Abstract
Background
We implemented and evaluated a large health system-wide hepatitis C virus (HCV) screening and linkage to care program for persons born between 1945 and 1965 (“baby boomers”).
Methods
An electronic health record (EHR) clinical decision support (CDS) tool for HCV screening for baby boomers was introduced in August 2015 for patients seen in the outpatient University of California, Los Angeles healthcare system setting. An HCV care coordinator was introduced in January 2016 to facilitate linkage to HCV care. We compared HCV testing in the year prior (August 2014–July 2015) to the year after (August 2015–July 2016) implementation of the CDS tool. Among patients with reactive HCV antibody testing, we compared outcomes related to the care cascade including HCV ribonucleic acid (RNA) testing, HCV RNA positivity, and linkage to HCV specialty care.
Results
During the study period, 19606 participants were screened for HCV antibody. Hepatitis C virus antibody screening increased 145% (from 5676 patients tested to 13930 tested) after introduction of the CDS intervention. Screening increased across all demographic groups including age, sex, and race/ethnicity, with the greatest increases among those in the older age groups. The addition of an HCV care coordinator increased follow-up HCV RNA testing for HCV antibody positive patients from 83% to 95%. Ninety-four percent of HCV RNA positive patients were linked to care postimplementation.
Conclusions
Introduction of an EHR CDS tool and care coordination markedly increased the number of baby boomers screened for HCV, rates of follow-up HCV RNA testing, and linkage to specialty HCV care for patients with chronic HCV infection.
Keywords: baby boomers, care cascade, hepatitis C virus, linkage to care, screening
The US Centers for Disease Control and Prevention and US Preventive Services Task Force (USPSTF) recommend a one-time hepatitis C virus (HCV) screening for adults born between 1945 and 1965 (a birth cohort known as “baby boomers”) [1, 2]. Approximately three-quarters of persons chronically infected with HCV are baby boomers, many of whom are unaware of their infection [2, 3].
Efforts to increase case identification and increase linkage to care and treatment have had mixed results [4, 5]. The biggest gaps are in screening and linkage to care [5]. It is essential that those particular areas are optimized, to increase downstream treatment rates and improve HCV-related patient outcomes, reduce future healthcare costs, and limit HCV transmission [6].
We implemented an HCV screening and linkage to care initiative within the University of California, Los Angeles (UCLA) Health, a large complex health system with a broad catchment area, following recommendations from the USPSTF for one-time HCV screening in persons born between 1945 and 1965 [1]. Our aim in this analysis was to report on the implementation of the program and to evaluate its impact on HCV screening and linkage to care.
METHODS
The Health System and Introduction of a Hepatitis C Virus Screening Reminder
UCLA Health consists of approximately 60 outpatient primary care practices throughout Southern California serving approximately 180000 patients, and it uses an Epic-based electronic health record (EHR) system (Epic Systems Corporation, Verona, WI). We first obtained approval from key stakeholders including hospital leadership, primary care group directors, the information technology unit, and the clinical laboratory. After those administrative approvals, in August 2015 HCV screening status was added to the routine health maintenance reminder, a clinical decision support (CDS) tool in the EHR, for patients born between 1945 and 1965. Within the health maintenance reminder, HCV screening was noted as “due” among patients with no laboratory evidence of HCV testing (HCV antibody or HCV ribonucleic acid [RNA]) in their EHR or if testing was not marked as “completed” by the provider. The CDS tool was available in all charts, but it only appeared on the patient’s dashboard if previously activated by the provider.
