Abstract
Altered microRNA regulation has been implicated in the pathogenesis of various disorders, including cerebral ischemia/reperfusion injury (I/RI). However, the regulatory mechanism of miR-130b in cerebral ischemia injury has not been reported. In this study, we explored the role of miR-130b in cerebral ischemia injury and investigated its potential mechanism. Levels of miR-130b were quantified by real-time PCR, and the protein level of AQP4 was detected by Western blotting. Cell apoptosis was detected by flow cytometry. In vitro, miR-130b levels in astrocytes were found significantly downregulated after OGD. Overexpression of miR-130b by miR-130b mimic decreased LDH release and apoptosis, but promoted cell health of astrocytes with OGD, thus playing a protective role in astrocyte I/RI. The level of miR-130b was also downregulated in ischemic tissues in MCAO model compared with the sham group, and the expression of miR-130b was gradually downregulated over time after reperfusion. AQP4 was upregulated both in two models, and as the reperfusion went on, AQP4 expression gradually upregulated. Our results indicated knockdown of AQP4 could ameliorate astrocyte injury induced by OGD. Finally, we found that miR-130b regulated astrocyte expression of AQP4, and rescue experiments further proved the protective role of miR-130b was mediated by AQP4 downregulation. Our study demonstrated that miR-130b might exert a neuroprotective effect following cerebral I/RI by regulating AQP4 expression at the post-transcriptional level. Therefore, miR-130b may be a potential therapeutic target for stroke treatment.
Keywords: Cerebral ischemia/reperfusion injury, miR-130b, AQP4, stroke, middle cerebral artery occlusion (MCAO)
Introduction
Cerebral ischemic injury (CII) is caused by cerebral ischemia and is further aggravated by sudden restoration of the blood supply. CII is the main pathological and physiological basis of ischemic stroke [1], a major disease affecting the global population with high incidence, mortality, and disability; numerous surviving stroke victims suffer from disability for the rest of their lives [2,3]. However, the precise mechanism of ischemia-induced cerebral injury remains unclear. Therefore, exploration of the potential mechanism of ischemia-related cerebral injury is of great significance for developing effective therapies for improving the prognosis of ischemic stroke.
MicroRNAs are endogenous ~22 nucleotide long highly conserved non-coding RNAs [4,5], which have emerged as important regulators of many diseases, including cancer, cerebrovascular disease, and metabolic disorders [6,7]. Increasing evidence has indicated that numerous miRNAs are involved in CII. For example, miR-383 may play a key role in focal cerebral ischemia by regulating PPARγ expression at the post-transcriptional level [8]. Knockdown of let-7a, the first discovered miRNA, was reported to inhibit the activation of p38 MAPK and JNK signaling pathways by upregulating MKP1 expression, and exerting a neuroprotective effect following CII [9]. However, the effects of miR-130b in CII have not yet been well understood.
Water channel protein aquaporin 4 (AQP4), a member of the aquaporin family of water channels, is dominantly expressed in astrocytes throughout the central nervous system (CNS), mainly at astrocyte endfeet at the blood-brain barrier [10]. Early studies have shown that AQP4 overexpression aggravates brain injury in a rat model of acute ischemic stroke [11]. AQP4 in cerebral ischemia enhanced cerebral edema in ischemic stroke and inhibition of AQP4 decreased infarct volume [12,13]. A previous study also showed that miR-29b overexpression reduced blood-brain barrier disruption after ischemic stroke via downregulating AQP4 [14]. Considering the essential role of AQP4 in cerebral ischemia, more methods exploring the role of AQP4 in ischemia are needed to improve cerebral ischemia injury.
In the current study, using the oxygen-glucose deprivation (OGD) model of cell ischemia in vitro and middle cerebral artery occlusion (MCAO) model of mouse focal cerebral ischemia, we investigated the role of miR-130b in regulating CII and its underlying mechanism. We found that miR-130b plays an essential role in CII protection. Furthermore, miR-130b was found to inhibit AQP4 expression during cerebral ischemia, thus alleviating AQP4-induced CII. Furthermore, miR-130b mimics were capable of downregulating AQP4 expression that might protect astrocytes from ischemia-induced cell injury.
