Abstract
Finding novel and effective antibiotics for treatment of Legionella disease is a challenging field. Treatment with antibiotics usually cures Legionella infection; however, if the resultant disease is not timely recognized and treated properly, it leads to poor prognosis and high case fatality rate. Legionella pneumophila DrrA protein (Defects in Rab1 recruitment protein A)/also known as SidM affects host cell vesicular trafficking through modification of the activity of cellular small guanosine triphosphatase )GTPase( Rab (Ras-related in brain) function which facilitates intracellular bacterial replication within a supporter vacuole. Also, Legionella pneumophila LepA and LepB (Legionella effector protein A and B) proteins suppress host-cell Rab1 protein’s function resulting in the cell lysis and release of bacteria that subsequently infect neighbour cells. Legionella readily develops resistant to antibiotics and, therefore, new drugs with different modes of action and therapeutic strategic approaches are urgently required among antimicrobial drug therapies;gene therapy is a novel approach for Legionnaires disease treatment. On the contrary to the conventional treatment approaches that target bacterial proteins, new treatment interventions target DNA (Deoxyribonucleic acid), RNA (Ribonucleic acid) species, and different protein families or macromolecular complexes of these components. The above approaches can overcome the problems in therapy of Legionella infections caused by antibiotics resistance pathogens. Targeting Legionella genes involved in manipulating cellular vesicular trafficking using a dendrimer-mediated antisense therapy is a promising approach to inhibit bacterial replication within the target cells.
Keywords: Legionella pneumophila, DrrA (SidM), LepA and LepB, Intracellular replication, Rab, Vesicular trafficking
Introduction
Legionnaires' disease is a serious form of pneumonia and lung inflammation, which is caused by intracellular bacterium Legionella. Although early therapeutic intervention using antibiotics usually cures Legionnaires’ disease, some patients experience complications that could lead to death.1 Legionella rapidly develops resistance to commonly used antibacterial agents.2 Therefore, there is an urgent demand for discovery of new antibacterial targets to overcome the resistance problem. Bacterial pathogens deliver effector proteins which interfere with host cell physiological functions and hijack their target cell machinery leading to specific clinical symptoms.3,4 To escape degradation by its host cells, a Legionella-containing vacuole (LCV) is formed and protects the bacterium from cell immune defense, possibly through secretion of bacterial proteins into the host cytosol.5 Therefore, specific antibiotics with high levels of permeability are required to pass cell membrane barrier and reach the bacterium within the cells.6 This review describes different gene therapy approaches including antisense therapy mediated by dendrimers to target and eliminate or disarm pathogen, novel method for specific targeting of effective types of antibiotics to intracellular L. pneumophila (Legionella pneumophila). We also describe antisense therapy for L. pneumophila treatment targeting bacterial protein synthesis aiming to disturb host trafficking pathway through interference with phagosome and lysosome fusion in macrophages, therefore targeting bacteria in the cytoplasm by different methods such as RNA interference type would be an alternative option to prevent bacterial growth and prevent the clinical symptoms.
Legionella pneumophila a medically important intracellular pathogen
The most recent major outbreak of L. pneumophila, or Legionnaires' disease happened in Portugal in 2014, and was referred to one of the biggest in European history. If this relatively rare infection is not timely recognized and properly treated, it can have poor prognosis and present a high case fatality rate.7
L. pneumophila is a gram-negative intracellular bacterium8 that causes Legionnairesis in humans. The natural host of L. pneumophila is unicellular protozoa and it infects human alveolar macrophages.9,10 Despite progress in the antimicrobial treatment, pneumonia is still one of the important infectious diseases, causing death in the developed countries.11-13 L. pneumophila co-infection with influenza could lead to influenza infection with possibly lethal prognosis.14
Following inhalation, L. pneumophila infects and replicates in alveolar macrophages, which contribute to inflammation and progress of the disease. L .pneumophila, with ability to deliver above 300 proteins to the host cell through its Type IVB, Icm/Dot (the intracellular multiplication/defective organelle trafficking) translocation system conserves the major recognized set of translocated substrates between all bacterial pathogens.15 Inside host cells, L. pneumophila avoids phagosome-lysosome fusion and influences host cell procedures to form a particular phagosome which is proper for intracellular replication.3,8,16 The Icm/Dot system is used by the bacterium to translocate its effectors.4,15
Legionella pathogenesis
The lungs are the main site of infection, where bacteria grow inside the lung macrophages.16-19 In the extra pulmonary forms of disease, organs such as heart, CNS, liver and intestines are involved and heart is the most common organ involved in the hospitalized patient. Recipients of organ transplant and patients with diabetes mellitus and chronic lung disease as well as aged people and cigarette smokers are suitable candidate for this disease.20
L. pneumophila Life Cycle
Intracellular pathogens use different mechanisms to manipulate the host cell system for intracellular replication. For example, L. pneumophila as an intracellular bacterial pathogen hijacks host vesicle trafficking pathway to stop phagosome and lysosome fusion inside the cell.4,21-23 Lysosomes are intracellular organelles with acidic pH in eukaryotic cells containing hydrolytic enzymes for digesting cellular waste products, bacteria and viruses. Normally when bacteria enter to cell by phagocytes, they are killed in lysosomes through digesting by the lysosomal enzymes.23
Host cells use the defense mechanism for limiting the intracellular infection.23 Following cell infection by L .pneumophila, some immune system cells such as macrophages surround the bacteria but bacteria manipulate host cells using membrane trafficking pathway. Inside the macrophages, pathogen utilizes the host cell proteins mediating intracellular trafficking pathway. This forms an organelle termed L. pneumophila - containing vacuole (LCV) which supports bacteria replication (Figure 1).9,16,21,23,24
Figure 1.