Patient Care Coordination
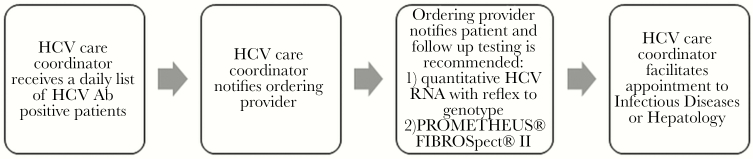
A full-time, nonclinically trained, dedicated HCV care coordinator was introduced in January 2016 in the UCLA Health hepatology unit to facilitate follow up and linkage to care for HCV positive patients. The UCLA microbiology laboratory pulled HCV antibody results from the ADVIA Centaur XP System (Siemens Medical Solutions USA, Inc., Malven, PA), and, through a secure intrahealth system, sent a daily list of all HCV antibody reactive patients to the HCV care coordinator by e-mail. The care coordinator used an internal electronic message to contact outpatient providers to recommend the following: (1) quantitative HCV RNA testing with reflex to HCV genotype testing and (2) noninvasive serum fibrosis testing by PROMETHEUS FIBROSpect II (PROMETHEUS Therapeutics and Diagnostics, San Diego, CA). The care coordinator did not order tests at any time. The provider informed patients of test results via telephone, the EHR patient portal, mailed letters, or at the patient’s next visit. After informing the patient, the provider contacted the care coordinator to facilitate linkage to care through a referral to hepatology or infectious diseases for patients with detectable HCV RNA. A flowchart depicting the HCV care coordination pathway is presented in Figure 1. The HCV care coordinator then telephoned the patient to help schedule follow-up visits. Patients indicating they did not wish to be linked to care during the initial call were given the care coordinator’s direct contact number for future follow up.
Figure 1.
Hepatitis C virus (HCV) linkage to care process. Ab, antibody; RNA, ribonucleic acid.
Data Collection
Data were abstracted from the EHR for 2 periods: August 1, 2014 through July 31, 2015 (preimplementation of the HCV screening intervention) and August 1, 2015 through July 31, 2016 (postimplementation). Patients were eligible for inclusion if they (1) were born between January 1, 1945 and December 31, 1965, (2) had a primary care visit between August 1, 2014 and July 31, 2016, (3) were seen at one of the outpatient clinics within UCLA Health, and (4) were tested for HCV during their visit. Patients without previous documentation of positive HCV antibody or HCV RNA testing within the EHR or a diagnosis in their medical record, as determined by electronic chart review, were included in the analysis. Data abstraction included age, sex, race/ethnicity, as well as HCV testing related data, including HCV RNA testing and linkage to care. Linkage to care was defined as whether a patient attended an initial appointment with an infectious diseases or hepatology specialist within 6 months of receiving the HCV antibody positive results as documented in the EHR. The UCLA Institutional Review Board approved the study before data collection.
Data Analysis
Summary statistics for demographic and clinical characteristics of HCV tested patients overall and by study period (pre- and postimplementation) were calculated. The χ2 tests were used to compare patient characteristics between the pre- and postimplementation period and between screened and unscreened patients. Number of HCV antibody tests, HCV antibody positivity rate among those tested, and rate of HCV RNA testing for HCV antibody positive patients were assessed for the overall cohort and by age, sex, and race/ethnicity. Differences in rates of HCV antibody positivity by these demographics were evaluated using χ2 tests. The change in the number of HCV antibody tests and rates of HCV antibody positivity over the period from preimplementation to postimplementation were assessed using different techniques of time series analysis including a Cochran-Armitage test for trend, a modified Mann-Kendall test, and a segmented regression analysis of interrupted time series [7]. The interrupted time series analysis allowed us to examine the intervention effect while taking into account time trends as well as any autocorrelations among the observations. The outcomes of interest (HCV testing and antibody positivity) were divided into 12 monthly data points before implementation of the intervention compared with the 12 monthly data points postimplementation. To evaluate the effectiveness of the HCV care coordinator, data were also analyzed comparing 2 periods: January 1, 2015–July 31, 2015 (pre-HCV care coordinator) and January 1, 2016–July 31, 2016 (post-HCV care coordinator), resulting in 87 antibody positives with a record available for review during the pre-HCV care coordinator period and 131 antibody positives during the post-HCV care coordinator period. All analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC).
