Abstract
Tremelimumab and ipilimumab are monoclonal antibodies directed against the extracellular domain of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and have been used as immunotherapies against immune checkpoints that suppress T-cell activation. Anti-CTLA-4 antibody-based therapies have been shown to be effective in treating various cancers including metastatic melanoma. However, a few immune-related adverse events including hypophysitis and thyroid disorder have been reported, mostly developed within the first year of receiving treatment. We report a case of tremelimumab-induced Graves hyperthyroidism in a 55-year-old man who was diagnosed with metastatic melanoma after 8 years of tremelimumab therapy. He had no personal or family history of thyroid or autoimmune diseases. His biochemical profile was in keeping with Graves disease, with raised serum free thyroid hormones, suppressed thyroid-stimulating hormone concentration, and raised thyrotropin receptor antibody level. He was treated with carbimazole as part of the block and replace therapy, without complications. Tremelimumab therapy was temporarily discontinued and recommenced when he was rendered biochemically euthyroid. There has been no further relapse of Graves hyperthyroidism since the discontinuation of block and replace therapy. The mechanistic profile of anti-CTLA-4-induced thyroid dysfunction and the long-term endocrine safety of this therapeutic approach remain unclear. It is important to monitor thyroid functions in patients receiving anti-CTLA-4 therapies, as their effects on endocrine systems could be more latent or prolonged than the data from current clinical trials suggest. Antithyroid drug therapy was safe and effective alongside anti-CTLA-4 therapy without compromising antitumour treatment efficacy.
Keywords: Tremelimumab, Graves disease, Hyperthyroidism
What Is Known about This Topic?
Anti-CTLA-4 immunotherapy used in cancer treatment can cause thyroiditis and Graves disease.
What Does This Case Report Add?
Transient thyroiditis and Graves disease usually take place within the first 12 weeks following anti-CTLA-4 therapy.
In this case, Graves disease developed after 8 years of tremelimumab therapy for metastatic melanoma.
Antithyroid drug therapy was safe and effective alongside anti-CTLA-4 therapy without compromising antitumour treatment efficacy.
Introduction
Tremelimumab and ipilimumab are monoclonal antibodies directed against the extracellular domain of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). CTLA-4 exerts a suppressive effect on the immune response by acting as a negative co-stimulator. It engages non-MHC cell surface receptors on antigen-presenting cells (known as B7s) to give an inhibitory signal to T-lymphocyte activation [1]. It acts as an important immune checkpoint to prevent the breakdown of self-tolerance. However, it also regulates “tumour immunity” in malignancy via the induction of immune tolerance towards tumour-associated antigens [2, 3]. Hence, strategies that could enhance immune responses against tumour are useful for cancer therapy. Anti-CTLA-4 antibody-based therapies are being increasingly used to treat various malignancies, with a licence to treat metastatic melanoma, where they have been shown to increase overall survival and clinical remission in this disease [4, 5]. However, immune-related adverse events, including endocrinopathies, are common with these therapies [6, 7]. Hypophysitis is among the most common dose-dependent endocrine adverse event in anti-CTLA-4 therapies, followed by thyroid disorders.
This report describes a case of Graves hyperthyroidism after 8 years of tremelimumab therapy. We speculate a causal relationship between tremelimumab therapy and the development of Graves disease, and highlight the importance of full diagnostic workup of cases of thyrotoxicosis in patients treated with anticancer drugs.
Clinical Case
We report a case of a 55-year-old man who was diagnosed with metastatic melanoma on the skin overlying the right parotid gland 14 years ago. The primary lesion was surgically excised. However, he developed nodal relapse in 2005, 7 years after the initial diagnosis, for which he underwent radiotherapy. On subsequent development of further nodal disease and lung metastases, he received 8 cycles of chemotherapy: temozolomide and a poly(ADP-ribose) polymerase inhibitor, rucaparib, in a clinical trial setting. He achieved a partial response after 4 cycles, but the disease progressed after 8 cycles of treatment. He was then enrolled into the phase II trial of tremelimumab, an anti-CTLA-4 monoclonal antibody therapy, as second-line metastatic treatment. He completed 8 cycles of 3-monthly tremelimumab in 2 years and then, in view of the excellent disease response, continued with rollover clinical trial protocol to receive the treatment every 6 months on an ongoing basis, with no evidence of further relapse. Thyroid function was monitored 6 monthly.