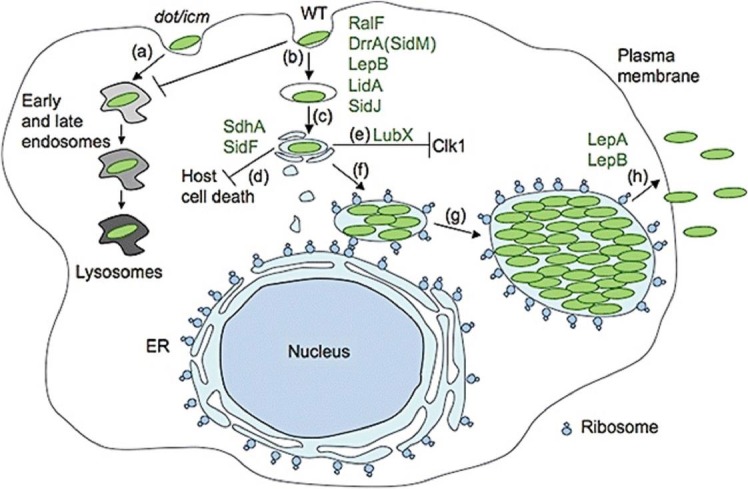
The replication of Legionella pneumophila inside the cell (Figure adapted from 23)
Wild-type and mutant form of Legionella use different ways for trafficking pathways(a) dot/icm mutants and trafficking pathway, (b) Wild-type of Legionella Pneumophila, the LCV formed and avoids endosomal fusion, (c) The Dot/Icm different effectors, (d) Host cell is prevented from death by the Dot/Icm effectors SdhA and Sid F, (e) the Dot/Icm effecter LubX effect the host cell factor Clk1, (f) the LCV was surrounded with Ribosomes, (g) Several cycle of Legionella Pneumophila replication,(h) Legionella Pneumophila Dot/Icm effectors LepA and B cause to Legionella pneumophila infect the other neighbour host cell Dot/Icm-translocated effectors is shown in green.23
Type IV secretion system of L. pneumophila which is encoded by the Icm/Dot genes enables bacteria to transfer its proteins into host cytosol.25-27 A number of different translocated substrates of Icm/Dot have been identified with similar functions to eukaryotic host cell proteins involving in vesicle trafficking pathway (Figure 1).9,23,27-31
Rab GTPase proteins in eukaryotic cells act as molecular switches and are important in cellular trafficking pathway. Following pathogen phagocytosis or endocytosis, host cell Rab GTPase proteins are essential for intracellular transportation. Bacterial effectors hijack Rab proteins at the molecular level act to escape degradation, be carried directly to specific intracellular locations, and control host vesicles carrying molecules requiring for a stable niche and/or bacterial development and differentiation.4,32,33
Development of antibacterial resistance by Legionella
The discovery and therapeutic use of antibiotics in the 1950s have certainly contributed to the one of the ultimate profits to human; however, because of the short life cycle and capacity to adjust rapidly to variations in the environmental condition, pathogenic bacteria continue to persevere by regularly overcoming the effect of drugs used to eliminate them. The growing drug resistance was the first problem resulting from the extensive, uncontrolled and inappropriate use of antibiotics. In spite of the entrance of new antibiotic into the market, drug resistance is detected in years or even months. At present, more than 70% of pathogenic bacteria are resistant to most antibiotics existing on the market and the mortality of some multi-resistant infections has extended to 50 - 80% and also the mortality rate as a result of bacterial infections is above 2 millions per year, worldwide.34,35 Furthermore, nowadays, some environmental bacterial pathogens such as Legionella spp. as a result of artificial ecosystems are a main problematic issue in industrialized countries, associated with Other factors such as modern medications and lifestyles have been caused an increased incidence of unintended pathogens in the form of emerging pathogens.36
According to the different approaches especially bacterial resistance and action of antibacterial medications presently used, different targets such as cellular structures, the cell wall biosynthesis, protein biosynthesis, DNA, different RNA families, biosynthetic pathway, new protein families or macromolecular complexes of these components have been suggested by commercial antibacterial companies and scientists.35,37 Therefore, targeting specific bacteria such as L. pneumophila which may present as Legionnaires’ diseases and cause case fatality rate of about 10% and even mortality rate higher than 25% in immune suppressed and nosocomial patients11 requires to be paid more attention.