Material and methods
Middle cerebral artery occlusion/reperfusion model
The Medical Faculty Ethics Committee of Zhejiang University approved the study protocol. All experimental procedures were performed in accordance with the Guide for the Care and Use of Experimental Animals. MCAO was performed according to previously published methods [12,15]. 6 weeks C57BL/6J mice (20-25 g, the Experimental Animal Center in the Zhejiang Academy of Medical Sciences) were subjected to MCAO and reperfusion or a sham operation. In brief, the mice were anesthetized with 4% chloral hydrate (Sigma, USA), and the left common carotid artery was exposed. After isolation and clamping of the artery and its branches, a silicone-coated 6-0 monofilament nylon suture (Doccol Corp., Redlands, CA, USA) was inserted into the left common carotid artery, and advanced through the carotid bifurcation until it occluded the origin of the MCA. After 1 h of MCAO, the mice were reperfused by removing the suture from the vessel. In the sham-operated groups, the left common carotid artery was surgically prepared for insertion of the filament but the filament was not inserted.
Primary astrocyte culture
Primary astrocytes were prepared from postnatal day 1 neonatal Sprague Dawley rats, as previously described [16]. Briefly, neocortices were dissected, treated with trypsin, and plated as a single-cell suspension, then plated onto poly-L-lysine coated 35 mm dishes with DMEM containing 10% bovine calf serum and incubated at 37°C with 5% CO2 in a humidified environment, and allowed to grow to confluence.
Cell transfection
When confluent, primary astrocytes were trypsinized and plated onto 24-well plates, before being transfected on Day5 with miR-130b mimics (RiboBio, Guangzhou, China), miR-130b inhibitor (RiboBio, Guangzhou, China) or siRNA against AQP4 (Santa Cruz, CA, USA) with appropriate controls using Lipofectamine 2000 (Invitrogen, Foster City, CA) according to the manufacturer’s instructions. The transfected cells were collected after 48 h for further experiments.
OGD model
After the cells were plated, primary astrocytes were transferred to an incubator with 5% CO2 and 95% atmospheric air, and cultivated at 37°C (i.e., normal conditions) for 24 h. Then, the cells were washed carefully three times with phosphate-buffered saline (PBS; 0.1 M; pH 7.4), the medium was changed to glucose-free DMEM, and the cultures were subjected to anoxic conditions (1% O2) for 6 h (OGD). Following OGD treatment, the medium was replaced with high-glucose DMEM (standard medium) and the cultures were returned to normal conditions for 24 h before being collected for further experiments.
Real-time PCR
Total RNA was isolated from cerebral tissues from MCAO mice or cultured primary astrocytes with TRIZOL reagent (Invitrogen, USA) according to manufacturer’s instructions. Total RNA (0.5 μg) was reversed to cDNA. SYBR Green PCR Master mix (Takara, Japan) was used to determine the mRNA level of miR-130b and AQP4. The procedure was as follows: 95°C for 5 min, followed by 30 cycles with 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by extension at 72°C for 5 min. U6 and β-actin were used as internal controls. The primers used were as follows: miR-130b 5’-ACTCTTTCCCTGTTGCACTAC-3’ mir-130b mimic Forward 5’- CAGUGCAAUGAUGAAAGGGCAU-3’ Reverse 5’-AUGCCCUUUCAUCAUUGCACUG-3’ mir-130b inhibitor 5’-AUGCCCUUUCAUCAUUGCACUG-3’.
