Abstract
The clinical utility of Traditional Chinese Medicine (TCM) herbs/roots extracts in osteoporosis (OP) and osteoarthritis (OA) has been described in multiple reports, but there have been few studies of TCM for preventing bone loss and cartilage degradation simultaneously. Six-month-old female Sprague-Dawley rats each were subjected to ovariectomized (OVX) or sham surgery and treated orally once daily with herbal extracts or vehicle. Body weight was recorded weekly, and blood samples were collected from fasting animals at different time points. Biochemical markers of bone resorption and cartilage degradation were analyzed. Changes in bone mineral density and calcium content were determined in the femoral center and femoral telocentric end of rats. Out of 56 TCM herbs/roots extracts, only kudzu root demonstrated consistent joint protective effects. OVX resulted in a marked increase in bone resorption and cartilage degradation, which could be significantly reversed by kudzu after three weeks of treatment. Compared to vehicle, kudzu induced a significant increase in bone mineral density in the femoral center and femoral telocentric end, and calcium content. The results show that kudzu exerts direct effects on articular cartilage in the OVX rat and can effectively prevent the acceleration of cartilage degradation induced by ovariectomy. Moreover, kudzu has demonstrated positive effects on metabolic health (cause a weight reduction) and may represent a possible treatment for OP and OA with high body mass index. Further studies are needed to investigate the potential effects of kudzu root in postmenopausal women.
Keywords: Osteoporosis, osteoarthritis, bone absorption, cartilage degradation, kudzu, SERMs
Introduction
Postmenopausal osteoporosis (OP) is a chronic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, which results in increased bone fragility and fracture risk. OP becomes a common disease with an increasing prevalence in women due to longer life expectancy [1,2].
Osteoarthritis (OA) is a degenerative joint disease and has a higher incidence in women after menopause than age-matched men. OA is also more likely to occur in people who are overweight [3,4]. OA is a disease that affects the whole joint, including cartilage, subchondral bone, synovium, tendons, and muscles. Estrogen deficiency during menopause can lead to health problems such as sleeping disorders, vaginal dryness, joint pain, reduced bone density, cardiovascular disease, etc [5,6]. OP and OA remain major and growing epidemiological problems worldwide.
Women receiving estrogen or selective estrogen receptor modulators (SERMs) therapy have a lower risk of developing radiographic knee and hip OA. Achieving a weight loss of 5% of total body weight within a 20-week period was useful for the treatment of knee OA [7]. However, current estrogen replacement therapy using synthetic estrogens has some side effects that include a slight but significant increase in the risk of developing breast and endometrial cancer due to unselective estrogenic action [8,9]. Kudzu root (also known as Ge Gen), the dry radix part of Puerariae Lobatae (Wild), is one of the most common ingredients in traditional Chinese herbs which have been applied in medicine for 1200 years [10]. Kudzu root contains abundant estrogen-like isoflavones in the forms of glycosides and aglycones, such as daidzein, daidzin, genistin, glycitein, and puerarin. Isoflavones, especially puerarin, which are believed to be the primary active constitutes in kudzu extract, belong to a class of phytochemicals called phytoestrogens or SERMs because they exhibit estrogenic and antiestrogenic properties [11,12]. They can act as estrogen agonists or antagonists, depending on endocrine estrogenic levels. Epidemiological studies have suggested that isoflavones may play a preventive role in many hormone-dependent diseases and symptoms that are usually associated with menopause such as hot flashes, OP, and OA [13]. Christgau S, et al. demonstrated that estrogen and SERMs could maintain joint health and suppress cartilage turnover in postmenopausal women and ovariectomized (OVX) rats [14]. The discovery that dietary isoflavones such as daidzein, genistein, biochanin A and formononetin can bind to estrogen receptors (ER) raised the possibility that the phytoestrogens may exert their beneficial effect by modulating estrogenic activity in vivo [15,16]. Much attention has been paid to phytoestrogens which could be used as potential SERMs to estrogen replacement therapy (ERT) for OP and OA [17,18].
Ovariectomy is an FDA-approved golden standard model for postmenopausal osteoporosis, and numerous animal studies indicate a relation between osteoarthritic changes and ovariectomy (OVX) in adult animals [14,19,20]. It is also reported that the estrogen and selective estrogen receptor modulators (SERMs) can prevent cartilage damage and bone loss in OVX model [21,22]. This model is commonly used both for the underlying pathophysiology of the disease and for the assessment of potential treatment modalities [23,24]. For mimicking postmenopausal OP and OA closely, the selection of aged OVX rats (>6 months old), which have already slow growth rates, is the most appropriate. The simplest methods for monitoring treatment effects include the use of biomarkers of osteoblast-mediated bone formation such as osteocalcin, Procollagen type I N-terminal propeptide (PINP), bone-specific alkaline phosphatase (bsALP), as well as osteoclast-mediated bone resorption such as N-terminal telopeptide of collagen type I (NTX-I) and C-terminal telopeptide of collagen type I (CTX-I), [23,25] which all can be measured in serum or urine samples. Validated assays for measuring different parameters of bone and cartilage are available and can be easily combined with this model.
In this study, 56 of CFDA listed Traditional Chinese Medicine (TCM) herbs/roots extracts were screened to determine their ability to prevent bone loss and cartilage degradation in OVX rats as a model of accelerated bone and cartilage degradation, and only one plant (wild kudzu) showed consistently superior joint protective effects. Finally, the kudzu capsules containing kudzu extract and calcium carbonate were investigated at Hunan provincial center for disease control and prevention (CDC, Changsha, Hunan Province, China) for safety and efficacy.