Targeting Legionella proteins by antibacterial
Finding new and effective antibiotics is a challenging research area driven by novel approaches required to tackle unconventional targets. Intracellular grown Legionella is extremely resistant to antibiotics.38,39 Although combination antibiotic therapy might be a choice in some conditions, it is not recommended for all patients and is a controversial and challenging issue.40L. pneumophila is able to manipulate vesicular trafficking by modification the activity of the small GTPase Rab1. L .pneumophila manipulates Rab1 function using some of its associate proteins such as DrrA (SidM). DrrA (SidM) has both guanine nucleotide exchange activity in Rab1 GDF (GDI dissociation factor) and Rab1 GEF (guanine nucleotide exchange factor).19,41-43 Also there is another protein of L. pneumophila named LepB which manipulate and inactivate host-cell Rab1 protein’s function.33,41 Following growing inside the LCV, a bacterium lyses the host cell and release to infect the neighbour cells. Two effectors, LepA and LepB, which show a role in the non-lytic release of Legionella from protozoa, are translocated by the Icm/Dot TFSS (Type Four Secretion System).42,44 Reduction of the Lep proteins through deletion of their genes contributes to better ability to lyse red blood cells. In contrast, overexpression of Lep-containing hybrid proteins seems to exactly block the activity of the Icm/Dot TFSS and may stop the transfer of other effectors which are critical for intracellular multiplication.33,42 Therefore, Legionella’s effectors which hijack host protein to escape degradation and replicate intracellularly could be targeted in antibacterial treatment. In addition, the LepA and the LepB proteins in Legionella are the other targets to induce infection the neighbour cells in host cells.
Gene therapy approach for treatment of Legionella infection
Antibiotic resistance is a health threat, worldwide. In spite of good progresses in genome sequencing and genetic manipulation tools, there are still problems to be used for effective therapeutic aims.45 Gene therapy is a technique which causes insertion, silencing or alteration of genes in a patient's cells to treat or prevent disease.46 RNA regulators are developed to overcome various restrictions of protein regulators such as simple structures and mechanisms causing their behaviour in different conditions anticipated with software tools. Also they propagate signals directly and fast as RNAs.47 The remodeling of RNA and DNA molecules with the aim of engineering antibiotic bunches to cause antibiotic overexpression is possible.48 Recently, combination of CRISPR (Clusters regularly interspaced short palindromic repeats) and antisense RNA system in order to control bacterial gene expression is introduced.47 Through blocking genes that manipulate and inactivate host-cell Rab1 protein’s function, Legionella can’t form LCV to support bacteria inside LCV for replication.
CRISPR-Cas system: a novel tool for treatment of Legionellosis
Not only CRISPR-Cas component is important in the natural history and pathogenesis of Legionnaires’ disease, but also L. pneumophila Cas2 has a role that is unique from the main view of CRISPR-Cas function.49 CRISPR , which were first discovered in bacteria in 1980s and then in archaea in 1990s, are a powerful genome editing tool. CRISPR function to facilitate adaptation of the organism to extreme environmental conditions and act as a part of bacterial immune system to defend against pathogens and harmful environment.50,51 CRISPR/Cas systems are powerful and efficient genome modifying tool in comparison to other genome modifying tools like zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). The system includes a nuclease (Cas) and small guide RNA that recruits the Cas to cut at a specific place in the genome. This system is able to induce targeted specific gene deletion, correction or mutation via RNA guided DNA cleavage. Short palindromic repeat 36 base pair (bp) lengths in the genome associated with the (Cas) gene carry out targeted editing in the proposed genome. It works by binding a RNA stem-loop structure when attached to a short target sequence (22-33bp) to guide the Cas protein to a specific spot in the genome. These adaptable sequences together with non-contiguous direct repeat attached to Cas gene forming the CRISPR Cas systems.52-57
CRISPR type II, based on Cas9 is the primary system used to genetically modify mammalian cells. Cas9 function in CRISPR system is central part of the tool and is guided to the target sequence by a trans activating crRNA (tracrRNA) to cleave target DNA – Cas9 cleaves supercoiled, relaxed and linear DNA – cleavage occurs 3bp upstream of Pam motif.54 This type of gene editing technology has been independently described by several groups and is termed RNA guided engineered nucleases (RGENs).58
CRISPR technology has some benefits over early methods of gene editing technology and is rapidly expanding in the area of genetic and biology research. It is a relatively rapid and cost effective genome editing technology that can be used to modify the genome in different organisms and various cell types. CRISPR Cas system target the bacterial lipoproteins transcript through dual RNA protein complexes (Figure 2).59
Figure 2.
