Abstract
Background
Membrane αKlotho (hereinafter called Klotho) is highly expressed in the kidney and functions as a coreceptor of FGF receptors (FGFRs) to activate specific fibroblast growth factor 23 (FGF23) signal pathway. FGF23 is produced in bones and participates in the maintenance of mineral homeostasis. The extracellular domain of transmembrane Klotho can be cleaved by secretases and released into the circulation as soluble Klotho. Soluble Klotho does not only weakly activate FGFRs to transduce the FGF23 signaling pathway, but also functions as an enzyme and hormonal substance to play a variety of biological functions. FGF23 exerts its biological effects through activation of FGFRs in a Klotho-dependent manner. However, extremely high FGF23 can exert its pathological action in a Klotho-independent manner.
Summary
The decline in serum and urinary Klotho followed by a rise in serum FGF23 at an early stage of chronic kidney disease (CKD) functions as an early biomarker for kidney dysfunction and can also serve as a predictor for risk of cardiovascular disease (CVD) and mortality in both CKD patients and the general population. Moreover, Klotho deficiency is a pathogenic factor for CKD progression and CVD. FGF23 may also contribute to CVD. Prevention of Klotho decline, reactivation of endogenous Klotho production, or supplementation of exogenous Klotho attenuate renal fibrosis, retard CKD progression, improve mineral metabolism, ameliorate cardiomyopathy, and alleviate vascular calcification in CKD. However, the poor CVD outcome after depletion of FGF23 with FGF23 antibody stimulates the generation of a more specific inhibitor of FGF23 for CKD treatment.
Key Message
Klotho/FGF23 may not only be diagnostic and/or prognostic biomarkers for CKD and CVD, but are also pathogenic contributors to CKD progression and CVD development. The Klotho/FGF23 axis should be a novel target for renal clinics.
Keywords: Biomarker, Cardiovascular disease, Chronic kidney disease, FGF23, Klotho
Introduction
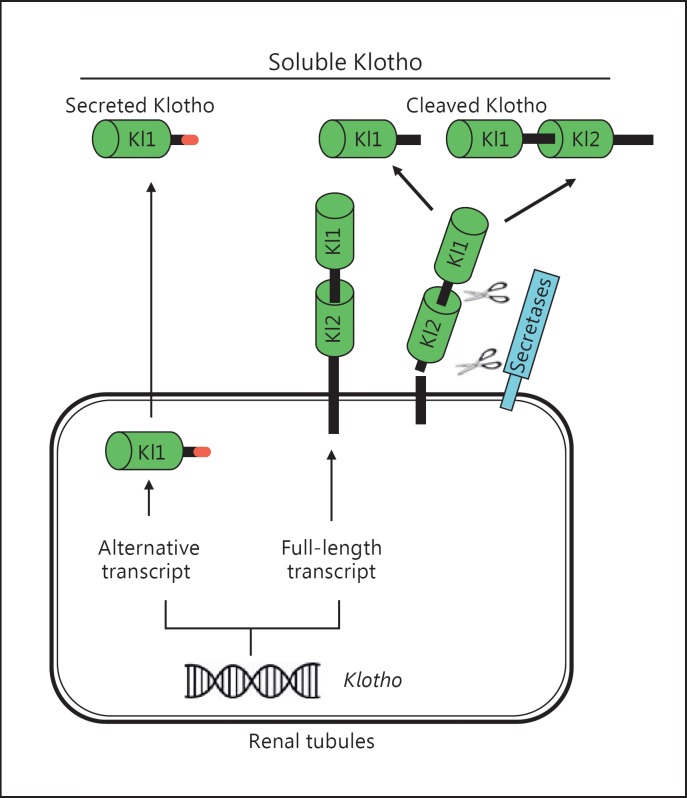
The Klotho gene was discovered in 1997 when mice with serendipitous silencing of this gene developed premature aging syndrome [1]. Recently, two other paralogs, βKlotho [2] and γKlotho [3], were identified, and Klotho was thereafter redesignated αKlotho [4] (hereinafter simply referred to as Klotho) to distinguish it from βKlotho and γKlotho. Klotho is highly expressed in the kidney, brain, and to a lesser extent in other organs [1]. The extracellular domain of membrane Klotho consisting of 2 repeat sequences (Kl1 and Kl2) can be shed by secretases to yield full fragment and Kl1 fragment of extracellular domain, respectively, and released into the circulation as cleaved Klotho [5,6]. Another form of Klotho protein encompassing the Kl1 fragment is generated by alternative transcript splicing and is called secreted Klotho [1,7]. Taken together with 2 cleaved Klotho fragments, these are collectively termed soluble Klotho (Fig. 1). Soluble Klotho, simply referred to as cleaved full fragment of Klotho protein, is the main functional form in the circulation [5], cerebrospinal fluid [8,9], and urine of mammals [10,11,12,13,14], and serves as the circulating substance exerting multiple biological actions on distant organs [15,16,17].
Fig. 1.
Source of soluble Klotho. The kidney is the main source of circulating Klotho under physiological conditions. Both renal proximal and distal tubules express membrane Klotho protein and also presumably produce secreted Klotho protein through alternative splicing. The secreted Klotho only contains a Kl1 domain and is directly secreted into the blood circulation (left panel); its biological function is not completely clear yet. Extracellular domain of membrane Klotho containing Kl1 and Kl2 repeats is shed and cleaved by secretases into either full extracellular domain or Kl1 repeat, and directly exerts its biological actions. Both cleaved Klotho fragments are in the blood circulation (right panel).