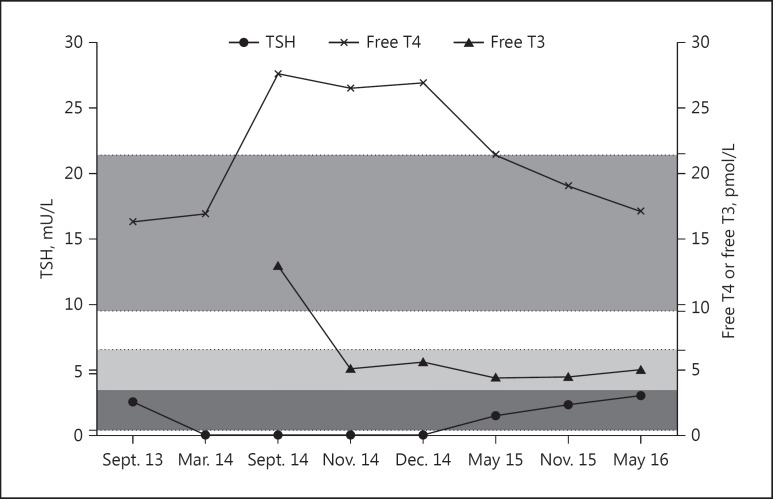
Following 8 years of tremelimumab therapy, the patient reported weight loss of 4 kg over a period of 6 months, despite having a good appetite. He had no past history of thyroid or other autoimmune diseases. There was also no family history of systemic or organ-specific autoimmune conditions. He has never smoked. On examination, he looked well, with warm hands and no tremor. He was in sinus rhythm with a pulse of 90 beats/min. Examination of the neck was difficult due to previous surgery and radiotherapy-related scarring; however, a large goitre was not palpable. There was no clinical evidence of Graves orbitopathy. Biochemical tests showed a serum thyroid-stimulating hormone (TSH) which was fully suppressed with a raised free T3 of 13.0 pmol/L (reference range 3.5–6.5) and free T4 of 27.6 pmol/L (reference range 9.5–21.5). Thyroid peroxidase antibodies were elevated at >600 kU/L (reference range 0–34), as was the thyrotropin receptor antibody level, measured at 5.0 IU/L (reference range1.0–1.8; BRAHMS Kryptor TRAK assay), consistent with Graves disease (Fig. 1). The patient was commenced on carbimazole 40 mg daily and, after 8 weeks, when his serum free T4 concentration normalised, levothyroxine at a dose of 125 μg daily was added. Tremelimumab treatment was initially suspended but recommenced when he was rendered biochemically euthyroid, 8 weeks following antithyroid drug treatment. The block and replace therapy for Graves disease was withdrawn after 12 months. He continues to receive 6-monthly tremelimumab treatment to date and has remained biochemically and clinically euthyroid since the discontinuation of antithyroid therapy 12 months ago.
Fig. 1.
The time course of free T4, free T3, and TSH profiles from 12 months prior to the diagnosis of Graves disease to 6 months after the discontinuation of block and replace therapy (last clinic review). Shaded horizontal areas represent the normal range for the following thyroid parameters: TSH (0.3–4.7 mU/L), free T4 (9.5–21.5 pmol/L), free T3 (3.5–6.5 pmol/L).