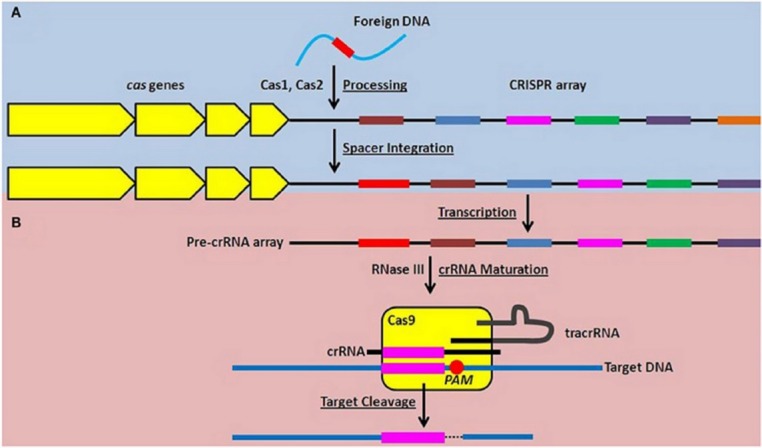
The Type II CRISPR-Cas system function in adaptive nucleic acid restriction (Figure adapted from59)
(A) Invading DNA is located by Cas1 and Cas2 and take it as new spacer sequence inside CRISPR array (immune completion blue). (B) To stop invading DNA, the pro-crRNA in constructed and matured into small targeting crRNAs to associate with Cas9 and the sequence of spacer within the crRNA can hybridize the matching DNA. Cas9 then cleavages target DNA just 3bp downstream of PAM site and generates double stranded break on target DNA.59
Antisense therapy of Legionellosis
Gene expression could be down regulated by RNA interference and antisense oligonucleotides (AS-ODNs) through inducing enzyme-dependent degradation of targeted mRNA.60 For treatment of different gene-specific diseases, oligonucleotide-based cure is a novel area of medicine to design new drugs. The antisense oligonucleotides and short interfering RNAs (siRNAs) are the more common forms, which often act against similar targets.61 Using antisense and other gene silencing technologies provide an efficient alternative way of treatment for cancer, genetic disorders or infection.62,63 The gene expression through changing mRNA splicing, arresting mRNA translation and inducing degradation of targeted mRNA are blocked through sequence-specific antisense oligonucleotides by RNase H.64 Antisense therapy is also convenient way of genetic alteration compare to more difficult methods such as generating gene knockout in cells and organisms. Antisense ODNs have been applied to efficiently block gene expression in eukaryotic cells and there is one antisense ODN-based product in the market and others in clinical trials.65,66 This gene therapy method is common for silencing the abnormal gene to stop the human disease such as cancer progression67-69 and neurodegenerative disorders70 as well as pneumonia.71
Antisense transcription was first revealed in bacteria about 50 years ago. The significant amount of antisense transcription is an important feature of gene expression in eukaryotes. This technique mostly uses DNA or RNA to inactivate circular segment of bacterial genome resembling RNA interference and prevents duplication of bacterial cells or kills them;62,71 however, antisense technology is not applied widely in prokaryotic systems. Gene regulations in prokaryotic cells have been done by antisense mechanisms of bacteria and also through increased antibiotic efficacy.35,45,62,72 Former reports indicated short antisense and modified antisense oligodeoxynucleotide (AODNs) could inhibit gene expression in bacteria.45,72,73 Others stated that gene expression in bacteria can be inhibited by peptide nucleic acid (PNA).71,74 The transcriptomes of bacteria such as Escherichia coli, Synechocystis sp. strain PCC6803, Helicobacter pylori, Bacillus subtilis, Mycoplasma pneumoniae, Sinorhizobiummeliloti, Vibrio cholerae, Chlamydia trachomatis, Pseudomonas syringae, and Staphylococcus aureus have been stated to have antisense RNA (asRNA) transcripts.35 The bacterial protein YidC is extremely preserved among pathogens and is necessary for membrane protein attachment and decrease of YidC production contributing to bacterial growth retardation. Therefore, it can be a novel potential target for therapeutic applications. Antisense RNA-mediated YidC down-expression in E. Colistrain resulted in identify antibacterial essential oils eugenol and carvacrol.75 The influence of the antisense oligomer is extremely particular to the targeted gene’s sequence, which is preserved in numerous bacterial types, and it does not have any noticeable toxicity against human cells.45 The combined CRISPR and asRNA system, can be applied to reversibly repress or derepress multiple target genes concurrently, permitting for rational reprogramming of cellular functions. Gene target are repressed/derepressed by CRISPR system from Streptococcus pyogenes and synthetic antisense RNAs (asRNAs) in Escherichia coli strains. In fact, when the CRISPR system represses the target gene, it can be derepressed by expressing asRNA which hide away a small guide RNA (sgRNA). In addition, up to 95% of derepression can be attained through designing as RNAs which target various regions of a sgRNA and by changing the hybridization free energy of the sgRNA–asRNA complex.47 RNA interference has been suggested to be an alternative method to prevent bacterial growth or demolish them. For this aim we suggest antisense therapy against type IV secretion system and Lep proteins synthesis in Legionella.