Fibroblast growth factor 23 (FGF23) was identified as a phosphaturic hormone in 2000 [18]; it is produced in the bone and controls mineral homeostasis by regulation of serum phosphate (Pi) [18,19], parathyroid hormone (PTH), and 1,25-(OH)2-vitamin D3 (1,25-(OH)2D3) [20]. Both Klotho and FGF23 interplay to participate in mineral homeostasis [21,22,23,24,25]. Soluble Klotho is dramatically decreased and FGF23 increased in chronic kidney disease (CKD) and end-stage renal disease (ESRD). They have also been proposed as sensitive biomarkers for adverse renal and extrarenal outcomes in patients with CKD and ESRD [25,26].
CKD is characterized by chronically progressive deterioration of renal function, which eventually progresses to ESRD. CKD is considered as a state of accelerated aging [27,28] with high risk for cardiovascular morbidity and mortality [29], and is the principal killer of CKD and ESRD patients. However, the mechanism underlying the progression from acute kidney injury (AKI) to CKD, ESRD, and cardiovascular disease (CVD) development is not completely illustrated. Aberrant Klotho/FGF23 axis is assumed to make a major contribution to them.
In this paper, we will discuss the recent literature on the Klotho/FGF23 axis in CKD/ESRD and then summarize preclinical data on Klotho supplementation for prevention of CKD progression and amelioration of CVD. Finally, we will discuss the potential effect of FGF23 on CKD. Hopefully Klotho/FGF23 will be a novel target for CKD.
Overview of the Klotho/FGF23 Axis
The Klotho gene encodes a single-pass transmembrane protein found in humans, mice, and rats. Klotho protein is expressed in multiple tissues but is found in particularly high levels in the kidney [1]. In the kidney, Klotho is prominently expressed in the distal convoluted tubules, but is also unequivocally found in the proximal convoluted tubule [13,30] and the inner medullary collecting duct-derived cell lines [31]. Under physiological conditions, the kidney makes a major contribution to the maintenance of soluble Klotho homeostasis through dual effects: (1) producing and releasing cleaved Klotho from membrane Klotho in the renal tubular epithelial cells into the circulation, and (2) clearing soluble Klotho from the blood into the urinary lumen through transcytosis to cross renal tubules [5]. Yet in CKD or ESRD, soluble Klotho is still detectable, although it is much lower than in healthy human beings [32,33,34], suggesting that there is a compensatory mechanism of production and release of Klotho into the circulation from extrarenal organ(s) or tissue(s). However, the site of this source is unclear.
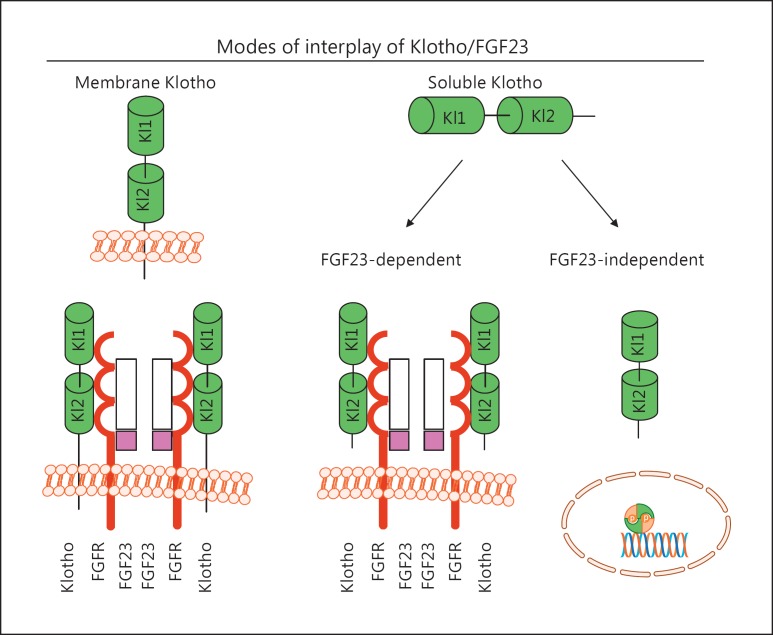
Unlike canonic FGF family members, the FGF19 subfamily consisting of 3 members - FGF19(15), FGF21, and FGF23 - has a unique structural feature: the lack of a heparin-binding domain that is preserved in all paracrine/autocrine FGFs impairs the capture in extracellular matrices and is believed to enable their endocrine release. Membrane Klotho functions as a coreceptor to enhance the binding of the endocrine FGF23 to FGF receptors (FGFRs) through the formation of a Klotho/FGFR/FGF23 complex, with subsequent activation of FGF signal transduction at their target organs (Fig. 2) [4,35,36].
Fig. 2.
Proposed modes of fibroblast growth factor 23 (FGF23) and Klotho action. Membrane Klotho (green) binds to the FGF receptor (FGFR) (brown), which in turn binds to FGF23 (white and pink) to form the 2FGF23/2FGFR2/Klotho complex. In this complex, Klotho functions as the receptor to replace the function of heparan sulfate to transduce the FGF23 signal. The potential FGFRs for FGF23 include FGFR1c, FGFR3c, and FGFR4 and serve as the high-affinity receptor for FGF23 (left panel). Soluble Klotho may form a similar complex and potentially prevent high FGF23-induced side (off-target) effects (middle panel). Soluble Klotho also exerts its multiple biological actions in an FGF23-independent manner via a yet-to-be-identified mechanism(s) (right panel).