Western blotting
Total proteins were extracted from MCAO mice’s ischemic hemisphere or cultured primary astrocytes, and quantified using the BCA Protein Assay Kit (Sigma, St. Louis, MO, USA). Equivalent amounts of protein (40 μg/lane) was separated on 10% SDS-PAGE gels and transferred to PVDF membrane. The membranes were blocked with 5% non-fat milk in Tris-buffered saline (TBS) and 0.1% Tween 20 (TBST) buffer for 1 h and incubated with AQP4 primary monoclonal antibody, sources of rabbits (Abcam, USA) at a dilution of 1:1000 overnight. After washing twice with TBST, the membranes were incubated with secondary antibody anti-rabbit conjugated to horseradish peroxidase at a 1:2000 dilution. Specific bands were visualized using enhanced chemiluminescence detection (Thermo Fisher Scientific, USA). The protein bands were visualized by autoradiography (Kodak, Rochester, NY, USA). GAPDH was used as an internal control.
Enzyme-linked immunosorbent assay (ELISA)
The cell supernatants from each group treated with different miR-130b or AQP4 treatment were collected to detect LDH level. Quantitation of LDH was performed using an ELISA kit (R&D Systems, US) according to the manufacturer’s instructions.
Cell apoptosis assay
Cell apoptosis was detected by flow cytometry with a FITC-conjugated Annexin-V and PI kit. Cultured primary astrocytes was treated for 48 h, and were collected trypsinization, washed twice with pre-chilled PBS and resuspended in 200 μl Annexin-V/PI pre-mix buffer. Astrocytes were incubated in the dark for 20 min at 4°C, then 300 μl binding buffer was added. The cells were examined with FACScan (BD, New Jersey, USA), and the data was analyzed by Cytomics FC500 Flow Cytometry CXP.
Cell health assay
Cell health was detected by calcein-AM/PI staining according to the manufacture’s protocol (CST, USA). In brief, 5 × 103 cells/well were plated into 96-well plates and treated with cells. After cells were treated, 100 µl/well of labeling solution was added and the cells were incubated at room temperature for 30 to 60 min while protected from light. Cell fluorescence was analyzed on a plate reader set with excitation/emission of 490/520 nm for live cells, and an excitation/emission setting of 535/620 nm for dead cells.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 software (SPSS, Chicago, IL, USA). Measurement data are expressed as the mean ± SD. Student’s t-test was used to compare the difference between two groups. One-way analysis of variance (ANOVA) and the least significant difference test were used among multiple groups. Results were considered significant if P < 0.05.
Results
Overexpression of miR-130b protected astrocytes against ischemia/reperfusion injury
To explore the role of miR-130b in cerebral ischemia injury, we employed the astrocyte OGD model to mimic CII in vitro and the mouse MCAO model to mimic CII in vivo. Our results demonstrated that the expression of miR-130b was significantly decreased in OGD astrocytes (P < 0.001) and MCAO mice (P < 0.01) compared with the control group (Figure 1A). Downregulation of miR-130b was maintained in MCAO models after reperfusion for 24 or 48 h (Figure 1B).
Figure 1.

Effects of miR-130b on OGD-treated primary cultured astrocytes function. A. Expression of miR-130b in OGD-treated primary cultured astrocytes or NC as assessed by real time PCR. U6 was used as the internal control. ***P < 0.001 vs. Control; **P < 0.01 vs. sham. B. Downregulation of miR-130b was maintained in MCAO models after reperfusion for 24 or 48 h. *P < 0.05, **P < 0.01 vs. 0 h. C. LDH release from OGD-treated primary astrocytes transfected with miR-130b mimic, miR-130b inhibitor or NC by ELISA. ***P < 0.001 vs. NC; ###P < 0.001 vs. OGD. D. Cell health in OGD-treated primary astrocytes transfected with miR-130b mimic, miR-130b inhibitor or NC as assessed by calcein/PI staining. PI positive cells represented dead cells, and the calcein positive cells represented healthy cells. ***P < 0.001 vs. NC; ###P < 0.001 vs. OGD. E. Cell apoptosis assay in OGD-treated primary astrocytes transfected with miR-130b mimic, miR-130b inhibitor or NC by flow cytometry. The cells in the B2 and B4 quadrants represented the apoptotic cells.