Dendrimers-based antisense delivery system approaches for treatment of Legionellosis
The important therapeutic aim in biotechnology is the capacity to safely and professionally transfer external DNA, RNA, antisense or drug76 into cells.63,77 The capability to deliver fragments of DNA or RNA to the required section of a cell is a challenging issue. Substrate-mediated transfection, which withstands the release of knocked DNA or vector/DNA complexes and provides cell growth, has been established to solve the problems associated with the extracellular obstacles in gene delivery system.78 Rapid transfection which can achieve by viral carrier, immunological and oncologic side effects connected to these vectors have remained as controversial issues. Nowadays, non-viral gene delivery system to transfer genetic material to targeted cells such as natural/synthetic molecules or physical forces are preferred methods. They have some benefits such as targeting capability, simplicity of fabrication, possibility for repeat administration and low immune rejection.79 There are different dendrimers such as peptide, and glycopeptide, has capability to bind bacterial polysaccharides representing interesting tools for both therapeutics and diagnostics applications. Nowadays, because of higher bacterial resistance for common antimicrobial drugs, discovery of new antibacterial medications and diagnostic tools are very important .80 It has been reported that acid-triethylene glycol (GATG) dendrimers is valuable and versatile platform to develop a novel antimicrobial materials targeting microbial viability and/or virulence.81,82 The anti-bacterial sequences (ABS) can be integrated into plasmids, viral, and other vectors and packaged in liposomes or cationic polymers such as polyethylenimine (PEL) to prevent or reduce the likelihood of infections leading to sepsis.65 Dendrimers as non-viral gene delivery tools which can be utilized to deliver sequence of DNA or RNA as oligonucleotides to the certain part of cells are challenging experiments and novel method.83,84 Dendrimers as Nano-sized synthetic polymers have positive charge with distinct, homogeneous, and monodisperse organization containing tree-like arms or branches.85 Dendrimers are suitable and safe for the successful application in biomedicine such as imaging, drug delivery, gene delivery and photodynamic therapy.86 The highest benefits are the progress in the antifungal properties and antibacterial action, for example decrease in toxicity, bioavailability, and target tissue which simplifies advanced therapeutic methods.87,88
Valuable effort is being performed to elucidate the techniques of using dendrimers for gene trafficking into the cells without any interference of damage to the cell’s DNA. It is important to maintain DNA activity in the course of dehydration so dendrimer/DNA complexes need to be encapsulated and compressed in a water resolvable polymers, subsequently they are deposited on or inserted in functional polymer films with a fast degradation rate to facilitate gene transfection. Based on this method, for substrate-mediated gene delivery, Polyamidoamine (PAMAM) dendrimer/DNA complexes have been applied to encapsulate functional biodegradable polymers. Many reports have revealed that the fast-degrading functional polymer with excessive potential for localized transfection is a good tool.78,86,89,90 Antisense has negative charge and conjugates with dendrimers as a positive charge polymer. We have established Epidermal growth factor receptor (EGFR) and c-Src antisense oligonucleotide encapsulated with PAMAM dendrimers in human colon cancer cell line and have showed its effects on signalling pathway.63,68,91 To confirm entry of antisense to the cell, fluorescent microscope and Fluorescence-activated cell sorting (FACS) analysis have been carried out and showed that Fluorescein isothiocyanate (FITS) are conjugated effectively to dendrimers. Our studies evaluated the antisense dendrimers mediated transfer into cells and showed the effective antisense entry inside the cell; however, the antisense alone is not able to enter the cells. As a result, dendrimers could be safe and suitable tool to antisense delivery system for L. pneumophila treatment. Therefore, antisense against the type IV secretion system and Lep protein synthesis in Legionella encapsulated with dendrimers could be a novel approaches in Legionnaires' disease.
Conclusion
Vesicle trafficking pathway in L. pneumophila could be as a target for eliminating or disarming pathogens via antisense therapy. Antisense therapeutic application for bacterial protein synthesis has role in mediating the intracellular trafficking pathway to avoid phagosome and lysosome fusion in macrophages. Some of these proteins have been shown to participate in the trafficking of the Legionella phagosome. By reducing these proteins through antisense therapy, bacteria could not be able to hijack host vesicle trafficking pathway, therefore phagosome and lysosome fuse inside the cell and they are killed in lysosomes through digesting by the lysosomal enzymes. Nowadays, instead of proteins based targeting as potential drug action, drug companies and researchers are interested in utilizing different RNA species, DNA, new protein families or macromolecular complexes of these components to treat and eliminate antibiotics resistance pathogens.
Acknowledgments
The author thanks to Prof. Jamie Rossjohn and Associate Prof. Travis Beddoe at Monash University, Melbourne, Australia, for their attitude regarding L. pneumophila.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Massis LM, Assis-Marques MA, Castanheira FV, Capobianco YJ, Balestra AC, Escoll P. et al. Legionella longbeachae is immunologically silent and highly virulent in vivo. J Infect Dis. 2017;215(3):440–51. doi: 10.1093/infdis/jiw560. [DOI] [PubMed] [Google Scholar]
- 2.Singh SB, Young K, Silver LL. What is an "ideal" antibiotic? Discovery challenges and path forward. Biochem Pharmacol. 2017;133:63–73. doi: 10.1016/j.bcp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Charpentier X, Gabay JE, Reyes M, Zhu JW, Weiss A, Shuman HA. Chemical genetics reveals bacterial and host cell functions critical for type iv effector translocation by Legionella pneumophila. PLoS Pathog. 2009;5(7):e1000501. doi: 10.1371/journal.ppat.1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein MP, Muller MP, Wandinger-Ness A. Bacterial pathogens commandeer Rab gtpases to establish intracellular niches. Traffic. 2012;13(12):1565–88. doi: 10.1111/tra.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenreich W, Heuner K. The life stage-specific pathometabolism of Legionella pneumophila. FEBS Lett. 2016;590(21):3868–86. doi: 10.1002/1873-3468.12326. [DOI] [PubMed] [Google Scholar]