FGF23 is produced mainly in osteocytes and osteoblasts by the regulation of serum Pi and iron, and mineral hormones including 1,25-(OH)2D3, PTH, and Klotho [4,37,38]. The FGF23 protein has 251 amino acids with a signal peptide of 24 amino acids [39]. The active FGF23 protein is proteolytically produced before or during the process of secretion between Arg179 and Ser180. Cleaved C-terminal peptides were shown to actively suppress FGF23 activity [35], but the biological function of N-terminal peptides remains to be illustrated. Since FGF23 was discovered, numerous studies have shown that excessive and deficient FGF23 results in hypo- and hyperphosphatemia, respectively. However, FGF23 was recently shown to be associated with CKD-mineral and bone disorder development and progression [40].
Two independent laboratories showed that Klotho is required to convert canonical FGFR into a specific receptor for FGF23 by functioning as an obligate coreceptor to form a constitutive binary complex with FGFR1c, FGFR3c, or FGFR4 to selectively increase the affinity of these FGFRs to FGF23 and not to other FGFs [41,42] (Fig. 2). Interestingly, soluble Klotho can also bind to FGF23/FGFR, but it prevents high FGF23-induced toxicity [43] (Fig. 2). Moreover, FGF23 can act independently of Klotho to induce pathogenic action in an off-target manner, particularly when present in supraphysiological concentrations [44,45,46], but the molecular mechanisms behind the direct effects of FGF23 remain to be elucidated.
Klotho/FGF23: A Novel Biomarker for CKD and CVD
Renal clinical practice requires highly sensitive and specific diagnostic and prognostic biomarkers for CKD. The early identification of CKD onset and risk stratification of CKD progression and/or CKD-related complications are essential for early treatment of CKD patients to ameliorate their comorbidity burden, particularly CVD, and prevent the development of ESRD [47,48]. Klotho and FGF23 might be future candidates as sensitive and specific markers for CKD and CVD.
Klotho Deficiency in CKD
Currently, CKD is considered as a pan state of Klotho deficiency and premature aging disease [14,27,49]. Low Klotho mRNA expression in the kidney was found in CKD patients and positively correlated with their estimated glomerular filtration rate (eGFR) [50,51,52]. Because of comparable change of levels of renal Klotho to those of serum and urinary Klotho, serum and urinary Klotho can be surrogate markers for renal Klotho [14]. It has been shown that serum Klotho levels start to decline in mild CKD patients (stages ≤2) and precede the elevation of serum FGF23, PTH, and Pi [49], suggesting that Klotho might be a sensitive biomarker for the decline of kidney function. Serum Klotho levels were progressively lower with more advanced CKD stages [53]. The adjusted decrease in serum Klotho was 3.2 pg/mL for each 1 mL/min/ 1.73 m2 decrease in eGFR [33]. In children under peritoneal dialysis, serum Klotho levels were also significantly lower than in healthy controls [54].
Urinary Klotho was even more sensitive because it was decreased in patients with CKD stage 1, and the magnitude of decrease in urinary Klotho was correlated with the severity of decline in eGFR in CKD patients [14]. Furthermore, eGFR significantly correlated with the amount of urinary excreted Klotho and serum Klotho levels. However, a stepwise multiple regression analysis identified eGFR to be a variable independently associated with only urinary excreted Klotho and not serum Klotho concentration, indicating that urinary Klotho might be more reliable than serum Klotho. A strong correlation between random urinary Klotho/creatinine ratio and 24-h urinary protein excretion suggested that urinary Klotho/creatinine is a surrogate for urinary Klotho excretion [10].
Growing results from animal experiments showed that Klotho deficiency causes vascular calcification, cardiac fibrosis, and cardiac hypertrophy in CKD [14,16,55]. A cross-sectional study of CKD patients with modest decline in renal function revealed that serum Klotho is independently associated with arterial stiffness measured by ankle-brachial pulse wave velocity [34]. In contrast, a larger cohort study of CKD stages 2-4 showed that plasma Klotho levels (highest versus lowest tertile) did not predict atherosclerotic or acute heart failure events or death after 2.6 years of follow-up [56]. Serum Klotho levels were significantly reduced in hypertensive patients with mild CKD when compared to healthy controls after adjustment for eGFR [57]. The role of Klotho as a predictor of adverse outcomes in CKD was confirmed in CKD patients (stages 1-5) after adjustment for cofounders. Among patients with low serum Klotho levels (≤396.3 pg/mL), 35.2% had poor and/or severe outcome (doubling serum creatinine, ESRD, or death), whereas only 15.7% of patients with high serum Klotho levels (>396.3 pg/mL) had adverse outcomes [33].
It is imperative to recognize that current Klotho clinical data are derived from small observational studies, despite being promising and somehow consistent, and still require subsequent extrinsic validation in a much bigger cohort. An additional difficulty of using Klotho as a biomarker is the lack of a standardized assay to measure soluble Klotho, often yielding inconsistent or even contradictory results [49,58]. The development of an assay that is accurate, reliable, and reproducible is mandatory to accelerate human studies in CKD. Furthermore, identification and characterization of circulating cleaved full-length Klotho, Kl1 or Kl2, and secreted Klotho may be biologically necessary to fully unveil the role of Klotho in kidney disease.