RESULTS
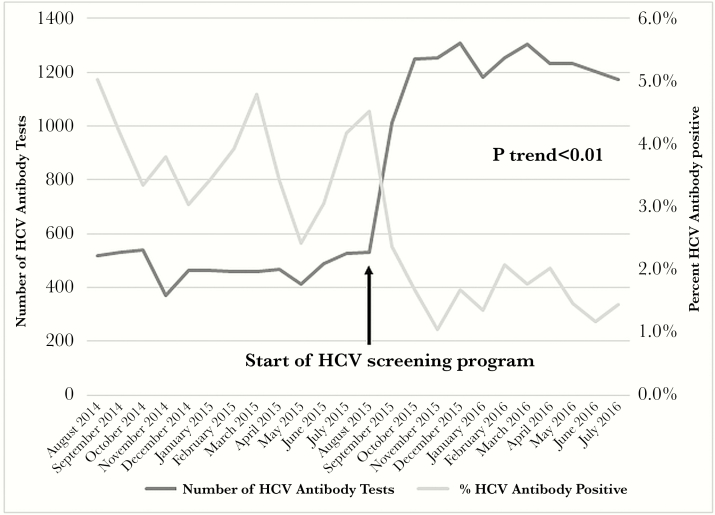
A total of 19606 patients were tested over the 2-year program evaluation period, with a greater proportion of women compared with men (54% vs 46%) and whites representing the single largest race/ethnic group (65%), followed by Hispanics/Latinos (12%) (Table 1). The number of those newly tested each month increased from 519 patients per month in August 2014 to 1117 patients per month by July 2016, with a dramatic increase noted immediately after program implementation in August 2015 (P for trend <.01), with testing frequency remaining stably high subsequently (Figure 2). Increases in HCV antibody testing were noted across all groups of age, sex, and race/ethnicity, with significant increases noted among those in the older age groups within the cohort (Table 1). A 205% increase in HCV testing was noted among those in the oldest age group within the birth cohort (ie, 65–71 years) when comparing the numbers tested for HCV antibody preprogram implementation to postimplementation. Among the racial/ethnic groups, testing increased most substantially among Asian and white racial/ethnic groups (by 121% and 151%, respectively). The distribution of HCV antibody testing by age and sex appeared to closely follow the demographic distribution of primary care visits for baby boomers during the time period of interest. Between August 2015 and July 2016, 54.5% of primary care visits among those in the birth cohort was among women and 45.5% of visits was among men, mirroring the distribution of HCV testing (53.5% women and 46.5% men).
Table 1.
HCV Testing Before and After Implementation of an HCV Screening Program Among “Baby Boomers”, UCLA Health System, August 2014–July 2016
| Patient Characteristics | Total (n = 19606) | August 2014–July 2015 (n = 5676)a | August 2015–July 2016 (n = 13930)a | %Increase in HCV Testing | P Value | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Age, years | <.01 | |||||||
| 49–54 | 5549 | 28.3 | 1854 | 32.7 | 3695 | 26.5 | 99.3 | |
| 55–59 | 5069 | 25.9 | 1529 | 26.9 | 3540 | 25.4 | 131.5 | |
| 60–64 | 4634 | 23.7 | 1219 | 21.5 | 3415 | 24.5 | 180.1 | |
| 65–71 | 4354 | 22.2 | 1074 | 18.9 | 3280 | 23.6 | 205.4 | |
| Sex | <.01 | |||||||
| Female | 10637 | 54.2 | 3192 | 56.2 | 7445 | 53.5 | 133.2 | |
| Male | 8969 | 45.8 | 2484 | 43.8 | 6485 | 46.5 | 161.1 | |
| Race/Ethnicity | <.01 | |||||||
| Asian | 1654 | 9.6 | 515 | 10.0 | 1139 | 9.4 | 121.2 | |
| Black or African American | 1172 | 6.8 | 419 | 8.1 | 753 | 6.2 | 79.7 | |
| Hispanic or Latino | 2033 | 11.8 | 687 | 13.4 | 1346 | 11.1 | 95.9 | |
| Other | 1230 | 7.1 | 353 | 6.9 | 877 | 7.3 | 148.4 | |
| White | 11144 | 64.6 | 3169 | 61.6 | 7975 | 66.0 | 151.7 | |
Abbreviations: HCV, hepatitis C virus; UCLA, University of California, Los Angeles.
aSum may not equal to total due to missing data
Figure 2.