Next, we examined cell supernatant LDH level, cell health and cell apoptosis in primary cultured astrocytes in NC (Negative control), OGD, miR-130b mimic + OGD and miR-130b inhibitor + OGD groups. Our results showed that addition of a miR-130b could prevent the OGD-induced release of LDH (Figure 1C) and prevent cell apoptosis (Figure 1E). MiR-130b also promoted astrocyte health (Figure 1D), thus suggesting a protective role in astrocyte ischemia injury. Inhibition of miR-130b had opposing effects (Figure 1C-E).
MiR-130b regulated AQP4 expression
By using the target gene prediction site TargetScan (www.targetscan.org), we identified AQP4 as a target gene of miR-130b (Figure 2A). We hypothesized that miR-130b may exert its protective effect by downregulating AQP4. We first assessed the protein level of AQP4 in astrocytes transfected with miR-130b mimic or miR-130b inhibitor or NC. Western blotting analysis showed significant downregulation of AQP4 protein levels in the miR-130b mimic group (P < 0.001), while miR-130b inhibitor significantly elevated AQP expression (P < 0.001, Figure 2B). To further investigate the effects of miR-130b on AQP4 expression, we investigated the expression of AQP4 in astrocytes models transfected with miR-130b mimics or miR-130b inhibitor or NC and exposed to OGD. We found that AQP4 significantly decreased in the miR-130b mimic + OGD group compared with the OGD group and NC group (P < 0.001), but there is no significant difference in miR-130b inhibitor + OGD group compared with OGD group and NC group (Figure 2C). These results indicated that miR-130b could prevent OGD-mediated AQP4 expression, thus preventing AQP4-mediated CII. We examined protein and mRNA expression of AQP4 in MCAO models with reperfusion. As expected, the expression of AQP4 moved in the opposite direction as miR-130b, in that AQP4 expression was increased as reperfusion time went on (Figure 2D, 2E).
Figure 2.

miR-130b regulates expression of AQP4 in vitro and vivo. A. Predicted matching miRNAs for the AQP4’UTR segment by TargetScan analysis. B. Expression of AQP4 protein in astrocytes transfected with miR-130b mimic or miR-130b inhibitor or NC by Western blotting; β-actin was used as the internal control, and the average band intensity value is represented as a histogram. ***P < 0.001 vs. NC. C. Expression of AQP4 protein in astrocytes models transfected with miR-130b mimics or miR-130b inhibitor or NC and exposed to OGD by Western Blot; β-actin was used as the internal control. *P < 0.05 vs. NC group. NC: negative control ###P < 0.001 vs. NC + OGD. D. The expression of protein AQP4 was increased in MCAO models after reperfusion for 24 or 48 h. *P < 0.05, **P < 0.01 vs. 0 h. E. The expression of AQP4 mRNA was increased in MCAO models after reperfusion for 24 or 48 h. *P < 0.05, **P < 0.01 vs. 0 h.
AQP4 overexpression aggravated astrocyte ischemia/reperfusion injury
Aquaporin-4 (AQP4) is a water channel expressed in astrocyte end-feet, and previous studies have demonstrated that AQP4 could promote CII [17]. To further prove our hypothesis that miR-130b may protect against cerebral IRI by downregulating AQP4, we examined the role of AQP4 in cerebral IRI. We first assessed the expression of AQP4 in in vitro OGD treated astrocytes, and in vivo MCAO models. Our results showed that AQP4 protein and mRNA were significantly upregulated in both OGD-treated astrocytes and MCAO mouse ischemic hemisphere compared with the control group (Figure 3A, 3B). To further study the role of AQP4 in OGD injury, we examined cell supernatant LDH level, cell health and cell apoptosis of astrocytes in NC, OGD, and AQP4 siRNA + OGD group. The Western blotting results showed good downregulation by AQP4 siRNA in untreated astrocytes (Figure 3C). Our results showed that knockdown of AQP4 significantly decreased LDH release and cell apoptosis, and promoted astrocyte health (Figure 3D-F). Thus, our results further suggest that expression of AQP4 may exacerbate CII.
Figure 3.