High FGF23 in CKD
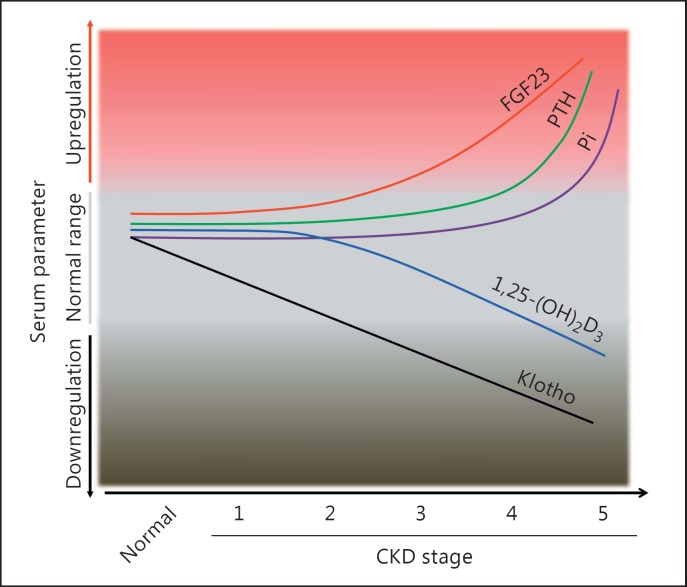
Currently the bulk of clinical observations and experimental animal studies show that FGF23 is a biomarker for CKD as it rises earlier than most other serum parameters, including serum creatinine, Pi, 1,25-(OH)2D3, and PTH, but may follow the reduction of Klotho in adult early-stage CKD [59] (Fig. 3). In mild CKD children (eGFR 30-90 mL/min/1.73 m2), a high plasma level of C-terminal fragment of FGF23 (cFGF23) is the earliest detectable abnormality [60]. Serum FGF23 can also be a predictor for poor outcomes in CKD as increased FGF23 is associated with fast progression to ESRD and increased cardiovascular morbidity and mortality. However, one population-based study analyzing alteration in the sequences of plasma intact fragment of FGF23 (iFGF23) and cFGF23 in the elderly (65 years or over) showed that iFGF23 increased significantly when eGFR was reduced to 51.6 mL/min/1.73 m2, while cFGF23 elevation occurred at an eGFR of 57.7 mL/min/1.73 m2[61], indicating that cFGF23 is more sensitive to detect eGFR decline in a non-CKD population. Circulating FGF23 is also independently associated with poor renal outcomes and mortality. After a median follow-up of 53 months, CKD patients with higher cFGF23 or iFGF23 at baseline had much faster progression to ESRD compared to those with low cFGF23 and iFGF23 [62]. Following that study, other epidemiologic studies confirmed that an elevated FGF23 level is an early biomarker of altered Pi metabolism in the initial stages of CKD and acts as a strong predictor of mortality in dialysis patients [61,63].
Fig. 3.
Proposed model and time profile of changes in serum Klotho, fibroblast growth factor 23 (FGF23), phosphate (Pi), and hormones relevant to mineral metabolism in relation to chronic kidney disease (CKD) progression. The decline in Klotho (black line) is an early event which is followed by other changes as CKD progresses. Low Klotho may induce FGF23 resistance causing a compensatory increase in blood FGF23 levels (red line) to maintain Pi homeostasis in CKD. The compensatory increase in FGF23 then suppresses 1,25-(OH)2-vitamin D3 (1,25-(OH)2D3) production (blue line). Low 1,25-(OH)2D3 and high blood Pi (purple line) due to progressive decline in renal function increase parathyroid hormone (PTH) (green line), which may contribute to high FGF23 in advanced CKD.
Patients with elevated FGF23 levels had higher aortic and coronary calcification scores than those with lower FGF23 levels [59,64]. Elevated levels of FGF23 were also shown to be clinically associated with endothelial dysfunction and arterial stiffness in CKD patients. In the non-CKD population, higher FGF23 is independently associated with an increased risk for major cardiovascular events [65]. Thus, an increase in FGF23 is an independent determinant for CVD in both kidney disease patients and the general population. An experimental study confirmed that preincubation of aortic rings with FGF23 increased superoxide levels, reduced NO levels, and abolished endothelium-dependent relaxation elicited by acetylcholine, which was prevented by the FGFR antagonist PD166866. Therefore, FGF23 can cause endothelial dysfunction and contribute to cardiovascular dysfunction [66]. Higher FGF23 is also associated with a greater risk of carotid atherosclerosis independent of CKD, and atherosclerosis may be a mechanism through which FGF23 increases cardiovascular events and stroke [67]. There is a close association between elevated FGF23 levels and increased mortality in the dialysis population [68] and in community participants over 65 years old [69]. Interestingly, these associations appear to be stronger in CKD patients [64].
Despite numerous epidemiologic studies showing a close relationship between FGF23 and the prevalence of cardiac events, including myocardial infarction, heart failure, and left ventricle hypertrophy, in both the CKD and the community population [70,71], there have been limited experimental studies revealing their causality. Faul and colleagues [44,72] showed that FGF23 may directly cause pathological cardiac remodeling in animal models and in isolated rat cardiomyocytes via FGFR- dependent activation of the calcineurin-NFAT signaling pathway, but this effect was independent of Klotho. Recently the same group further indicated that the pathogenic effect of FGF23 on the induction of cardiac hypertrophy might be mediated through upregulation of FGFR4 expression and activity [73].
