubMed] [Google Scholar]
- 23.Pitoia F, Jerkovich F, Urciuoli C, Schmidt A, Abelleira E, Bueno F, Cross G, Tuttle RM. Implementing the modified 2009 American Thyroid Association Risk stratification system in thyroid cancer patients with low and intermediate risk of recurrence. Thyroid. 2015;25:1235–1242. doi: 10.1089/thy.2015.0121. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Miyauchi A, Kihara M. Relationship between prognosis of papillary thyroid carcinoma patient and age: a retrospective single-institution study. Endocr J. 2012;59:399–405. doi: 10.1507/endocrj.ej12-0044. [DOI] [PubMed] [Google Scholar]
- 26.Sugino K, Kure Y, Iwasaki H, Ozaki O, Mimura T, Matsumoto A, Ito K. Metastases to the regional lymph nodes, lymph node recurrence, and distant metastases in nonadvanced papillary thyroid carcinoma. Surg Today. 1995;25:324–328. doi: 10.1007/BF00311254. [DOI] [PubMed] [Google Scholar]
- 27.Hollenbeak CS, Boltz MM, Schaefer EW, Saunders BD, Goldenberg D. Recurrence of differentiated thyroid cancer in the elderly. Eur J Endocrinol. 2013;168:549–556. doi: 10.1530/EJE-12-0848. [DOI] [PubMed] [Google Scholar]
- 28.Orlov S, Orlov D, Shaytzag M, Dowar M, Tabatabaie V, Dwek P, Yip J, Hu C, Freeman JL, Walfish PG. Influence of age and primary tumor size on the risk for residual/recurrent well-differentiated thyroid carcinoma. Head Neck. 2009;31:782–788. doi: 10.1002/hed.21020. [DOI] [PubMed] [Google Scholar]
- 29.Jukkola A, Bloigu R, Ebeling T, Salmela P, Blanco G. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocr Relat Cancer. 2004;11:571–579. doi: 10.1677/erc.1.00826. [DOI] [PubMed] [Google Scholar]
- 30.Tuttle RM. Optimal management of a biochemical incomplete response to therapy in differentiated thyroid cancer: aggressive treatment or cautious observation? Endocrine. 2014;46:363–364. doi: 10.1007/s12020-014-0213-2. [DOI] [PubMed] [Google Scholar]
- 31.Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, Vaisman M, Tuttle RM. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf) 2012;77:132–138. doi: 10.1111/j.1365-2265.2012.04342.x. [DOI] [PubMed] [Google Scholar]
- 32.Pitoia F, Abelleira E, Tala H, Bueno F, Urciuoli C, Cross G. Biochemical persistence in thyroid cancer: is there anything to worry about? Endocrine. 2014;46:532–537. doi: 10.1007/s12020-013-0097-6. [DOI] [PubMed] [Google Scholar]