Materials and methods
Preparation of test extracts
A total of 56 plants were purchased from Anqing herbal medicine market (Anhui, China) (Table 1). The plant extracts were dissolved in either ethanol or water and used as test articles for in-vivo screening. 17β-estradiol was dissolved in ethanol and used as a positive control.
Table 1.
List of 56 herbal extracts
| Plant family | Plant species | Plant part used in screening |
|---|---|---|
| Solanaceae | Fructus Lycii | Fruit |
| Araliaceae | Dlender Acanthopanax | Root |
| Panax Notoginseng | Root | |
| Adoxaceae | Sambucus Williamsii Hance | Rhizome |
| Umbelliferae | Foeniculum Vulgare Mill | Seed |
| Orobanchaceae | Desertliving Cistanche | Rhizome |
| Labiatae | Rosemary | Leaf |
| Salvia Miltiorrhiza | Root | |
| Prunella Vulgaris L | Cluster | |
| Berberidaceae | Epimediim | Leaf |
| Dipsacaceae | Dipsacus Asperoides | Root |
| Convolvulaceae | Semen Cuscutae | Seed |
| Eucommiaceae Engler | Cortex Eucommiae | Cortex |
| Citrus crosses | Australia Citrus | Fruit |
| Polypodiaceae | Rhizoma Drynariae | Rhizome |
| Scrophulariaceae | Radix Rehmanniae | Root |
| Poaceae | Wild Oat | Seed |
| Rutaceae | Citrus Medica | Fruit |
| Pericarpium Citri Reticulatae | Cortex | |
| Pomelos/Teaka | Fruit | |
| Grapefruit | Fruit | |
| Tangelo | Fruit | |
| Tangerine | Fruit | |
| Granulated Sugar Tangerine | Fruit | |
| Kumquat | Fruit | |
| Lu Citrus | Fruit | |
| Ponkan | Fruit | |
| Brazil Navel Orange | Fruit | |
| Rock Candy Navel Orange | Fruit | |
| Chinese Navel Orange | Fruit | |
| Newhall Navel Orange | Fruit | |
| Amaranthaceae | Radix Achyranthis Bidentatae | Root |
| Solanaceae | Solanum nigrum L | Rhizome |
| Solanum Melongena L | Root | |
| Polygonaceae | Fallopia Multiflora | Root |
| Rosaceae | Fructus Mume | Fruit |
| Palmleaf Raspberry | Fruit | |
| Prunus persica (L.) Batsch | Cortex | |
| Semen Pruni | Seed | |
| Chinese Red Plum | Fruit | |
| America Plum | Fruit | |
| Chinese Black Plum | Fruit | |
| Plum Seeds | Seed | |
| Plum Leaves | Leaf | |
| Plum Bark | Cortex | |
| Dicksoniaceae | Cibotium Barometz | Rhizome |
| Papilionaceae | Black Soybean | Seed |
| Fabaceae | Root of Lobed Kudzu vine | Root |
| Flos Sophora Immaturus | Flower | |
| Trifolium Repens Linn | Leaf | |
| Fructus Psoraleae | Seed | |
| Leguminosae sp. | Astragalus Complanatus | Root |
| Radix Astragali | Root | |
| Aristolochiaceae | Solanum Lyratum Thunb | Rhizome |
| Magnoliopsida | Chinese Green Plum | Fruit |
| Agavaceae | Rhizoma Anemarrhenae | Rhizome |
Preparation and purification of kudzu extracts
Kudzu root was comminuted and weighed. The pieces were put into a round flask filling less than 3/5 of the bottle volume. Eight times the amount of the powder material of 70% ethanol were added to the flask. The flask was connected to a condenser, and heated at 80°C, for 3 hours twice. The liquid extract was filtered by filter gauze to get clear liquid which was concentrated by rotary evaporator at 50°C to produce a concentrated extract having a density around 1.1-1.2 g/cm3.
The concentrated extract was diluted with water to a solid concentration corresponding to the extractable content of 0.26-0.28 g of raw material per ml of water and a pH value of 5-6. This calculated concentration was used for the estimation of loading volume of the solution to an Ab-8 macroporous adsorption resin column. The diluted extract was purified on the AB-8 to enrich the active ingredients of the extract further and to get rid of some of the impurity substances. The run through was discarded, and the active ingredients of the extract in water solution were retained in AB-8 resin column and were eluted with 70% ethanol. The aqueous solution was absorbed by the AB-8 resin at 2 ml/min, and then desorbed using 70% ethanol at 2 ml/min; the volume of 70% ethanol was six times the amount of resin. We collected the eluted fractions and tested for reactivity with 1% FeCl3. Fractions changing color from yellow to dark green indicating anti-oxidant character were collected. The 70% ethanol elution was rotary evaporated, and concentrated under low pressure at 50°C to produce a concentrated extract which was put into flasks or plates, and frozen at -20°C. The flasks or plates were subsequently put in low-temperature vacuum freeze dryer to produce a dry powder. The yield of solid drug/kg of raw material varied from 50 to 200 g between lots.
Standard puerarin (9.9 mg) was dissolved in 25 ml of 30% ethanol for 3 min, and then ultra-soundly treated for 20 min at 40°C. Take out 2.0 ml, 1.0 ml, 0.5 ml, 0.25 ml, 0.125 ml, 0.0625 ml of the mixture, fill them up to 10 ml with 30% ethanol, and ultra-soundly treat for 20 min at room temperature. Ten milligrams of kudzu extract was dissolved in 50 ml of 30% ethanol, mixed for 3 min, and then ultra-soundly treated for 20 min at 40°C. Filter all the solution by 0.45 μm organic membranes. Ten microliters of the standard and kudzu extract were injected to high-performance liquid chromatography (HPLC, LC-20A, Shimadzu) respectively. The weight/weight yields regarding dry starting material were calculated.