FGF23 circulates as a full-length protein, and after proteolytic cleavage, a C-terminal fragment. FGF23 C-terminal enzyme-linked immunosorbent assay (ELISA) yields the sum of the C-terminal fragment and intact FGF23 [74,75], whereas FGF23 intact ELISA simply yields iFGF23, which constitutes the biological activity of FGF23 and is involved in pathophysiological processes in subjects with CKD and other phosphatemic disorders [74,76,77]. Animal studies have shown that an increase in cFGF23 by C-terminal ELISA may be due to the increase in C-terminal fragments acting as an acute-phase reactant under stressful conditions because iFGF23 was not elevated [78]. This concept in CKD patients is still uncertain and needs to be confirmed.
The Klotho/FGF23 Axis Is a Novel Target for CKD and CVD Treatment
Replacement of Klotho
The kidney is the major organ contributing to circulating Klotho [5,79]. Decreased Klotho production renders the kidney more susceptible to a variety of kidney insults [12,17], retards kidney recovery from AKI, promotes the AKI-to-CKD transition [80], and exacerbates uremic cardiomyopathy [16] and vascular calcification [14]. Conceivably, any therapy that restores Klotho levels through reactivation of endogenous Klotho or administration of exogenous Klotho might be a novel treatment strategy for CKD.
It has been shown that epigenetic processes such as methylation and acetylation in the Klotho gene promoter can modulate endogenous Klotho expression. In CKD, accumulated indoxyl sulfate or p-cresyl sulfate in the blood induces hypermethylation of the Klotho gene and decreased Klotho expression in CKD [81,82]. The hyperacetylation of histone in the Klotho promoter also inhibits Klotho expression [83]. TNF and TNF-like weak inducer of apoptosis-downregulated renal Klotho expression can be blunted by inhibition of histone deacetylase [84], indicating that cytokine-induced elevation of histone acetylation is associated with downregulation of Klotho expression. Other epigenetic mechanisms such as microRNA-mediated regulation also contribute to the downregulation of Klotho expression in diabetic nephropathy and CKD [85,86]. However, the therapeutic efficiency of epigenetic modulation in the upregulation of renal Klotho is worth confirming in kidney diseases.
To date, several categories of drugs in the market, including peroxisome proliferator-activated receptor-gamma agonists [87], angiotensin II-type I receptor antagonists [88], 3-hydroxy-3-methylglutaryl CoA reductase inhibitors (statin) [89], vitamin D-active derivatives [11,90], and intermedin [91], have been shown to upregulate Klotho expression in vivo and/or in vitro. Hence, the upregulation of Klotho doubtlessly has beneficial effects on target organs of experimental CKD models, although the detailed molecular mechanisms whereby Klotho is upregulated remain to be explored [11].
The alternative way to increase soluble Klotho is the administration of soluble Klotho protein, which is a more direct, safer, and more potent way to restore endocrine Klotho deficiency than reactivation of endogenous Klotho, particularly in advanced CKD or ESRD. Animal studies have already provided encouraging proof-of-concept data that bolus administration of soluble Klotho protein is safe and effective in protecting against kidney injury [12] and in inducting phosphaturia [13]. Furthermore, repeated administration of soluble Klotho protein attenuates AKI-to-CKD progression when it is given after ischemia-reperfusion [80] or after ureteral ligation [92]. Although intravenous delivery of a transgene encoding soluble Klotho was shown to ameliorate cardiac hypertrophy in CKD mice with Klotho-deficiency [55], the biosafety in clinical practice is of top concern.
FGF23 Antagonism
Elevated FGF23 is a strong predictor of adverse cardiovascular outcomes and mortality in CKD and ESRD. Theoretically, FGF23 might be a novel target for treatment of CKD and ESRD patients to attenuate cardiovascular events and reduce mortality. Traditionally, interventions to lower Pi uptake from the intestine or low-Pi diet were among the basic regimens to reduce cardiovascular risk and subsequently improve survival in CKD patients. Now they have been shown to lower serum FGF23 [93]. Sevelamer, a Pi binder, in combination with a Pi-restricted diet decreased FGF23 levels in mild and moderate CKD patients [94]. In advanced CKD, sevelamer carbonate treatment still reduced serum Pi, but not serum FGF23 [95]. When compared to calcium carbonate, lanthanum carbonate induced lower serum FGF23 and less urinary Pi excretion in CKD stage 4-5 patients [96]. In addition to Pi binder and restriction of Pi intake, dietary protein restriction also reduces serum FGF23 [97].
Shalhoub et al. [98] demonstrated that FGF23 antibodies successfully ameliorated the development and progression of most features of secondary hyperparathyroidism in a rat model of CKD, however at the expense of hyperphosphatemia, progressive vascular calcification, and death. Another beneficial effect of FGF23-neutralizing antibody was to attenuate bone disease and improve bone quality [99]. Such “negative” consequences following neutralizing antibody do not discourage one's effort to explore the clinical potential of FGF23, but rather they stimulate one to define FGF23's pathophysiological action and especially delineate the narrow window between adaptation and maladaptation of high FGF23 in the CKD scenario [100]. When one uses FGF23 antibody to deplete all the FGF23 in the circulation, i.e., creating an FGF23 knockout model, one should be aware that the phenotypes in FGF23 knockout mice will occur, such as vascular calcification and hyperphosphatemia [1,101]. Therefore, in CKD patients who have low Klotho levels, complete suppression of FGF23 by removing it from the circulation would worsen uremic symptoms. The best strategy for the management of high FGF23 is to maintain a low level of FGF23 instead of completely depleting it, and at the same time block FGF23 off-target action C-terminal fragment of FGF23 is a natural antagonist to FGF23 activity [35], and whether it will be a therapeutic agent for treatment of CVD in CKD is worthy of investigation.