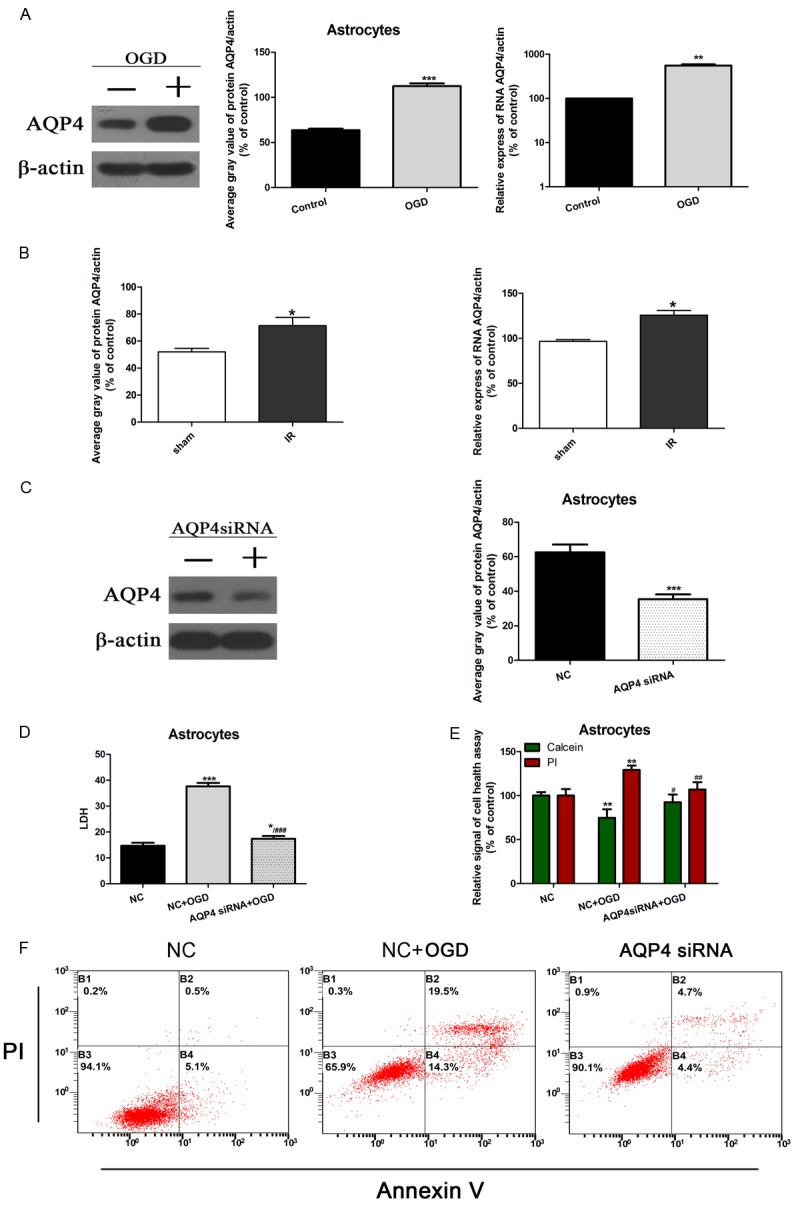
Effect of AQP4 on OGD-treated primary astrocytes and MCAO mouse ischemic hemisphere. A: Expression of AQP4 protein in OGD-treated primary astrocytes or NC by Western blotting; β-actin was used as the internal control, and the average band intensity is represented as a histogram. **P < 0.01, ***P < 0.001 vs. Control. B: AQP4 protein and mRNA in MCAO mouse ischemic hemisphere compared with sham group were determined by Western Blot and PCR. *P < 0.05 vs. sham. C: Western blot showing AQP4 expression in primary astrocytes transfected with AQP4 siRNA or a negative control siRNA Beta-actin was used as the internal control, and the average grey value was represented as a histogram. ***P < 0.001 vs. NC group. NC: negative control. D: LDH release from OGD-treated primary astrocytes transfected with AQP4 siRNA or NC by ELISA. *P < 0.05, ***P < 0.001 vs. NC group. NC: negative control. ###P < 0.001 vs. NC + OGD. E: Cell health in OGD-treated primary astrocytes transfected with AQP4 siRNA or NC by calcein/PI staining. PI positive cells represent dead cells, and calcein positive cells represent healthy cells. *P < 0.05, **P < 0.01, vs. NC group. NC: negative control. ##P < 0.01. F: Cell apoptosis in OGD-treated primary astrocytes transfected with AQP4 siRNA or NC by flow. The cells in the B2 and B4 quadrants represented the apoptotic cells.