Animals
Female Sprague-Dawley rats (Vitalriver, Beijing, China), six months of age, were housed at the animal facilities at Nordic Bioscience Beijing Ltd. Animals were acclimatized, weighed and stratified into groups of seven animals per group and mean ± 95% confidence interval (95% CI) body weights from 342 ± 19 g to 352 ± 48 g. The rats were housed two by tow in standard type III H cages with sawdust bedding and nesting material (HFK Bioscience, Beijing, China). They were fed ad libitum with a standard diet (HFK Bioscience, Beijing, China) and had access to purified water (Milli-Q system; Millipore) ad libitum. Rats were maintained under conditions of a 12-hour light/dark cycle.
Sixty female Sprague-Dawley rats (Tianqin Biotech, Changsha, China), six months of age, were housed at the animal facilities of Hunan provincial center for disease control and prevention (CDC). They were fed ad libitum with a standard diet (low calcium casein 23.0%, DL- methionine 0.3%, corn starch 32.0%, sugar 30.0%, fiber 5.0%, corn oil 5.0%, mixed mineral salts 3.5%, mixed vitamin 1.0%, choline bitartrate 0.2%).
Animal study design
The studies were approved by the ethical committees of Nordic Bioscience Beijing, China and Hunan provincial center for disease control and prevention, Changsha, China, respectively.
Screening of herbal extracts in OVX rats
The 6-month-old rats were subjected to surgery and administered orally once daily for three weeks post-surgery with 56 herbal extract articles. The body weight of each animal was recorded once a week and quantity of items adjusted weekly according to new weight recordings. Blood samples for biochemical marker analysis were collected from fasting animals at baseline, and after three weeks. The blood sample was gathered in the morning hours throughout the study. The blood samples were left at room temperature for minimum 30 min to clot and then centrifuged at 1500 g for 10 min and stored at -20°C until use. On completion of the treatment period, the animals were asphyxiated with carbon dioxide (CO2) and killed by exsanguination. The absence and presence of ovaries were checked at necropsy for the OVX and sham-operated animals, respectively. The uterus from each rat was isolated and wet weight was measured at the termination of the study to confirm the success of OVX model. The RatLaps ELISA (IDS, UK) measures collagen type I C-telopeptide degradation products (CTX-I) according to the manufacturer’s instructions [26]. Cross-linked fragments of CTX-II in the serum were measured using the Serum Pre-Clinical CartiLaps ELISA (IDS, UK) [27,28]. All samples were measured in duplicate and samples from the same rat were included on the same streptavidin-coated microtiter plate.
Investigation of kudzu extract in OVX rats
Six groups with seven 6-month-old female SD rats each were subjected to ovariectomy (OVX) or sham surgery and treated orally once daily for 6 weeks with kudzu or vehicle (V) in the following intervention groups: (1) sham + V; (2) OVX + 17β-estradiol; (3) OVX + V; (4) OVX + 1.8 g/kg/day kudzu; (5) OVX + 0.9 g/kg/day kudzu; (6) OVX + 0.45 g/kg/day kudzu. The concentration of kudzu root used in the animal study was decided from previous in-house experiments (data not shown). Body weights were recorded weekly, and blood samples for biochemical marker analysis were collected from fasting animals at baseline, three and six weeks. CTX-I and CTX-II were measured. Only biomarkers data from baseline and three weeks are reported hereafter.
BMD improvement study of kudzu capsules in OVX rats
Rats were subjected to ovariectomy after anesthesia by intraperitoneally injecting 30 mg/kg/day of sodium pentobarbital. They were divided into six groups and administered by gavage once daily with 0.633 g/kg/day CaCO3, deionized water, 0.315, 0.630, and 1.890 g/kg/day kudzu capsule for 90 days. The calcium content in CaCO3 is equivalent to the amount in high-dose kudzu capsule. The kudzu dosages are equivalent to 5, 10, 30 times as much as the recommended doses in human respectively. Since the start of the test, each group of animals was caged, fed with formula feed. The weights were recorded weekly. The rats were sacrificed by cervical dislocation at the end of the experiment, and stripped of the right femur, baked to constant weight. Bone mineral density was measured at the midpoint of the femur and distal bone using SD-1000C Bone mineral measuring instrument (Beijing Research Institute of Uranium Geology). Bone calcium content was measured using atomic absorption spectrophotometer (GBC Avanta M) (flames method).
Statistical analysis
The statistical analysis for the different group comparisons was performed with GraphPad Prism statistic software (version 6.04). Statistical analyses were performed using two-way ANOVA analysis with Dunnett’s post-test. Differences between mean values were considered as statistically significant if P<0.05. Expression data are shown as mean with standard error of the mean (S.E.M.).
Results
Screening of herbal extracts
The overview of 56 herbal extracts was summarized in Table 1. All the herbs/roots extracts were screened to determine their ability to prevent bone loss and cartilage degradation in OVX rats, and only wild kudzu showed consistently superior joint protective effects (data not shown).
Quantification of puerarin in kudzu root extract
The retention time of puerarin in kudzu extract was determined as 11.03 minutes, which is following the theoretical value of standard puerarin (Figure 1A, 1C). The calibration curve was established by plotting the peak area against the concentration of standard puerarin (Figure 1B). The concentration of puerarin in kudzu extract was interpolated accordingly (Figure 1B, 1C). The puerarin concentration and yield are summarized in Table 2, indicating that the mean puerarin yield was 148.1 ± 1.380 g/kg kudzu extract (i.e. 14.8%).