High Pi, low serum vitamin D, and high FGF23 are negative regulators of Klotho expression in the kidney and circulating Klotho, as well as positive inducers of FGF23 production in the bone and circulating FGF23, as such exogenous soluble Klotho administration in conjunction with FGF23 antagonism, vitamin D supplementation, and Pi control could better prevent or ameliorate the burden of CKD or even restore mineral metabolism homeostasis and halt CKD-related complications.
Summary
CKD is a public health epidemic. An increased risk of death and CVD, including uremic cardiomyopathy and vascular calcification, was found in ESRD and even mild or moderate CKD patients. Klotho deficiency and FGF23 elevation are associated with poor outcomes and complications in CKD/ESRD patients and the general population. Recent advances in the understanding of Klotho and FGF23 biology definitely support the notion that aberrant function of the Klotho/FGF23 axis promotes CKD progression and CVD development. Klotho and FGF23 might be novel diagnostic targets for the early diagnosis of renal dysfunction and prediction of chronic complications, including CVD in CKD. In addition, they are also potential therapeutic targets for the treatment of CKD patients. Obviously, better understanding of the cellular and molecular mechanisms behind the effect of Klotho and FGF23 on CKD progression and CVD development will help in developing and implementing novel therapeutic strategies. Collaborative and translational research are crucial to enhance bench-to-bedside transition.
Conflict of Interest Statement
There are no potential conflicts of interest.
Acknowledgments
The research work in M.C. Hu's research laboratory is in part supported by the NIH (R01-DK091392, R01-DK092461, R01-DK092461-S1), the George M. O'Brien Kidney Research Center (P30-DK-07938), the Charles and Jane Pak Foundation, and the Pak Center Innovative Research Support. The authors thank Ms. Tamara Crowe for her secretarial assistance in preparing the manuscript.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Ito S, Kinoshita S, Shiraishi N, et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98:115–119. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 3.Ito S, Fujimori T, Hayashizaki Y, et al. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576:341–345. doi: 10.1016/s0167-4781(02)00281-6. [DOI] [PubMed] [Google Scholar]
- 4.Hu MC, Shiizaki K, Kuro-o M, et al. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu MC, Shi M, Zhang J, et al. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol. 2016;27:79–90. doi: 10.1681/ASN.2014101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CD, Tung TY, Liang J, et al. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry. 2014;53:5579–5587. doi: 10.1021/bi500409n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumura Y, Aizawa H, Shiraki-Iida T, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, Li H, Liang J, et al. The anti-aging and tumor suppressor protein klotho enhances differentiation of a human oligodendrocytic hybrid cell line. J Mol Neurosci. 2015;55:76–90. doi: 10.1007/s12031-014-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semba RD, Moghekar AR, Hu J, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer's disease. Neurosci Lett. 2014;558:37–40. doi: 10.1016/j.neulet.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akimoto T, Yoshizawa H, Watanabe Y, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012;13:155. doi: 10.1186/1471-2369-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau WL, Leaf EM, Hu MC, et al. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82:1261–1270. doi: 10.1038/ki.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33:118–129. doi: 10.1016/j.semnephrol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu MC, Shi M, Cho HJ, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26:1290–1302. doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panesso MC, Shi M, Cho HJ, et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. 2014;85:855–870. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 19.Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010;25:591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krajisnik T, Bjorklund P, Marsell R, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 21.Bian A, Xing C, Hu MC. Alpha Klotho and phosphate homeostasis. J Endocrinol Invest. 2014;37:1121–1126. doi: 10.1007/s40618-014-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol. 2013;9:650–660. doi: 10.1038/nrneph.2013.111. [DOI] [PubMed] [Google Scholar]
- 23.Myrvang H. Basic research: role of renal Klotho in mineral metabolism. Nat Rev Nephrol. 2012;8:553. doi: 10.1038/nrneph.2012.174. [DOI] [PubMed] [Google Scholar]
- 24.Huang CL, Moe OW. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 2011;462:185–193. doi: 10.1007/s00424-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez OM. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: updating the “trade-off” hypothesis. Clin J Am Soc Nephrol. 2010;5:1710–1716. doi: 10.2215/CJN.02640310. [DOI] [PubMed] [Google Scholar]
- 26.Scholze A, Liu Y, Pedersen L, et al. Soluble α-klotho and its relation to kidney function and fibroblast growth factor-23. J Clin Endocrinol Metab. 2014;99:E855–E861. doi: 10.1210/jc.2013-4171. [DOI] [PubMed] [Google Scholar]
- 27.Stenvinkel P, Larsson TE. Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis. 2013;62:339–351. doi: 10.1053/j.ajkd.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Kooman JP, Broers NJ, Usvyat L, et al. Out of control: accelerated aging in uremia. Nephrol Dial Transplant. 2013;28:48–54. doi: 10.1093/ndt/gfs451. [DOI] [PubMed] [Google Scholar]