Hepatitis C virus (HCV) antibody testing and percentage positivity before and after implementation of an HCV screening program among baby boomers tested through UCLA Health system, August 2014–July 2016.
Not surprisingly, as testing increased, HCV antibody positivity declined from an average of 4.1% preprogram implementation to 1.5% after program implementation (P value for trend <.01) (Figure 2). In the year before program implementation, a total of 5676 patients were tested for HCV antibody; in the year after program implementation, the number tested increased to 13930 patients, representing a 145% overall increase in testing. Hepatitis C virus antibody positivity varied by age and sex, with a higher prevalence among those aged 60–64 years than 49–54 years (2.6% vs 1.6%; P value < .01) and a higher prevalence among males compared with females (3.1% vs 1.5%; P < .01) (Table 2). Hepatitis C virus antibody positivity differed by race/ethnicity, with the highest prevalence noted among African Americans (4.4%) followed by Hispanics (4.1%) in the postimplementation period (P < .01). Hepatitis C virus RNA was detected (postimplementation) in 44% of those testing antibody positive. No differences were noted in HCV RNA detection by age or race/ethnicity, although the proportion with detectable HCV RNA was higher among men compared with women (50% vs 39%; P = .01).
Table 2.
HCV Antibody Positivity Before and After Implementation of an HCV Screening Program Among “Baby Boomers”, UCLA Health System, August 2014–July 2016
| Patient Characteristics | August 2014–July 2015 (n = 5676)a | August 2015–July 2016 (n = 13930)a | Total (n = 19606) | P Value* | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, years | <.01 | ||||||
| 49–54 | 45 | 2.4 | 43 | 1.2 | 88 | 1.6 | |
| 55–59 | 63 | 4.1 | 53 | 1.5 | 116 | 2.3 | |
| 60–64 | 45 | 3.7 | 76 | 2.2 | 121 | 2.6 | |
| 65–71 | 37 | 3.5 | 68 | 2.1 | 105 | 2.4 | |
| Sex | <.01 | ||||||
| Female | 66 | 2.1 | 90 | 1.2 | 156 | 1.5 | |
| Male | 124 | 5.0 | 150 | 2.3 | 274 | 3.1 | |
| Race/Ethnicity | <.01 | ||||||
| Asian | 14 | 2.7 | 13 | 1.1 | 27 | 1.6 | |
| Black or African American | 23 | 5.5 | 29 | 3.9 | 52 | 4.4 | |
| Hispanic or Latino | 36 | 5.2 | 46 | 3.4 | 82 | 4.1 | |
| Other | 14 | 4.0 | 19 | 2.2 | 33 | 2.7 | |
| White | 89 | 2.8 | 102 | 1.3 | 191 | 1.7 | |
Abbreviations: HCV, hepatitis C virus; UCLA, University of California, Los Angeles.
aSum may not equal to total due to missing data.
*P value reflects comparisons of HCV antibody positivity for each demographic characteristic.
Although the proportion of HCV antibody positivity declined with increased testing, there was a modest increase in the absolute number of those testing HCV antibody positive during the postimplementation period, most notably among 60- to 71-year-olds (from 72 positive tests preimplementation to 146 positive tests postimplementation, a 103% increase) and women (from 66 to 90 positive tests, a 36% increase).