Figure 1.
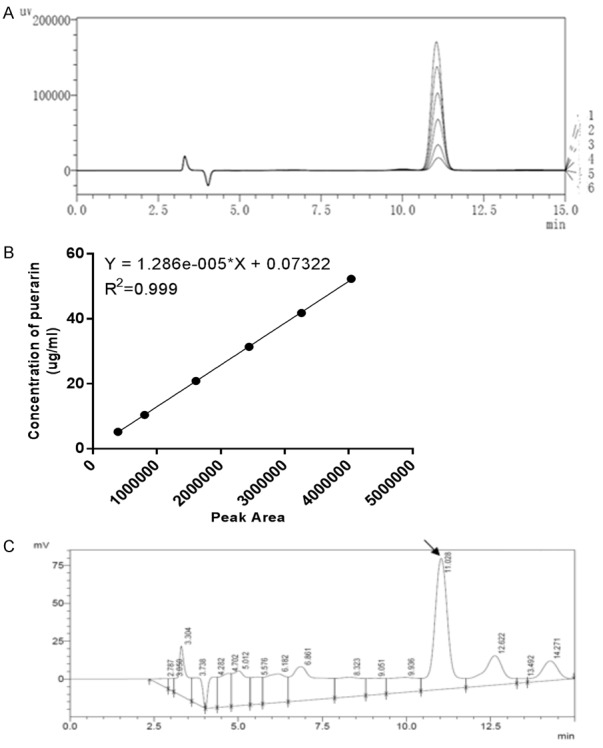
High-performance liquid chromatography (HPLC) analysis of puerarin in kudzu extract. A: The HPLC graph of standard puerarin; B: Calibration curve of standard puerarin; C: The HPLC graph of puerarin in kudzu extract. HPLC parameters: C18 shim-pack VP-ODS (150 L × 4.6 8,052,494); mobile phase: methanol-water (25:75); column temperature: 40°C; flow velocity: 0.8 ml/min; detection wavelength: 250 nm.
Table 2.
Puerarin yield of kudzu extract (batch no.: C11)
| Test nr. | Peak area | Puerarin concentration (µg/ml) | Puerarin yield (g/kg kudzu extract) | Mean puerarin yield (g/kg ± SEM) |
|---|---|---|---|---|
| 1 | 2444504 | 31.51 | 147.8 | 148.1 ± 1.380 (14.8%) |
| 2 | 2431259 | 31.34 | 147.0 | |
| 3 | 2446196 | 31.53 | 147.9 | |
| 4 | 2415641 | 31.14 | 146.1 | |
| 5 | 2471267 | 31.85 | 149.4 | |
| 6 | 2483781 | 32.01 | 150.2 |
Y = C × V × 1000/(M × 1000 × 1000). Y: puerarin yield, unit: g/kg kudzu extract. C: puerarin concentration determined by high performance liquid chromatography (HPLC), unit: μg/mL. V: sample volume, unit: mL. M: sample mass, unit: g.
Quantification of puerarin in kudzu capsule
Kudzu capsules containing kudzu extract, calcium carbonate were characterized by Hunan Provincial CDC. Three different lots (20130125, 20130126, and 20130127) showed puerarin yield (6.31 ± 0.03 g/100 g capsule) and calcium content (13.3 ± 0.06 g/100 g capsule) consistently (Table 3).
Table 3.
Puerarin and calcium content in three batches of kudzu capsule
| Batch 1 (20130125) | Batch 2 (20130126) | Batch 3 (20130127) | Mean ± SEM | |
|---|---|---|---|---|
| Puerarin (g/100 g) | 6.32 | 6.28 | 6.33 | 6.31 ± 0.03 |
| Calcium (g/100 g) | 13.3 | 13.4 | 13.3 | 13.3 ± 0.06 |
Bone loss
Serum CTX-I levels were determined as a biochemical parameter of bone resorption and measured at the baseline just before OVX operation (0 weeks) and three weeks after OVX operation. Three different batches of kudzu extracts (A11, B11, C11) demonstrated the consistent effect on bone loss (Figure 2). Ovariectomy resulted in a marked increase in serum levels of CTX-I (P<0.0001, Figure 3), which could be effectively reversed by 17β-estradiol supplementation (P<0.0001, Figure 3). Treatment with the highest dosage of kudzu counteracted serum CTX-I (P<0.001, Figure 3) 3 weeks after treatment similarly.
Figure 2.
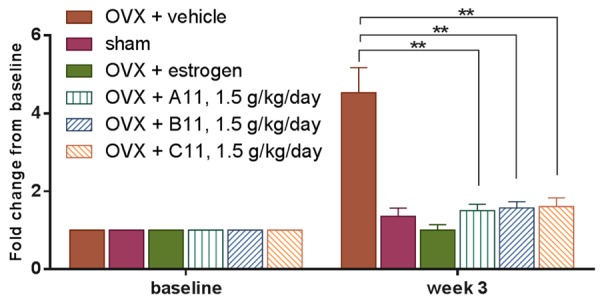
Bone turnover in ovariectomized (OVX) rats treated with vehicle alone (OVX + vehicle), estrogen (OVX + estrogen), or different batches of kudzu extract, given in a high dose (1.5 g/kg. per day). Bone resorption was determined by measurement of matrix metalloproteinase (MMP)-mediated collagen type I fragments (CTX-I). Values for vehicle-treated sham-operated rats (Sham) are also included. The dried extract was re-dissolved in water at a concentration of 120 mg/ml and given to rat daily by gavage. Measurements were made at the weekly intervals shown. Data are mean ± standard error of the mean (SEM) of 10 replicate rats. Two-way ANOVA was used to compare each bar with the vehicle. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Figure 3.