- 29.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 30.Ide N, Olauson H, Sato T, et al. In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney Int. 2016;90:348–362. doi: 10.1016/j.kint.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Mitobe M, Yoshida T, Sugiura H, et al. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. 2005;101:e67–e74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 32.Fliser D, Seiler S, Heine GH, et al. Measurement of serum soluble Klotho levels in CKD 5D patients: useful tool or dispensable biomarker? Nephrol Dial Transplant. 2012;27:1702–1703. doi: 10.1093/ndt/gfs076. [DOI] [PubMed] [Google Scholar]
- 33.Kim HR, Nam BY, Kim DW, et al. Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis. 2013;61:899–909. doi: 10.1053/j.ajkd.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa M, Sugiyama H, Morinaga H, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goetz R, Nakada Y, Hu MC, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki M, Uehara Y, Motomura-Matsuzaka K, et al. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22:1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuyama R, Stockmans I, Torrekens S, et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116:3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrow EG, Yu X, Summers LJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA. 2011;108:E1146–E1155. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukagawa M, Kazama JJ. With or without the kidney: the role of FGF23 in CKD. Nephrol Dial Transplant. 2005;20:1295–1298. doi: 10.1093/ndt/gfh827. [DOI] [PubMed] [Google Scholar]
- 41.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 43.Six I, Okazaki H, Gross P, et al. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One. 2014;9:e93423. doi: 10.1371/journal.pone.0093423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grabner A, Faul C. The role of fibroblast growth factor 23 and Klotho in uremic cardiomyopathy. Curr Opin Nephrol Hypertens. 2016;25:314–324. doi: 10.1097/MNH.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Marco GS, Reuter S, Kentrup D, et al. Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrol Dial Transplant. 2014;29:2028–2035. doi: 10.1093/ndt/gfu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 48.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 49.Barker SL, Pastor J, Carranza D, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30:223–233. doi: 10.1093/ndt/gfu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 51.Asai O, Nakatani K, Tanaka T, et al. Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–547. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 52.Sakan H, Nakatani K, Asai O, et al. Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One. 2014;9:e86301. doi: 10.1371/journal.pone.0086301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavik I, Jaeger P, Ebner L, et al. Secreted Klotho and FGF23 in chronic kidney disease stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013;28:352–359. doi: 10.1093/ndt/gfs460. [DOI] [PubMed] [Google Scholar]
- 54.Cano FJ, Freundlich M, Ceballos ML, et al. Longitudinal FGF23 and Klotho axis characterization in children treated with chronic peritoneal dialysis. Clin Kidney J. 2014;7:457–463. doi: 10.1093/ckj/sfu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie J, Yoon J, An SW, et al. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015;26:1150–1160. doi: 10.1681/ASN.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seiler S, Rogacev KS, Roth HJ, et al. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin J Am Soc Nephrol. 2014;9:1049–1058. doi: 10.2215/CJN.07870713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park MY, Herrmann SM, Saad A, et al. Biomarkers of kidney injury and klotho in patients with atherosclerotic renovascular disease. Clin J Am Soc Nephrol. 2015;10:443–451. doi: 10.2215/CJN.07290714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devaraj S, Syed B, Chien A, et al. Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol. 2012;137:479–485. doi: 10.1309/AJCPGPMAF7SFRBO4. [DOI] [PubMed] [Google Scholar]
- 59.Desjardins L, Liabeuf S, Renard C, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23:2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 60.Portale AA, Wolf M, Juppner H, et al. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol. 2014;9:344–353. doi: 10.2215/CJN.05840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chudek J, Kocelak P, Owczarek A, et al. Fibroblast growth factor 23 (FGF23) and early chronic kidney disease in the elderly. Nephrol Dial Transplant. 2014;29:1757–1763. doi: 10.1093/ndt/gfu063. [DOI] [PubMed] [Google Scholar]
- 62.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 63.Chathoth S, Al-Mueilo S, Cyrus C, et al. Elevated fibroblast growth factor 23 concentration: prediction of mortality among chronic kidney disease patients. Cardiorenal Med. 2015;6:73–82. doi: 10.1159/000440984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, Yan J, Zhu M, et al. Fibroblast growth factor 23 predicts coronary calcification and poor prognosis in patients with chronic kidney disease stages 3-5D. Ann Clin Lab Sci. 2015;45:17–22. [PubMed] [Google Scholar]
- 65.Arnlov J, Carlsson AC, Sundstrom J, et al. Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin J Am Soc Nephrol. 2013;8:781–786. doi: 10.2215/CJN.09570912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silswal N, Touchberry CD, Daniel DR, et al. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab. 2014;307:E426–E436. doi: 10.1152/ajpendo.00264.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah NH, Dong C, Elkind MS, et al. Fibroblast growth factor 23 is associated with carotid plaque presence and area: the Northern Manhattan Study. Arterioscler Thromb Vasc Biol. 2015;35:2048–2053. doi: 10.1161/ATVBAHA.115.305945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarmento-Dias M, Santos-Araujo C, Poinhos R, et al. Fibroblast growth factor 23 is associated with left ventricular hypertrophy, not with uremic vasculopathy in peritoneal dialysis patients. Clin Nephrol. 2016;85:135–141. doi: 10.5414/CN108716. [DOI] [PubMed] [Google Scholar]