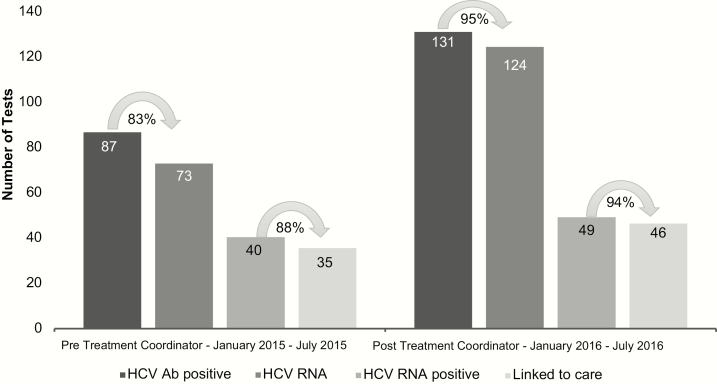
In addition, inclusion of an HCV care coordinator appeared instrumental in maintaining high levels of uptake across the HCV care continuum. In the period after the addition of the HCV care coordinator, the percentage of HCV antibody positive patients who received HCV RNA testing increased from 83% (pretreatment coordinator) to 95% (P < .01) (Figure 3). The absolute number of those identified with active HCV infection (ie, HCV RNA positive) increased from 40 in the preimplementation to 49 in the postimplementation period (Figure 3). The HCV care coordinator was successful in facilitating linkage to care for 94% of those identified as HCV RNA positive, a modest (nonsignificant) increase from the preimplementation period.
Figure 3.
Absolute number of hepatitis C virus (HCV) antibody positive, HCV ribonucleic acid (RNA) tested, HCV RNA positive, and linked to care, before and after addition of a HCV care coordinator.
DISCUSSION
Introduction of an HCV screening reminder through an EHR CDS tool led to a marked increase in HCV testing among the birth cohort, demonstrating the effectiveness of an EHR intervention to facilitate uptake of guideline-based recommendations throughout a large health system. The increase in testing suggests acceptance of the EHR reminder by providers. Acceptance of the reminder was likely facilitated by (1) a substantial investment in engaging and obtaining the support of key stakeholders and health system leadership and (2) minimal disruption to physician workflow as HCV screening was added to an existing health maintenance reminder. Clinical decision support tools have been implemented for other screening and prevention recommendations including colorectal cancer screening, resulting in higher screening rates over time among physicians receiving EHR reminders [8].
The EHR intervention increased HCV screening similarly to electronic interventions in other studies [9, 10]. The simplicity and low cost of the EHR intervention and widespread use of EHR systems with health maintenance reminders or other CDS tools suggests that our EHR intervention may work across different health systems. The impact of a dedicated care coordinator on follow up of HCV RNA testing and linkage to care is also likely generalizable to other health systems, because the function of the coordinator can easily be reproduced and the use of a care coordinator might be cost effective. Our study demonstrated that a clinically trained coordinator is not required to link individuals to care effectively and thus nonclinically trained professionals may be more affordable for other health systems.
In the BEST-C Study, the cost of different approaches to screening was assessed, comparing an electronic best practices alert (BPA) as well as medical assistant placement of an HCV test order with repeated mailing and patient solicitation [10]. The authors found that the BPA intervention had the lowest incremental cost per HCV antibody test completed, an approach for which, similar to the intervention in the current study, there were initial start-up costs for information technology related development of the BPA and staff training, but no subsequent costs assessed for implementation. It is notable that in BEST-C, but not in the approach reported in the current study, personnel effort was included in the intervention to initiate an HCV test order, but the ongoing cost of the medical assistant (beyond initial training) was not included in the calculation of the cost of the BPA arm. The cost of programming the EHR health maintenance reminder is expected to be lower than that of programming a BPA, but the uptake of a health maintenance reminder and BPA may differ and influence its cost effectiveness.