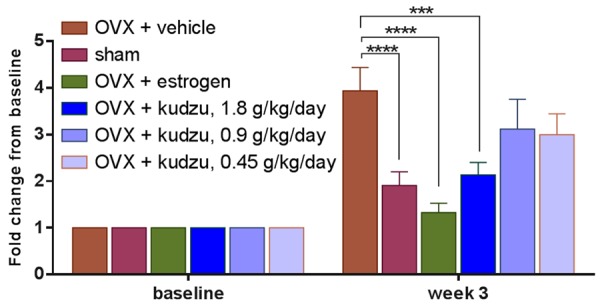
Bone turnover in ovariectomized (OVX) rats treated with vehicle alone (OVX vehicle), estrogen (OVX estrogen), or kudzu extract, given in a low dose (0.45 g/kg. per day), mid dose (0.9 g/kg. per day) and a high dose (1.8 g/kg. per day). Bone resorption was determined by measurement of MMP-mediated collagen type I fragments (CTX-I). Values for vehicle-treated sham-operated rats (Sham) are also included. The dried extract was re-dissolved in water at a concentration of 120 mg/ml and given to rat daily by gavage. Measurements were made at the weekly intervals shown. Data are mean ± standard error of the mean (SEM) of seven replicate rats. Two-way ANOVA was used to compare each bar with the vehicle. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Articular cartilage degradation
Serum CTX-II levels were determined as a biochemical parameter of cartilage degradation and measured at the baseline just before OVX operation (0 weeks) and three weeks after OVX operation. Three different batches of kudzu extracts (A11, B11, C11) demonstrated the consistent effect on cartilage degradation (Figure 4). OVX induced estrogen deficiency results in significantly increased levels of CTX-II at week three after ovariectomy compared to sham group (P<0.0001, Figure 5). The estrogen treatment was able to completely normalize CTX-II levels in the OVX rats (P<0.0001, Figure 5). The kudzu extract showed a significant effect on the reduction of the cartilage degradation product of CTX-II (P<0.0001, Figure 5) no matter what dose of kudzu was administered.
Figure 4.
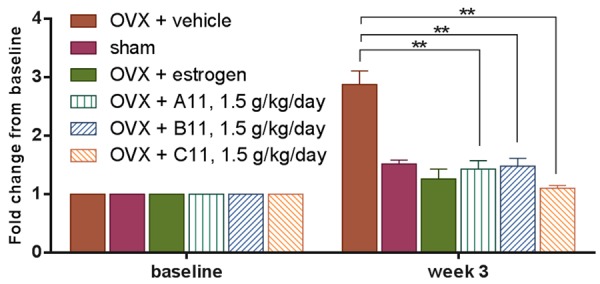
Cartilage turnover in ovariectomized (OVX) rats treated with vehicle alone (OVX + vehicle), estrogen (OVX + estrogen), or different batches of kudzu extract, given in a high dose (1.5 g/kg. per day). Cartilage degradation was assessed using MMP-mediated collagen type II fragments (CTX-II) as a marker. Values for vehicle-treated sham-operated rats (Sham) are also included. The dried extract was re-dissolved in water at a concentration of 120 mg/ml and given to rat daily by gavage. Measurements were made at the weekly intervals shown. Data are mean ± standard error of the mean (SEM) of 10 replicate rats. Two-way ANOVA was used to compare each bar with the vehicle. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Figure 5.
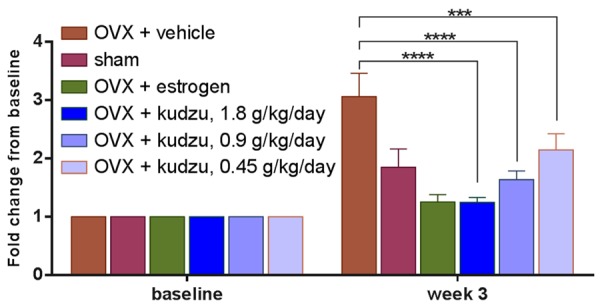
Cartilage turnover in ovariectomized (OVX) rats treated with vehicle alone (OVX + vehicle), estrogen (OVX + estrogen), or kudzu extract, given in a low dose (0.45 g/kg. per day), mid dose (0.9 g/kg. per day) and a high dose (1.8 g/kg. per day). Values for vehicle-treated sham-operated rats (Sham) are also included. The dried extract was re-dissolved in water at a concentration of 120 mg/ml and given to rat daily by gavage. Cartilage degradation was assessed using MMP-mediated collagen type II fragments (CTX-II) as a marker. Measurements were made at the weekly intervals shown. Data are mean ± standard error of the mean (SEM) of seven replicate rats. Two-way ANOVA was used to compare each bar with the vehicle. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Body weight and uterus weight
Ovariectomy induced significant weight gain in the animals, reaching 7.1% in the OVX + vehicle group at week six after ovariectomy, in accordance with the established effects of deprivation of endogenous estrogen production. The corresponding changes in the OVX + estrogen-treated group and the OVX and kudzu extract treated group was -4.1%, -12.4%, -10.7%, and -7.4% respectively, which differ from the OVX + vehicle control group (Figure 6). The uterus from each rat was isolated and wet weight was measured at the termination of the study, confirming that the OVX model was successfully set up, and estrogen, as well as a high dose of kudzu, can encounter the effect of OVX on uterus weight (Figure 7).