- 6.Chahin A, Opal SM. Severe pneumonia caused by Legionella pneumophila: Differential diagnosis and therapeutic considerations. Infect Dis Clin North Am. 2017;31(1):111–21. doi: 10.1016/j.idc.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob M, Ramos HC, Morgado B. Severe Legionella pneumophila infection in an immunocompetent patient: A success story 300 kilometers away. Cureus. 2016;8(12):e937. doi: 10.7759/cureus.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz MA, Silverstein SC. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980;66(3):441–50. doi: 10.1172/jci109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ensminger AW, Isberg RR. Legionella pneumophila dot/icm translocated substrates: A sum of parts. Curr Opin Microbiol. 2009;12(1):67–73. doi: 10.1016/j.mib.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields BS. The molecular ecology of legionellae. Trends Microbiol. 1996;4(7):286–90. doi: 10.1016/0966-842X(96)10041-X. [DOI] [PubMed] [Google Scholar]
- 11.Burillo A, Pedro-Botet ML, Bouza E. Microbiology and epidemiology of legionnaire's disease. Infect Dis Clin North Am. 2017;31(1):7–27. doi: 10.1016/j.idc.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Lamoth F, Greub G. Amoebal pathogens as emerging causal agents of pneumonia. FEMS Microbiol Rev. 2010;34(3):260–80. doi: 10.1111/j.1574-6976.2009.00207.x. [DOI] [PubMed] [Google Scholar]
- 13.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC. et al. Infectious diseases society of america/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magira EE, Zakynthinos S. Legionnaire's disease and influenza. Infect Dis Clin North Am. 2017;31(1):137–53. doi: 10.1016/j.idc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Urbanus ML, Quaile AT, Stogios PJ, Morar M, Rao C, Di Leo R. et al. Diverse mechanisms of metaeffector activity in an intracellular bacterial pathogen, Legionella pneumophila. Mol Syst Biol. 2016;12(12):893. doi: 10.15252/msb.20167381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz MA. The legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158(6):2108–26. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser DW, McDade JE. Legionellosis. Sci Am. 1979;241(4):82–99. doi: 10.1038/scientificamerican1079-82. [DOI] [PubMed] [Google Scholar]
- 18.Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, Sharrar RG. et al. Legionnaires' disease: Description of an epidemic of pneumonia. N Engl J Med. 1977;297(22):1189–97. doi: 10.1056/nejm197712012972201. [DOI] [PubMed] [Google Scholar]
- 19.Ninio S, Roy CR. Effector proteins translocated by Legionella pneumophila: Strength in numbers. Trends Microbiol. 2007;15(8):372–80. doi: 10.1016/j.tim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Cunha BA, Burillo A, Bouza E. Legionnaires' disease. Lancet. 2016;387(10016):376–85. doi: 10.1016/s0140-6736(15)60078-2. [DOI] [PubMed] [Google Scholar]
- 21.Heidtman M, Chen EJ, Moy MY, Isberg RR. Large-scale identification of Legionella pneumophila dot/icm substrates that modulate host cell vesicle trafficking pathways. Cell Microbiol. 2009;11(2):230–48. doi: 10.1111/j.1462-5822.2008.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee S, Liu X, Arasaki K, McDonough J, Galan JE, Roy CR. Modulation of Rab gtpase function by a protein phosphocholine transferase. Nature. 2011;477(7362):103–6. doi: 10.1038/nature10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 2008;10(6):1209–20. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 24.Neild AL, Roy CR. Immunity to vacuolar pathogens: What can we learn from legionella? Cell Microbiol. 2004;6(11):1011–8. doi: 10.1111/j.1462-5822.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 25.Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7(1):7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 26.Hilbi H, Segal G, Shuman HA. Icm/Dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol Microbiol. 2001;42(3):603–17. doi: 10.1046/j.1365-2958.2001.02645.x. [DOI] [PubMed] [Google Scholar]
- 27.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: Making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7(1):13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machner MP, Isberg RR. Targeting of host Rab gtpase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11(1):47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8(9):971–7. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- 30.Voth DE, Broederdorf LJ, Graham JG. Bacterial Type IV secretion systems: Versatile virulence machines. Future Microbiol. 2012;7(2):241–57. doi: 10.2217/fmb.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michard C, Doublet P. Post-translational modifications are key players of the Legionella pneumophila infection strategy. Front Microbiol. 2015;6:87. doi: 10.3389/fmicb.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103(32):11821–7. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfeffer S. Microbiology: Pathogen drop-kick. Nature. 2007;450(7168):361–2. doi: 10.1038/450361a. [DOI] [PubMed] [Google Scholar]
- 34.Berdy J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J Antibiot (Tokyo) 2012;65(8):385–95. doi: 10.1038/ja.2012.27. [DOI] [PubMed] [Google Scholar]
- 35.Rao CVS, De Waelheyns E, Economou A, Anne J. Antibiotic targeting of the bacterial secretory pathway. Biochim Biophys Acta. 2014;1843(8):1762–83. doi: 10.1016/j.bbamcr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 36. Rivera-Perez JI, Gonzalez AA, Toranzos GA. From evolutionary advantage to disease agents: Forensic reevaluation of host-microbe interactions and pathogenicity. Microbiol Spectr 2017;5(1). [DOI] [PMC free article] [PubMed]
- 37.Lange RP, Locher HH, Wyss PC, Then RL. The targets of currently used antibacterial agents: Lessons for drug discovery. Curr Pharm Des. 2007;13(30):3140–54. doi: 10.2174/138161207782110408. [DOI] [PubMed] [Google Scholar]
- 38.Barker J, Scaife H, Brown MR. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39(12):2684–8. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonas D, Engels I, Hartung D, Beyersmann J, Frank U, Daschner FD. Development and mechanism of fluoroquinolone resistance in Legionella pneumophila. J Antimicrob Chemother. 2003;51(2):275–80. doi: 10.1093/jac/dkg054. [DOI] [PubMed] [Google Scholar]
- 40.Tangden T. Combination antibiotic therapy for multidrug-resistant gram-negative bacteria. Ups J Med Sci. 2014;119(2):149–53. doi: 10.3109/03009734.2014.899279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450(7168):365–9. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- 42.Muller MP, Peters H, Blumer J, Blankenfeldt W, Goody RS, Itzen A. The legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329(5994):946–9. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- 43.Tan Y, Luo ZQ. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature. 2011;475(7357):506–9. doi: 10.1038/nature10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Massip C, Descours G, Ginevra C, Doublet P, Jarraud S, Gilbert C. Macrolide resistance in Legionella pneumophila: The role of lpeab efflux pump. J Antimicrob Chemother 2017. [DOI] [PubMed]
- 45.Ayhan DH, Tamer YT, Akbar M, Bailey SM, Wong M, Daly SM. et al. Sequence-specific targeting of bacterial resistance genes increases antibiotic efficacy. PLoS biol. 2016;14(9):e1002552. doi: 10.1371/journal.pbio.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ermak G. Emerging medical technologies. World Scientific: 2015.