Testing increased most notably in select subgroups of older age, Asian, and whites, groups that may have previously been perceived as lower risk by healthcare providers. As testing increased, we saw an expected decrease in HCV antibody positivity, consistent with a shift to screening based predominantly on birth cohort status, whereas preprogram implementation screening may have included a combination of risk factor-driven testing (enriching the population with HCV infection) with some birth cohort-based screening. The previous approach would have been more subjected to provider bias or lack of awareness regarding risk factors for HCV infection, leading to underscreening of the target population [11].
The prevalence of HCV antibody positivity in our cohort (1.5%) is lower than that observed in other studies of HCV screening in baby boomers, likely due to differences in the sociodemographic makeup of the UCLA Health patient population compared with other cohorts. Jonas et al [9] described a prevalence of 3.25% in a Kaiser Permanente cohort from Maryland, Virginia, and the District of Columbia, and Goel et al [12] described a prevalence of 3.3% in a New York City university-based primary care cohort of persons born between 1945 and 1965; both groups showed a greater proportion of black and Hispanic patients. A review of HCV seroprevalence in state prisons showed a wide range of seroprevalence from 7.5% to 41.2% between states, and the prevalence among Hispanic subpopulations also varies by Hispanic/Latino background and city, with the highest prevalence described in the Bronx and lower prevalence in Chicago, Miami, and San Diego [3, 13]. The lower HCV seroprevalence in our study may also be explained by differences in the selection of the study population, whereby we used both previous laboratory testing in the EHR as well as chart review to exclude previously diagnosed cases of HCV. Other cohorts only excluded cases based on laboratory testing available in the EHR, sometimes within limited periods, thus potentially including more patients with known HCV infection. We did observe a higher prevalence of HCV antibody positivity among African Americans and men in our cohort, consistent with other studies [9, 14–16].
A lower HCV RNA positivity than expected was also observed in our cohort, with a proportion of 44% in the postimplementation/postcare coordination period compared with 75.9% and 84% in other studies [9, 12]. Those differences may also be explained in part by differences in the racial/ethnic makeup of the cohorts, as black or African American individuals have a lower rate of spontaneous HCV clearance compared with whites, which is partly explained by host genetic differences [17], and our cohort had a smaller proportion of black patients.
Our findings support the use of CDS and dedicated HCV care coordination to successfully scale-up HCV screening and linkage to care within a health system. Barriers to successful linkage were minimal in the postimplementation period, as noted in Figure 3. The only barrier to linkage identified was patients stating that they were not ready for an appointment with an HCV specialist at the time they received a call from the HCV care coordinator.
There were other external factors to consider as possible limitations to the interpretation of our findings, such as ongoing advertisements in the media and other news reports as well as changes in legislation, litigation, and policy related to HCV that we were unable to control for in this study. Another factor influencing higher screening might have been changes in the number of clinical sites served over the observation period. Those factors may have influenced higher screening and linkage to care rates as awareness about HCV and available treatments in the general population increased. Although those factors might have influenced higher screening and linkage to care rates, the sharp increase in screening and lack of continued increase over time suggest that the impact of those factors was minimal. A further limitation is that the CDS tool required activation as well as purposeful review by providers, and thus not all providers would have been reminded of the screening recommendation. Follow-up HCV RNA testing to assess for active HCV infection also required a provider to order HCV RNA testing and that the patient return for follow-up specimen collection. To address those barriers to screening and confirmation of HCV infection, the allowance of a dedicated staff member to activate the CDS tool on all patients and the use of reflex HCV RNA testing for positive HCV antibody tests could further improve outcomes [9]. Implementation of the screening program required (1) health system leadership and (2) participation from various departments that might limit feasibility in a more resource-constrained health delivery setting.
An additional limitation of the study was that the preimplementation data were collected retrospectively. Approximately 20% of the anti-HCV positive patients in that period had missing HCV RNA testing data. It is possible that some patients in the preimplementation period had screening or follow up outside of UCLA that was not captured.