Figure 6.
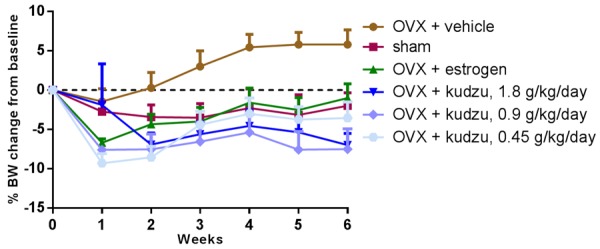
Body weight in ovariectomized (OVX) rats treated with vehicle alone (OVX + vehicle), estrogen (OVX + estrogen), or kudzu extract, given in a low dose (0.45 g/kg. per day), mid dose (0.9 g/kg. per day) and a high dose (1.8 g/kg. per day). Values for vehicle-treated sham-operated rats (Sham) are also included. Measurements were made at the weekly intervals shown. Data are mean ± standard error of the mean (SEM) of seven replicate rats.
Figure 7.
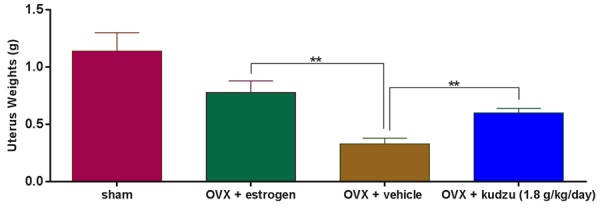
Uterus weights in ovariectomized (OVX) rats treated with vehicle alone (OVX + vehicle), estrogen (OVX + estrogen), or kudzu extract, given in a high dose (1.8 g/kg. per day). Values for vehicle-treated sham-operated rats (Sham) are also included. Data are mean ± standard error of the mean (SEM) of seven replicate rats. Two-way ANOVA was used to compare each bar with OVX + vehicle. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Bone mineral density (BMD) and calcium content
The maximum dosage of kudzu capsules (1.890 g/kg/day) group and the sham group had significantly higher BMD of center femoral and femoral telocentric end (P<0.05, Figure 8) and calcium content (P<0.05, data not shown) in comparison to those from vehicle group. However, CaCO3 had impact neither on BMD nor calcium content, indicating kudzu has the effect of increasing bone mineral density and calcium content in rats.
Figure 8.
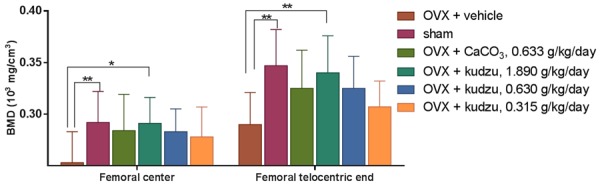
Femoral bone mineral densities in ovariectomized (OVX) rats treated with vehicle alone (OVX + vehicle), CaCO3 (OVX + CaCO3), or kudzu extract, given in a low dose (0.315 g/kg. per day), mid dose (0.630 g/kg. per day) and a high dose (1.890 g/kg. per day). Values for vehicle-treated sham-operated rats (Sham) are also included. Femoral bone mineral densities were determined at the end of the 90-day experimental period. Data are mean ± standard error of the mean (SEM). *P<0.05 vs. OVX + vehicle.
Discussion
In the present study, OVX induced estrogen deficiency resulted in significantly higher levels of MMP-mediated collagen type I and type II fragments, CTX-I, and CTX-II, after ovariectomy when compared to the sham group. This observation was in agreement with the expected increase in bone turnover induced by ovariectomy [14,27]. The estrogen implants were able to normalize CTX-I and CTX-II levels in the OVX rats completely. The kudzu extract increased bone mineral density [12] and demonstrated significant effects on the reduction of bone resorption [29,30], which is in line with the current literature. Surprisingly, it also suppressed the cartilage degradation (CTX-II) in the OVX rats at week 3. Our laboratory previously demonstrated a strong link between CTX-II values and articular cartilage degradation in the ovariectomized rat (see Figure S1, Table S1) [14,27]. In this rodent model where ovariectomy induced an aggressive destruction of joint cartilage as assessed by histological erosion scores and circulating CTX-II was dramatically elevated simultaneously. Both erosion scores and CTX-II were significantly reduced if exogenous estrogen was administered. Our colleagues also observed that two types of SERMs (i.e. levormeloxifene, and cis-3,4-diaryl-hydroxychromanes) suppressed the elevated cartilage turnover (CTX-II) in ovariectomized rats [14,27]. Therefore, we suggest that the reduction of CTX-II in OVX + kudzu rats (kudzu is also a type of SERMs) reflects prevention of destruction of joint cartilage compared to the OVX + vehicle rats with minimal reduction in CTX-II levels.
To our knowledge, this is the first time that kudzu extract demonstrates the chondroprotective effect. It is reported that puerarin may block nuclear factor-kappa B (NF-κB) and tumor necrosis factor-alpha (TNF-α) pathways in human [31]. Our laboratory previously discovered that proinflammatory cytokines (e.g. TNF and Oncostatin M) could induce the expression of MMPs (see Figure S2). The up-regulation of MMPs could be attenuated by TNF pathway inhibitor histologically and biochemically (see Figures S3, S4) [32]. In other words, low CTX-II level represents a down-regulated expression of MMPs. Therefore, we retrospectively speculate that the decreased MMP-degraded fragments, CTX-II, in kudzu-treated rats were due to the suppression of proinflammatory cytokines by kudzu [33]. The observed suppression of circulating levels of CTX-II fragments observed in the ovariectomized rats upon treatment with the kudzu extract is a reflection of reduced degradation of cartilage, and that this regaining of metabolic cartilage balance in an otherwise healthy animal, is unrelated to the relief of any other disease stage or state. Importantly, at the end of the study, animals that received estrogen and kudzu therapy had apparent lower body weights compared with ovariectomized animals, prompting us to address the potential relief effect on weight wearing area of joint in OA patients with high body mass index (BMI). Although significantly reduction of BMI has been observed in obese human by Pueraria thomsonii flower extract [34], Pueraria Lobatae (kudzu root) has not been reported to have an impact on BMI yet.