- 71.Mirza MA, Larsson A, Melhus H, et al. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Faul C. Fibroblast growth factor 23 and the heart. Curr Opin Nephrol Hypertens. 2012;21:369–375. doi: 10.1097/MNH.0b013e32835422c4. [DOI] [PubMed] [Google Scholar]
- 73.Grabner A, Amaral AP, Schramm K, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22:1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith ER. The use of fibroblast growth factor 23 testing in patients with kidney disease. Clin J Am Soc Nephrol. 2014;9:1283–1303. doi: 10.2215/CJN.10941013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith ER, Cai MM, McMahon LP, et al. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab. 2012;97:3357–3365. doi: 10.1210/jc.2012-1811. [DOI] [PubMed] [Google Scholar]
- 76.Amatschek S, Haller M, Oberbauer R. Renal phosphate handling in human - what can we learn from hereditary hypophosphataemias? Eur J Clin Invest. 2010;40:552–560. doi: 10.1111/j.1365-2362.2010.02286.x. [DOI] [PubMed] [Google Scholar]
- 77.Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goebel S, Lienau J, Rammoser U, et al. FGF23 is a putative marker for bone healing and regeneration. J Orthop Res. 2009;27:1141–1146. doi: 10.1002/jor.20857. [DOI] [PubMed] [Google Scholar]
- 79.Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25:2169–2175. doi: 10.1681/ASN.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi M, Flores B, Gillings N, et al. αKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol. 2016;27:2331–2345. doi: 10.1681/ASN.2015060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen J, Zhang X, Zhang H, et al. Elevated Klotho promoter methylation is associated with severity of chronic kidney disease. PLoS One. 2013;8:e79856. doi: 10.1371/journal.pone.0079856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young GH, Wu VC. KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 2012;81:611–612. doi: 10.1038/ki.2011.461. [DOI] [PubMed] [Google Scholar]
- 83.Rubinek T, Shulman M, Israeli S, et al. Epigenetic silencing of the tumor suppressor klotho in human breast cancer. Breast Cancer Res Treat. 2012;133:649–657. doi: 10.1007/s10549-011-1824-4. [DOI] [PubMed] [Google Scholar]
- 84.Moreno JA, Izquierdo MC, Sanchez-Nino MD, et al. The inflammatory cytokines TWEAK and TNFα reduce renal Klotho expression through NFκB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehi SJ, Maltare A, Abraham CR, et al. MicroRNA-339 and microRNA-556 regulate Klotho expression in vitro. Age (Dordr) 2014;36:141–149. doi: 10.1007/s11357-013-9555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang WL, Xu GS. Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci Rep. 2016;6:19979. doi: 10.1038/srep19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang HC, Deleuze S, Zuo Y, et al. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol. 2009;20:2380–2388. doi: 10.1681/ASN.2008111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoon HE, Ghee JY, Piao S, et al. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011;26:800–813. doi: 10.1093/ndt/gfq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Narumiya H, Sasaki S, Kuwahara N, et al. HMG-CoA reductase inhibitors up-regulate anti-aging klotho mRNA via RhoA inactivation in IMCD3 cells. Cardiovasc Res. 2004;64:331–336. doi: 10.1016/j.cardiores.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 90.Ritter CS, Zhang S, Delmez J, et al. Differential expression and regulation of Klotho by paricalcitol in the kidney, parathyroid, and aorta of uremic rats. Kidney Int. 2015;87:1141–1152. doi: 10.1038/ki.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu MC. Klotho connects intermedin1-53 to suppression of vascular calcification in chronic kidney disease. Kidney Int. 2016;89:534–537. doi: 10.1016/j.kint.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Isakova T, Ix JH, Sprague SM, et al. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol. 2015;26:2328–2339. doi: 10.1681/ASN.2015020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adema AY, de Jong MA, de Borst MH, et al. Phosphate binding therapy to lower serum fibroblast-growth-factor-23 concentrations in chronic kidney disease: rationale and study design of the Sevelamer on FGF23 Trial (SoFT) Nephron. 2016 doi: 10.1159/000448184. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spatz C, Roe K, Lehman E, et al. Effect of a non-calcium-based phosphate binder on fibroblast growth factor 23 in chronic kidney disease. Nephron Clin Pract. 2013;123:61–66. doi: 10.1159/000351811. [DOI] [PubMed] [Google Scholar]
- 96.Soriano S, Ojeda R, Rodriguez M, et al. The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol. 2013;80:17–22. doi: 10.5414/CN107764. [DOI] [PubMed] [Google Scholar]
- 97.Goto S, Nakai K, Kono K, et al. Dietary phosphorus restriction by a standard low-protein diet decreased serum fibroblast growth factor 23 levels in patients with early and advanced stage chronic kidney disease. Clin Exp Nephrol. 2014;18:925–931. doi: 10.1007/s10157-014-0947-4. [DOI] [PubMed] [Google Scholar]
- 98.Shalhoub V, Ward SC, Sun B, et al. Fibroblast growth factor 23 (FGF23) and alpha-Klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89:140–150. doi: 10.1007/s00223-011-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun N, Guo Y, Liu W, et al. FGF23 neutralization improves bone quality and osseointegration of titanium implants in chronic kidney disease mice. Sci Rep. 2015;5:8304. doi: 10.1038/srep08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ketteler M, Biggar PH, Liangos O. FGF23 antagonism: the thin line between adaptation and maladaptation in chronic kidney disease. Nephrol Dial Transplant. 2013;28:821–825. doi: 10.1093/ndt/gfs557. [DOI] [PubMed] [Google Scholar]
- 101.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]