CONCLUSIONS
Implementation of national guidelines for HCV screening at the health system level through a multipronged approach, including decision support tools within the EHR and dedicated care coordination, although requiring substantial initiative, has the potential to rapidly scale up HCV screening and reduce gaps at every step in the HCV care cascade. With the current availability of highly effective and well tolerated curative HCV therapy, such an investment is likely to have an impact on decreasing the HCV-related disease burden, improving patient outcomes, and reducing future healthcare costs.
Acknowledgments
We thank Dr. Omai Garner and the UCLA Microbiology Laboratory staff for help and support with data retrieval. We also thank Arash Shamsian from UCLA Information Services & Solutions.
Financial support. The National Center for Advancing Translational Sciences at the National Institutes of Health with the University of California, Los Angeles (UCLA) Clinical and Translational Science Institute (UL1TR001881) provided funding for data extraction and preparation. UCLA Health provided funding for the modification of the EHR. Gilead Sciences, Incorporated as Frontlines of Communities in the United States (FOCUS) program partners (20161077) provided funding for a bachelor level coordinator as well as part of the time and effort of the principal investigator and data analyst.
Potential conflicts of interest. K. W. C. reports other funding from the Gilead Focus Award, during the conduct of the study; and grants from Merck Sharp & Dohme, outside the submitted work. S. S. is a part of the advisory board, a consultant, and on the speaker bureau for AbbVie, BMS, Gilead, Janssen, and Merck.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Moyer VA; U.S. Preventive Services Task Force Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 159:349–57. [DOI] [PubMed] [Google Scholar]
- 2. Smith BD, Morgan RL, Beckett GA et al. . Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep 2012; 61:1–32. [PubMed] [Google Scholar]
- 3. Edlin BR, Eckhardt BJ, Shu MA et al. . Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015; 62:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linas BP, Barter DM, Leff JA et al. . The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One 2014; 9:e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ward JW. The epidemiology of chronic hepatitis C and one-time hepatitis C virus testing of persons born during 1945 to 1965 in the United States. Clin Liver Dis 2013; 17:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 8. Sequist TD, Zaslavsky AM, Marshall R et al. . Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med 2009; 169:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jonas MC, Rodriguez CV, Redd J et al. . Streamlining screening to treatment: the hepatitis C cascade of care at Kaiser Permanente Mid-Atlantic States. Clin Infect Dis 2016; 62:1290–6. [DOI] [PubMed] [Google Scholar]
- 10. Brady JE, Liffmann DK, Yartel A et al. . Uptake of hepatitis C screening, characteristics of patients tested, and intervention costs in the BEST-C study. Hepatology 2017; 65:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrante JM, Winston DG, Chen PH, de la Torre AN. Family physicians’ knowledge and screening of chronic hepatitis and liver cancer. Fam Med 2008; 40:345–51. [PubMed] [Google Scholar]
- 12. Goel A, Sanchez J, Paulino L et al. . A systematic model improves hepatitis C virus birth cohort screening in hospital-based primary care. J Viral Hepat 2017; 24:477–85. [DOI] [PubMed] [Google Scholar]
- 13. Kuniholm MH, Jung M, Everhart JE et al. . Prevalence of hepatitis C virus infection in US Hispanic/Latino adults: results from the NHANES 2007–2010 and HCHS/SOL studies. J Infect Dis 2014; 209:1585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spradling PR, Rupp L, Moorman AC et al. . Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis 2012; 55:1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cartwright EJ, Rentsch C, Rimland D. Hepatitis C virus screening practices and seropositivity among US veterans born during 1945–1965. BMC Res Notes 2014; 7:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coyle C, Viner K, Hughes E et al. . Identification and linkage to care of HCV-infected persons in five health centers–Philadelphia, Pennsylvania, 2012–2014. MMWR Morb Mortal Wkly Rep 2015; 64:459–63. [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas DL, Thio CL, Martin MP et al. . Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]