AQP4 downregulation mitigated miR-130b-mediated cerebral ischemic protection
To further prove whether the effect of miR-130b in protecting against CII protection is mediated by regulation of AQP4, we co-transfected AQP4 siRNA with miR-130b mimics, miR-130b inhibitor or NC in OGD-treated astrocytes. The interfering efficiency of AQP4 siRNA was confirmed by western blotting and represented as a grey value (Figure 4A, 4B). Our results showed that the effect of miR-130b on LDH releasing release level and cell apoptosis of in OGD-treated primary astrocytes vanished after AQP4 was interfered silenced (Figure 4C, 4D). These findings verify that the cerebral ischemic protective role of miR-130b is mediated by inhibition of AQP4 expression.
Figure 4.

AQP4 knockdown prevented the protective effect of miR-130b. A: Determination of AQP4 siRNA efficiency by Western blotting; β-actin was used as the internal control, and the average band intensity is represented as a histogram. B: LDH release in OGD-treated primary astrocytes transfected with AQP4 siRNA and miR-130b mimic, AQP4 siRNA and miR-130b inhibitor or AQP4 siRNA alone. ***P < 0.001 vs. NC group. NC: negative control. C: LDH release from OGD-treated primary astrocytes transfected with AQP4 siRNA and miR-130b mimic, AQP4 siRNA and miR-130b inhibitor or AQP4 siRNA alone by ELISA. D: Cell apoptosis in OGD-treated primary astrocytes transfected with AQP4 siRNA and miR-130b mimic, AQP4 siRNA and miR-130b inhibitor or AQP4 siRNA alone. Apoptosis was determined by flow cytometry with Annexin V-FITC/PI kit. Cells in the B2 and B4 quadrants represented the apoptotic cells.
Discussion
In the present study, we provide evidence that miR-130b was inactivated in cerebral ischemia in the mouse MACO model and in OGD-treated primary astrocytes. MiR-130b overexpression protected rat astrocytes from ischemic injury. We found that miR-130b levels were changed in opposition to AQP4 expression levels in mice MCAO cerebral tissues and OGD-treated primary astrocytes. Furthermore, miR-130b could inhibit AQP4 expression, thus relieving AQP4-mediated CII. Our study provides novel insights into the molecular mechanism of miR-130b-mediated brain protection in cerebral ischemia, and identifies miR-130b as a potential treatment for ischemic stroke.
A subset of miRNAs are abundantly expressed in the human brain [18], and play important roles in numerous brain diseases [19-23]. Increasing evidence has indicated that miRNAs play critical roles in response to cerebral ischemia [22,24-26]. Both increased and decreased miRNA levels may be important either in prevention or treatment of stroke. For example, increasing miR-30a expression could relieve CII through decreasing Beclin-1-mediated autophagy [27]. Knockdown of miR-155 could reduce infarct volume, improving CII in an animal model [28]. Downregulation of miR-383 could upregulate PPARγ expression at an early stage after ischemia, and exert anti-inflammatory effects, providing neuroprotection against ischemic brain injury [8]. Finally, knockdown of miR-124 could promote the expression of the p53 family proteins and reduce infarction size in mouse focal cerebral ischemia [29]. In the present study, we show for the first time that miR-130b expression was downregulated in primary cultured astrocytes subjected to OGD and MCAO models, and that miR-130b provides a protective effect against ischemia injury by directly regulating the expression of AQP4.