Moreover, kudzu extract was proved non-toxic in acute oral toxicity test and negative in genetic toxicity tests including Ames test, mouse bone marrow polychromatic erythrocyte micronucleus test, and mice sperm abnormality test. Kudzu had no apparent side effects on stimulating reproductive organs after 30 days feeding in newborn rats (data not shown).
Collectively, these observations clearly demonstrate that kudzu exerts direct effects on articular cartilage in the OVX rat and can effectively prevent the acceleration of cartilage degradation induced by ovariectomy. However, the mechanism by which kudzu exerts its effects is still incompletely understood. We propose the estrogen receptors in chondrocytes are involved in the action of kudzu. Further studies are needed to investigate the potential effects of kudzu extract in postmenopausal women.
In summary, we provided experimental evidence that kudzu can confer chondro-protective effects, as indicated by protection against bone and type II collagen loss in ovariectomized rats. Furthermore, kudzu has demonstrated positive effects on metabolic health (cause a weight reduction), and may, therefore, represent a possible treatment opportunity for OP and subchondral bone turnover-driven OA, with an unhealthy phenotype (e.g. high BMI). Therefore, kudzu may be promising for OP and OA treatment that could affect both articular cartilage and subchondral bone. Additionally, we confirmed that ovariectomized rats could be used as an experimental model for the testing of the chondro-protective effect of novel drug candidates.
Acknowledgements
The authors wish to thank Rui Zhang for help with TCM extraction and animal experiments. The research has received funding from the Danish Science Foundation (Den Danske Forskningsfond).
Disclosure of conflict of interest
None.
Abbreviations
- BMD
Bone Mineral Density
- BMI
Body Mass Index
- bsALP
bone-specific alkaline phosphatase
- CTX-I
C-terminal telopeptide of collagen type I
- CTX-II
C-terminal telopeptide of collagen type II
- ER
Estrogen receptor
- ERT
Estrogen Replacement Therapy
- HPLC
high-performance liquid chromatography
- NTX-I
N-terminal telopeptide of collagen type I
- OA
Osteoarthritis
- OP
Osteoporosis
- OVX
Ovariectomy
- PINP
Procollagen type I N-terminal propeptide
- SEM
standard error of mean
- SERMs
Selective Estrogen Receptor Modulators
- TCM
Traditional Chinese Medicine
Supporting Information
References
- 1.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6:99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 2.Reginster JY, Pelousse F, Bruyère O. Safety concerns with the long-term management of osteoporosis. Expert Opin Drug Saf. 2013;12:507–22. doi: 10.1517/14740338.2013.793669. [DOI] [PubMed] [Google Scholar]
- 3.Abramson SB, Attur M, Yazici Y. Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol. 2006;2:304–312. doi: 10.1038/ncprheum0193. [DOI] [PubMed] [Google Scholar]
- 4.Bay-Jensen AC, Hoegh-Madsen S, Dam E, Henriksen K, Sondergaard BC, Pastoureau P, Qvist P, Karsdal MA. Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis? Rheumatol Int. 2010;30:435–442. doi: 10.1007/s00296-009-1183-1. [DOI] [PubMed] [Google Scholar]
- 5.Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP, Winston M. Cardiovascular disease in women. Circulation. 1993;88:1999–2009. doi: 10.1161/01.cir.88.4.1999. [DOI] [PubMed] [Google Scholar]
- 6.Rymer J, Wilson R, Ballard K. Making decisions about hormone replacement therapy. Br Med J. 2003;326:322–326. doi: 10.1136/bmj.326.7384.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the nonsurgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kauppila A. The use of oestrogens and progestin and the risk of breast cancer in post-menopausal women. Pharmacol Res. 1995;32:327. doi: 10.1016/s1043-6618(05)80035-0. [DOI] [PubMed] [Google Scholar]
- 9.Bolton JL, Pisha E, Zhang F, Qiu S. Role of quinoids in estrogen carcinogenesis. Chem Res Toxicol. 1998;11:1113–1126. doi: 10.1021/tx9801007. [DOI] [PubMed] [Google Scholar]
- 10.Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K. Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol. 2011;134:584–607. doi: 10.1016/j.jep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Kang SC, Lee CM, Choi H, Lee JH, Oh JS, Kwak JH, Zee OP. Evaluation of oriental medicine herbs for estrogenic and anitproliferative activities. Phyther Res. 2006:1017–1019. doi: 10.1002/ptr.1987. [DOI] [PubMed] [Google Scholar]
- 12.Zheng G, Zhang X, Zheng J, Meng Q, Zheng D. Estrogen-like effects of puerarin and total isoflavones from Pueraria lobata. Zhong Yao Cai. 2002;25:566–568. [PubMed] [Google Scholar]
- 13.Woo J, Lau E, Ho SC, Cheng F, Chan C, Chan ASY, Haines CJ, Chan TY, Li M, Sham A. Comparison of pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause. 2003;10:352–61. doi: 10.1097/01.GME.0000054764.94658.33. [DOI] [PubMed] [Google Scholar]