- 47.Lee YJ, Hoynes-O'Connor A, Leong MC, Moon TS. Programmable control of bacterial gene expression with the combined CRISPR and antisense RNA system. Nucleic Acids Res. 2016;44(5):2462–73. doi: 10.1093/nar/gkw056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guzman-Trampe S, Ceapa CD, Manzo-Ruiz M, Sanchez S. Synthetic biology era: Improving antibiotic's world. Biochem Pharmacol. 2017;134:99–113. doi: 10.1016/j.bcp.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Gunderson FF, Cianciotto NP. The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae. MBio. 2013;4(2):e00074–13. doi: 10.1128/mBio.00074-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mojica FJ, Diez-Villasenor C, Soria E, Juez G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol. 2000;36(1):244–6. doi: 10.1046/j.1365-2958.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakata A, Amemura M, Makino K. Unusual nucleotide arrangement with repeated sequences in the Escherichia coli K-12 chromosome. J Bacteriol. 1989;171(6):3553–6. doi: 10.1128/jb.171.6.3553-3556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151(8):2551–61. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- 53.Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327(5962):167–70. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 54.Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. eLife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60(2):174–82. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- 56.Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 2005;151(Pt 3):653–63. doi: 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- 57.Weinberger AD, Gilmore MS. CRISPR-Cas: To take up DNA or not-that is the question. Cell Host Microbe. 2012;12(2):125–6. doi: 10.1016/j.chom.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15(5):321–34. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 59.Sampson TR, Weiss DS. CRISPR-Cas systems: New players in gene regulation and bacterial physiology. Front Cell Infect Microbiol. 2014;4:37. doi: 10.3389/fcimb.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kole R, Krainer AR, Altman S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012;11(2):125–40. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chi X, Gatti P, Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov Today 2017. [DOI] [PubMed]
- 62.Georg J, Hess WR. Cis-antisense RNA, another level of gene regulation in bacteria. Microbiol Mol Biol Rev. 2011;75(2):286–300. doi: 10.1128/mmbr.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Najar AG, Pashaei-Asl R, Omidi Y, Farajnia S, Nourazarian AR. EGFR antisense oligonucleotides encapsulated with nanoparticles decrease EGFR, MAPK1 and STAT5 expression in a human colon cancer cell line. Asian Pac J Cancer Prev. 2013;14(1):495–8. doi: 10.7314/APJCP.2013.14.1.495. [DOI] [PubMed] [Google Scholar]
- 64.Burnett JC, Rossi JJ. RNA-based therapeutics: Current progress and future prospects. Chem Biol. 2012;19(1):60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen Y, Tan XX. Nucleotides for prevention and treatment of bacterial and fungal pathologies. US 8889397 B2; 2014.