AQP4 is the principle bidirectional water transporting channel expressed at astrocyte endfeet at the blood-brain barrier [10]. Increasing evidence indicates that AQP4 plays an important role in promoting brain ischemia [30]. In rat ischemic stroke models, AQP4 aggravated brain ischemia by enhancing cerebral edema [31-33]. In this study, we found AQP4 was a potential target for regulation by miR-130b at the post-transcriptional level by miR-130b binding to the 3’-UTR of AQP4 mRNA. In addition, we also showed that miR-130b could negatively regulate AQP4 expression; miR-130b mimics decreased expression of AQP4 in primary astrocytes under normoxic and OGD conditions. Furthermore, knockdown of endogenous AQP4 expression removed miR-130b mediated protection during OGD. Taken together, these results provide strong evidence that miR-130b contributes to CII protection by negatively regulating AQP4 levels. Our study further confirmed the importance of miR-130b in CII protection, and suggests that miR-130b might be a novel intervention target for cerebral ischemic stroke.
In conclusion, we have provided evidence that miR-130b expression decreased after cerebral ischemia. Upregulation of miR-130b in primary cultured astrocytes could alleviate OGD-induced injury by downregulating AQP4 expression. Our findings highlight the importance of miR-130b in treating CII. Further study of these mechanisms has the potential to lead to targeted clinical therapy.
Acknowledgements
This work was supported by Zhejiang Medical Technology & Education (2014KYA088) Yueying Zheng; Department of education of Zhejiang Province, Y201223957 (Jianhong Xu); National Nature Science Foundation of China 81571147 (Xiaoxing Xiong).
Disclosure of conflict of interest
None.
References
- 1.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 2.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8:345–354. doi: 10.1016/S1474-4422(09)70023-7. [DOI] [PubMed] [Google Scholar]
- 3.Xu AD, Wang YJ, Wang DZ. Consensus statement on the use of intravenous recombinant tissue plasminogen activator to treat acute ischemic stroke by the Chinese Stroke Therapy Expert Panel. CNS Neurosci Ther. 2013;19:543–548. doi: 10.1111/cns.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Volný O, Kašičková L, Coufalová D, Cimflová P, Novák J. microRNAs in cerebrovascular disease. Adv Exp Med Biol. 2015;888:155–195. doi: 10.1007/978-3-319-22671-2_9. [DOI] [PubMed] [Google Scholar]
- 8.Pei L, Meng S, Yu W, Wang Q, Song F, Ma L. Inhibition of MicroRNA-383 ameliorates injury after focal cerebral ischemia via targeting PPAR gamma. Cell Physiol Biochem. 2016;39:1339–1346. doi: 10.1159/000447838. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZK, Liu FF, Wang Y, Jiang XM, Yu XF. Let-7a gene knockdown protects against cerebral ischemia/reperfusion injury. Neural Regen Res. 2016;11:262–269. doi: 10.4103/1673-5374.177734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu LS, Fan YY, Ye G, Li J, Feng XP, Lin K, Dong M, Wang Z. Curcumin alleviates brain edema by lowering AQP4 expression levels in a rat model of hypoxia-hypercapnia-induced brain damage. Exp Ther Med. 2016;11:709–716. doi: 10.3892/etm.2016.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han D, Sun M, He PP, Wen LL, Zhang H, Feng J. Ischemic postconditioning alleviates brain edema after focal cerebral ischemia reperfusion in rats through down-regulation of aquaporin-4. J Mol Neurosci. 2015;56:722–729. doi: 10.1007/s12031-015-0504-y. [DOI] [PubMed] [Google Scholar]