- 14.Christgau S, Tankó LB, Cloos PA, Mouritzen U, Christiansen C, Delaissé JM, Høegh-Andersen P. Suppression of elevated cartilage turnover in postmenopausal women and in ovariectomized rats by estrogen and a selective estrogen-receptor modulator (SERM) Menopause. 2004;11:508–518. doi: 10.1097/01.wcb.0000121484.18437.98. [DOI] [PubMed] [Google Scholar]
- 15.Bao L, Zou S, Zhang S. Dose-dependent effects of daidzein in regulating bone formation through estrogen receptors and peroxisome proliferator-activated receptor γ. Zhong Xi Yi Jie He Xue Bao. 2011;9:165–72. doi: 10.3736/jcim20110209. [DOI] [PubMed] [Google Scholar]
- 16.Meng QS, Zhu XY, Tang XL, Ma B, Ni X. Effect of isoflavones in regulating the transcription of target genes through estrogen receptors. J Chin Integr Med. 2007;5:577–580. doi: 10.3736/jcim20070521. [DOI] [PubMed] [Google Scholar]
- 17.Wang HJ, Zhuang HR, Xiangying Zhang JF. The application and investigation of puerarin in kneen osteoarthritis treatment. Hainan Med. 2006;17:95–96. [Google Scholar]
- 18.Kim SN, Kim HS, Nam GS, Hwang SW, Hwang SY. Inhibition of inflammatory-cytokines production and prostaglandin E2 activity by puerariae radix extracts. J Korean Soc Food Sci Nutr. 2006;35:28–34. [Google Scholar]
- 19.Cake MA, Appleyard RC, Read RA, Smith MM, Murrell GAC, Ghosh P. Ovariectomy alters the structural and biomechanical properties of ovine femoro-tibial articular cartilage and increases cartilage iNOS. Osteoarthr Cartil. 2005;13:1066–1075. doi: 10.1016/j.joca.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, G S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr Cartil. 2007;15:695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Turner AS, Athanasiou KA, Zhu CF, Alvis MR, Bryant HU. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthr Cartil. 1997;5:63–69. doi: 10.1016/s1063-4584(97)80032-5. [DOI] [PubMed] [Google Scholar]
- 22.Tsai CL, Liu TK. Estradiol-induced knee osteoarthrosis in ovariectomized rabbits. Clin Orthop Relat Res. 1993:295–302. [PubMed] [Google Scholar]
- 23.Karsdal MA, Henriksen K, Sørensen MG, Gram J, Schaller S, Dziegiel MH, Heegaard AM, Christophersen P, Martin TJ, Christiansen C, Bollerslev J. Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am J Pathol. 2005;166:467–476. doi: 10.1016/S0002-9440(10)62269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nic Amhlaoibh R, Hoegh-Andersen P, Brünner N, Sørensen A, Winding B, Holst-Hansen C, Karsdal MA, Engsig MT, Delaissé JM, Heegaard AM. Measurement of tumor load and distribution in a model of cancer-induced osteolysis: a necessary precaution when testing novel antiresorptive therapies. Clin Exp Metastasis. 2004;21:65–74. doi: 10.1023/b:clin.0000017205.49933.fe. [DOI] [PubMed] [Google Scholar]
- 25.Christgau S, Bitsch-Jensen O, Hanover Bjarnason N, Gamwell Henriksen E, Qvist P, Alexandersen P, Bang Henriksen D. Serum CrossLaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone. 2000;26:505–511. doi: 10.1016/S8756-3282(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 26.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaissé JM. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18:859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 27.Høegh-Andersen P, Tankó LB, Andersen TL, Lundberg CV, Mo JA, Heegaard AM, Delaissé JM, Christgau S. Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res Ther. 2004;6:R169–R180. doi: 10.1186/ar1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouritzen U, Christgau S, Lehmann HJ, Tankó LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62:332–336. doi: 10.1136/ard.62.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Sun S, Tong E. Experimental study on the protective effect of puerarin to Parkinson disease. J Huazhong Univ Sci Technol Med Sci. 2003;23:148–150. doi: 10.1007/BF02859940. [DOI] [PubMed] [Google Scholar]
- 30.Weaver CM, Martin BR, Jackson GS, McCabe GP, Nolan JR, McCabe LD, Barnes S, Reinwald S, Boris ME, Peacock M. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using 41Ca methodology. J Clin Endocrinol Metab. 2009;94:3798–3805. doi: 10.1210/jc.2009-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu XJ, Zhao J, Gu XY. The effects of genistein and puerarin on the activation of nuclear factor-kappaB and the production of tumor necrosis factor-alpha in asthma patients. Pharmazie. 2010;65:127–31. [PubMed] [Google Scholar]
- 32.Sondergaard BC, Schultz N, Madsen SH, Bay-Jensen AC, Kassem M, Karsdal MA. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation-divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthr Cartil. 2010;18:279–288. doi: 10.1016/j.joca.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Jiang XX, Li Y, Wu YB. Effect of puerarin preconditioning on cytokine levels in patients undergoing cardiopulmonary bypass in perioperative period. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:1089–1091. [PubMed] [Google Scholar]
- 34.Kamiya T, Takano A, Matsuzuka Y, Kusaba N, Ikeguchi M, Takagaki K, Kondo K. Consumption of pueraria flower extract reduces body mass index via a decrease in the visceral fat area in obese humans. Biosci Biotechnol Biochem. 2012;76:1511–7. doi: 10.1271/bbb.120235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