- 66.Uhlmann E. Oligonucleotide technologies: Synthesis, production, regulations and applications. 29-30th November 2000, Hamburg, Germany. Expert Opin Biol Ther. 2001;1(2):319–28. doi: 10.1517/14712598.1.2.319. [DOI] [PubMed] [Google Scholar]
- 67.Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. 2005;5(6):468–79. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- 68.Nourazarian AR, Najar AG, Farajnia S, Khosroushahi AY, Pashaei-Asl R, Omidi Y. Combined EGFR and c-Src antisense oligodeoxynucleotides encapsulated with PAMAM denderimers inhibit HT-29 colon cancer cell proliferation. Asian Pac J Cancer Prev. 2012;13(9):4751–6. doi: 10.7314/APJCP.2012.13.9.4751. [DOI] [PubMed] [Google Scholar]
- 69.Posey KL, Coustry F, Veerisetty AC, Hossain M, Gattis D, Booten S. et al. Antisense reduction of mutant COMP reduces growth plate chondrocyte pathology. Mol Ther. 2017;25(3):705–14. doi: 10.1016/j.ymthe.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evers MM, Toonen LJ, van Roon-Mom WM. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Deliv Rev. 2015;87:90–103. doi: 10.1016/j.addr.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Cazzola M, Matera MG, Page CP. Novel approaches to the treatment of pneumonia. Trends Pharmacol Sci. 2003;24(6):306–14. doi: 10.1016/s0165-6147(03)00129-9. [DOI] [PubMed] [Google Scholar]
- 72.Simons RW, Kleckner N. Biological regulation by antisense RNA in prokaryotes. Annu Rev Genet. 1988;22:567–600. doi: 10.1146/annurev.ge.22.120188.003031. [DOI] [PubMed] [Google Scholar]
- 73.Gasparro FP, Edelson RL, O'Malley ME, Ugent SJ, Wong HH. Photoactivatable antisense DNA: Suppression of ampicillin resistance in normally resistant escherichia coli. Antisense Res Dev. 1991;1(2):117–40. doi: 10.1089/ard.1991.1.117. [DOI] [PubMed] [Google Scholar]
- 74.Good L, Nielsen PE. Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol. 1998;16(4):355–8. doi: 10.1038/nbt0498-355. [DOI] [PubMed] [Google Scholar]
- 75.Patil SD, Sharma R, Srivastava S, Navani NK, Pathania R. Downregulation of yidC in escherichia coli by antisense RNA expression results in sensitization to antibacterial essential oils eugenol and carvacrol. PLoS One. 2013;8(3):e57370. doi: 10.1371/journal.pone.0057370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fathi M, Entezami AA, Pashaei-Asl R. Swelling/deswelling, thermal, and rheological behavior of PVA-g-NIPAAm nanohydrogels prepared by a facile free-radical polymerization method. J Polym Res. 2013;20(5):125. doi: 10.1007/s10965-013-0125-5. [DOI] [Google Scholar]
- 77.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18(1):33–7. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 78.Fu HL, Cheng SX, Zhang XZ, Zhuo RX. Dendrimer/DNA complexes encapsulated functional biodegradable polymer for substrate-mediated gene delivery. J Gene Med. 2008;10(12):1334–42. doi: 10.1002/jgm.1258. [DOI] [PubMed] [Google Scholar]
- 79.Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139–50. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sebestik J, Niederhafner P, Jezek J. Peptide and glycopeptide dendrimers and analogous dendrimeric structures and their biomedical applications. Amino Acids. 2011;40(2):301–70. doi: 10.1007/s00726-010-0707-z. [DOI] [PubMed] [Google Scholar]
- 81.Leire E, Amaral SP, Louzao I, Winzer K, Alexander C, Fernandez-Megia E. et al. Dendrimer mediated clustering of bacteria: Improved aggregation and evaluation of bacterial response and viability. Biomater Sci. 2016;4(6):998–1006. doi: 10.1039/c6bm00079g. [DOI] [PubMed] [Google Scholar]
- 82.Sousa-Herves A, Novoa-Carballal R, Riguera R, Fernandez-Megia E. GATG dendrimers and PEGylated block copolymers: From synthesis to bioapplications. AAPS J. 2014;16(5):948–61. doi: 10.1208/s12248-014-9642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bae Y, Rhim HS, Lee S, Ko KS, Han J, Choi JS. Apoptin gene delivery by the functionalized polyamidoamine dendrimer derivatives induces cell death of U87-MG glioblastoma cells. J Pharm Sci 2017. [DOI] [PubMed]
- 84.Dufes C, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57(15):2177–202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 85. Sampathkumar SG, Yarema KJ. Dendrimers in cancer treatment and diagnosis. In: Kumar CS, editor. Nanotechnologies for the Life Sciences. Germany: Wiley; 2007.
- 86.Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW. et al. Dendrimers: Synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. doi: 10.1186/1556-276x-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voltan AR, Quindos G, Alarcon KPM, Fusco-Almeida AM, Mendes-Giannini MJS, Chorilli M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int J Nanomedicine. 2016;11:3715–30. doi: 10.2147/ijn.s93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winnicka K, Wroblewska M, Wieczorek P, Sacha PT, Tryniszewska EA. The effect of PAMAM dendrimers on the antibacterial activity of antibiotics with different water solubility. Molecules. 2013;18(7):8607–17. doi: 10.3390/molecules18078607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu HL, Cheng SX, Zhang XZ, Zhuo RX. Dendrimer/DNA complexes encapsulated in a water soluble polymer and supported on fast degrading star poly(DL-lactide) for localized gene delivery. J Control Release. 2007;124(3):181–8. doi: 10.1016/j.jconrel.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 90.Sarisozen C, Pan J, Dutta I, Torchilin VP. Polymers in the co-delivery of siRNA and anticancer drugs to treat multidrug-resistant tumors. J Pharma Investig. 2017;47(1):37–49. doi: 10.1007/s40005-016-0296-2. [DOI] [Google Scholar]
- 91.Nourazarian AR, Pashaei-Asl R, Omidi Y, Najar AG. C-Src antisense complexed with PAMAM denderimes decreases of c-Src expression and EGFR-dependent downstream genes in the human HT-29 colon cancer cell line. Asian Pac J Cancer Prev. 2012;13(5):2235–40. doi: 10.7314/APJCP.2012.13.5.2235. [DOI] [PubMed] [Google Scholar]