Discussion
Tremelimumab is a fully human anti-CTLA-4 non-complement-fixing monoclonal antibody (IgG2 isotype), which is being investigated as a treatment in prostate cancer [8], malignant mesothelioma [9], metastatic melanoma [10], and non-small cell lung cancer [11]. CTLA-4 is a type 1 glycoprotein expressed on the surface of T cells and serves as a co-inhibitory molecule in T-cell activation [12]. The mature protein of CTLA-4 is constitutively expressed in the CD4+CD25+ regulatory T (Treg) cells but is only found on the surface of activated T (CD4+ and CD8+) lymphocytes, B cells, monocytes, and dendritic cells [13 14 15]. It competes with the co-stimulatory molecule CD28 for the B7 ligands (CD80 and CD86) on antigen-presenting cells, leading to a reduction in B7-CD28 co-stimulatory signals and T-cell activation [13]. CTLA-4 induces Treg cell suppression and anergy by blocking the costimulatory signals in antigen-presenting cells and attenuates T-cell response (T-cell response hypo-signalling) via direct negative signalling through its cytoplasmic tail [16]. Although anti-CTLA-4 therapy has been proven to be effective in tumour rejection and in the reduction of tumour-mediated immune tolerance via enhanced T-cell activation, it has the potential effect of disrupting systemic immune regulation, particularly in the breakdown of self-tolerance. A spectrum of immune-related adverse events has been reported among patients receiving anti-CTLA-4 therapy, including colitis, arthritis, hepatitis, cutaneous reactions, uveitis, pancreatitis, and nephritis [6]. From an endocrine perspective, cases of lymphocytic hypophysitis, thyroiditis, and adrenalitis have been reported [6, 7].
A single nucleotide polymorphism CT60A/G (rs3087243) in the 3′ untranslated region of human CTLA-4 locus on chromosome 2q33 has been associated with multiple autoimmune diseases, including type 1 diabetes, Graves disease, rheumatoid arthritis, and celiac disease [17 18 19 20 21]. The CTLA-4 and the HLA locus together confer up to 50% of the inherited susceptibility to Graves disease in UK cohorts [22]. From clinical experience, thyroid dysfunction is commonly reported following anti-CTLA-4 therapy [7]. Ipilimumab has been shown to induce hypothyroidism/thyroiditis [23], euthyroid Graves ophthalmopathy [24], and Graves thyrotoxicosis [25]. In a single centre specialising in immune modulatory therapy, 6% of the 256 patients were found to have hypothyroidism/thyroiditis following ipilimumab therapy, with a male to female ratio of 2: 3 [26]. One patient treated with tremelimumab was reported to have developed asymptomatic thyroiditis followed by hypothyroidism, which required long-term thyroxine replacement therapy [27]. The previously reported cases of anti-CTLA-4-induced thyroid dysfunction occurred within a relatively short period following ipilimumab infusion, after 2–4 cycles or 6–12 weeks of treatment.
The data on autoimmunity toxicity remain sparse for tremelimumab as it is only used in clinical trials. Transient thyroiditis is commonly seen within the first year of tremelimumab treatment [28], and it is rather surprising to observe the “latent autoimmune toxicity” as in this case. However, much prolonged onset of autoimmune thyroid dysfunction has been observed with other immunotherapy, such as alemtuzumab, a human anti-CD52 monoclonal antibody therapy, with onset ranging from 6 to 61 months after the first course of treatment [29, 30]. One might suggest that our case was an incidental finding of isolated Graves disease rather than CTLA-4-induced thyroid disease. However, the absence of personal or family history of autoimmune diseases renders strong autoimmune predisposition less likely. The mechanistic profile of anti-CTLA-4-induced thyroid dysfunction and the long-term endocrine safety of this therapeutic approach remain unclear and should be explored further.
It is difficult to predict when and who will develop anti-CTLA-4-induced Graves disease, but endocrine adverse effects may be delayed. Hence, it is important to monitor thyroid function regularly (at least 6 monthly) while patients are on an immunomodulatory agent, preferably with baseline thyroid function and thyroid autoantibodies prior to commencing anti-CTLA-4 therapy. Although the majority of dysthyroid episodes may be due to thyroiditis with a self-limiting clinical course, Graves disease can also occur. A full diagnostic workup by an endocrinologist aiming to characterise the aetiology of thyrotoxicosis is paramount, as management will be dictated by a precise diagnosis.
To conclude, this report illustrates a case of Graves hyperthyroidism following prolonged anti-CTLA-4 therapy, and highlights the importance of thyroid function monitoring and of making a precise diagnosis of the cause of thyrotoxicosis. Antithyroid drug therapy was safe and effective alongside anti-CTLA4 therapy without compromising anti-tumour treatment efficacy.
Disclosure Statement
We declare that all authors have no competing interests.