- 13.Yao X, Derugin N, Manley GT, Verkman AS. Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci Lett. 2015;584:368–372. doi: 10.1016/j.neulet.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Huang J, Ma Y, Tang G, Liu Y, Chen X, Zhang Z, Zeng L, Wang Y, Ouyang YB, Yang GY. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J Cereb Blood Flow Metab. 2015;35:1977–1984. doi: 10.1038/jcbfm.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, He Q, Baral S, Mao L, Li Y, Jin H, Chen S, An T, Xia Y, Hu B. MicroRNA-493 regulates angiogenesis in a rat model of ischemic stroke by targeting MIF. Febs J. 2016;283:1720–1733. doi: 10.1111/febs.13697. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, Giffard RG. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia. 2013;61:1784–1794. doi: 10.1002/glia.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assentoft M, Larsen BR, MacAulay N. Regulation and function of AQP4 in the central nervous system. Neurochem Res. 2015;40:2615–2627. doi: 10.1007/s11064-015-1519-z. [DOI] [PubMed] [Google Scholar]
- 18.Saugstad JA. MicroRNAs as effectors of brain function. Stroke. 2013;44:S17–19. doi: 10.1161/STROKEAHA.113.000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saugstad JA. Non-coding RNAs in stroke and neuroprotection. Front Neurol. 2015;6:50. doi: 10.3389/fneur.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L, Yu JT, Hu N, Tan L. Non-coding RNAs in Alzheimer’s disease. Mol Neurobiol. 2013;47:382–393. doi: 10.1007/s12035-012-8359-5. [DOI] [PubMed] [Google Scholar]
- 22.Volný O, Kašičková L, Coufalová D, Cimflová P, Novák J. microRNAs in cerebrovascular disease. Adv Exp Med Biol. 2015;888:155–195. doi: 10.1007/978-3-319-22671-2_9. [DOI] [PubMed] [Google Scholar]
- 23.Volvert ML, Rogister F, Moonen G, Malgrange B, Nguyen L. MicroRNAs tune cerebral cortical neurogenesis. Cell Death Differ. 2012;19:1573–1581. doi: 10.1038/cdd.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Sun G, Zhang L, Shi L, Zeng Y. Circulating MicroRNAs as biomarkers of acute stroke. Int J Mol Sci. 2014;15:1418–1432. doi: 10.3390/ijms15011418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan H, Fang M, Liu XY. Role of microRNAs in stroke and poststroke depression. Scientific-WorldJournal. 2013;2013:459692. doi: 10.1155/2013/459692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Sandhu HK, Zhi F, Hua F, Wu M, Xia Y. Effects of hypoxia and ischemia on MicroRNAs in the brain. Curr Med Chem. 2015;22:1292–1301. doi: 10.2174/0929867322666150209154755. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Liang J, Li Y, Li J, Yang X, Zhang X, Han S, Li S, Li J. Down-regulation of miRNA-30a alleviates cerebral ischemic injury through enhancing beclin 1-mediated autophagy. Neurochem Res. 2014;39:1279–1291. doi: 10.1007/s11064-014-1310-6. [DOI] [PubMed] [Google Scholar]
- 28.Wen Y, Zhang X, Dong L, Zhao J, Zhang C, Zhu C. Acetylbritannilactone modulates MicroRNA-155-mediated inflammatory response in ischemic cerebral tissues. Mol Med. 2015;21:197–209. doi: 10.2119/molmed.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Li F, Zhao S, Luo Y, Kang J, Zhao H, Yan F, Li S, Ji X. MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke. 2013;44:1973–1980. doi: 10.1161/STROKEAHA.111.000613. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Chen J, Lu H. Expression of aquaporin-4 and pathological characteristics of brain injury in a rat model of traumatic brain injury. Mol Med Rep. 2015;12:7351–7357. doi: 10.3892/mmr.2015.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuda AM, Badaut J. Aquaporin 4: a player in cerebral edema and neuroinflammation. J Neuroinflammation. 2012;9:279. doi: 10.1186/1742-2094-9-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, Takano T, Peng W, Wang F, Rangroo Thrane V, Enger R, Haj-Yasein NN, Skare Ø, Holen T, Klungland A, Ottersen OP, Nedergaard M, Nagelhus EA. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A. 2011;108:846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng HK, Wang QS, Deng YY, Fang M, Chen CB, Fu YH, Jiang WQ, Jiang X. Hypertonic saline ameliorates cerebral edema through downregulation of aquaporin-4 expression in the astrocytes. Neuroscience. 2010;166:878–885. doi: 10.1016/j.neuroscience.2009.12.076. [DOI] [PubMed] [Google Scholar]