References
- 1.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 2.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119:254–264. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richrads J, Maio M, Hauschild A, Miller W, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok J. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 6.Cousin S, Italiano A. Molecular pathways: immune checkpoint antibodies and their toxicities. Clin Cancer Res. 2016;22:4550–4555. doi: 10.1158/1078-0432.CCR-15-2569. [DOI] [PubMed] [Google Scholar]
- 7.Torino F, Corsello SM, Salvatori R. Endocrinological side-effects of immune checkpoint inihibitors. Curr Opin Oncol. 2016;28:278–287. doi: 10.1097/CCO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 8.McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, Liu G. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61:1137–1147. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L, Maio M. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 10.Millward M, Underhill C, Lobb S, McBurnie J, Meech SJ, Gomez-Navarro J, Marshall MA, Huang B, Mather CB. Phase I study of tremelimumab (CP-675 206) plus PF-3512676 (CPG 7909) in patients with melanoma or advanced solid tumours. Br J Cancer. 2013;108:1998–2004. doi: 10.1038/bjc.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatloukal P, Heo DS, Park K, Kang J, Butts C, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2009;27:8071. [Google Scholar]
- 12.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily – CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 13.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Carrega P, Saverino D, Piccioli P, Camoriano M, Morabito A, Dozin B, Fontana V, Simone R, Mortara L, Mingari MC, Ferlazzo G, Pistillo MP. CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum Immunol. 2010;71:934–941. doi: 10.1016/j.humimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 18.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–2777. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhernakova A, Stahl EA, Trynka G, Raychaudhuri S, Festen EA, Franke L, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011;7:e1002004. doi: 10.1371/journal.pgen.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furugaki K, Shirasawa S, Ishikawa N, Ito K, Ito K, Kubota S, Kuma K, Tamai H, Akamizu T, Hiratani H, Tanaka M, Sasazuki T. Association of the T-cell regulatory gene CTLA4 with Graves' disease and autoimmune thyroid disease in the Japanese. J Hum Genet. 2004;49:166–168. doi: 10.1007/s10038-003-0120-5. [DOI] [PubMed] [Google Scholar]
- 21.de Jong VM, Zaldumbide A, van der Slik AR, Laban S, Koeleman BPC, Roep BO. Variation in the CTLA4 3′UTR has phenotypic consequences for autoreactive T cells and associates with genetic risk for type 1 diabetes. Genes Immun. 2016;17:75–78. doi: 10.1038/gene.2015.51. [DOI] [PubMed] [Google Scholar]
- 22.Vaidya B, Imrie H, Perros P, Young ET, Kelly WF, Carr D, Large DM, Toft AD, McCarthy MI, Kendall-Taylor P, Pearce SH. The cytotoxic T lymphocyte antigen-4 is a major Graves' disease locus. Hum Mol Genet. 1999;8:1195–1199. doi: 10.1093/hmg/8.7.1195. [DOI] [PubMed] [Google Scholar]
- 23.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164:303–307. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McElnea E, Ní Mhéalóid A, Moran S, Kelly R, Fulcher T. Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit. 2014;33:424–427. doi: 10.3109/01676830.2014.949792. [DOI] [PubMed] [Google Scholar]
- 25.Azmat U, Liebner D, Joehlin-Price A, Agrawal A, Nabhan F. Treatment of ipilimumab induced Graves' disease in a patient with metastatic melanoma. Case Rep Endocrinol. 2016;2016:2087525. doi: 10.1155/2016/2087525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryder M, Callahan M, Postow MA, Wolchol J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti- cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, Palmer J, Margolin DH, Hollenstein J. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J Clin Endocrinol Metab. 2014;99:80–89. doi: 10.1210/jc.2013-2201. [DOI] [PubMed] [Google Scholar]
- 30.Tuohy O, Costelloe L, Hill-Cawthorne G, Bjornson I, Harding K, Robertson N, May K, Button T, Azzopardi L, Kousin-Ezewu O, Fahey MT, Jones J, Compston DA, Coles A. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry. 2015;86:208–215. doi: 10.1136/jnnp-2014-307721. [DOI] [PubMed] [Google Scholar]