Summary
Acute kidney injury (AKI) occurs commonly in human immunodeficiency virus–infected patients with cryptococcal meningitis treated with 2 weeks of amphotericin B deoxycholate and is associated with mortality. Urine protein may play a role in earlier diagnosis of AKI but needs further evaluation.
Keywords: acute kidney injury, amphotericin B, biological marker, cystatin C, neutrophil gelatinase-associated lipocalin, protein, tissue inhibitor of metalloproteinase-2
Abstract
Background
Cryptococcus is the most common etiology of adult meningitis in Africa. Amphotericin B deoxycholate remains paramount to treatment, despite toxicities, including acute kidney injury (AKI). We assessed the ability of the following urine markers to predict AKI in patients who received amphotericin B: urine neutrophil gelatinase-associated lipocalin (NGAL), cystatin C (CysC), tissue inhibitor of metalloproteinases-2 (TIMP-2), and protein.
Methods
One hundred and thirty human immunodeficiency virus (HIV)–infected participants with cryptococcal meningitis were enrolled and received amphotericin and fluconazole for 2 weeks. We defined AKI as glomerular filtration rate (GFR) < 60 mL/min/1.73 m2; measured urine NGAL, CysC, TIMP-2, and protein; and explored AKI incidence, risk factors, and associations with mortality using Cox proportional hazards models.
Results
Participants were 48% female with a median age of 35 years, a median CD4 count of 21 cells/μL, and 44% died within 12 months. Incident AKI occurred in 42% and was associated with mortality (adjusted hazard ratio [aHR] = 2.8; P < .001). Development of AKI was associated with female sex (P = .04) and with higher CD4 count (49 vs 14 cells/μL; P < .01). Urine protein level in the highest quartile independently predicted AKI and mortality (aHR = 1.64, P = .04; aHR = 2.13, P = .02, respectively). Urine NGAL levels in the highest quartile independently predicted AKI (aHR = 1.65; P = .04).
Conclusions
Acute kidney injury occurred in 42% of patients, and AKI was associated with mortality. Urine biomarkers, specifically urine protein, may be useful for antecedent prediction of amphotericin-associated AKI but need further evaluation.
Cryptococcal meningitis is a disseminated fungal infection caused by Cryptococcus species and remains a major cause of mortality in human immunodeficiency virus (HIV)–infected persons, especially in resource-limited settings [1]. Cryptococcosis causes approximately 2% of deaths of HIV-infected persons in the United States [2] and approximately 15% of AIDS-related deaths in sub-Saharan Africa [1, 3, 4]. Liposomal amphotericin formulations remain unavailable in resource-limited settings due to cost; thus amphotericin B deoxycholate (hereafter amphotericin) remains the key treat ment for cryptococcosis despite numerous toxicities. The most common and severe side effect of amphotericin is renal toxicity, which has been reported in 49%–65% of patients [5]. Renal toxicity is caused through a combination of mechanisms, which are incompletely understood. First, systemic and renal afferent arteriolar vasoconstriction decrease kidney perfusion [6], and the resulting decrease in glomerular filtration rate (GFR) may be mitigated by aggressive saline hydration [7, 8]. Second, regardless of hydration status, direct cumulative damage to renal tubular cells occurs from induction of proinflammatory cytokines, channel formation across renal cell membranes, and apoptosis [9, 10].
Acute kidney injury (AKI) is a clinical syndrome, which has wide etiologies and was defined, staged, and classified in 2012 [11]. Acute kidney injury predicts mortality, independent of underlying disease [12], and AKI is a common complication of HIV infection [13, 14]. Definition and staging of AKI depends on serum creatinine or urine output [11], which are both late indicators of kidney injury. This has led to a wide interest in new urine and serum biomarkers that could detect AKI earlier in critically ill patients [15]. Neutrophil gelatinase-associated lipocalin (NGAL; also known as Lipocalin-2) is a 25-kDa protein produced in many tissues, including renal epithelial cells, and is an established sensitive early marker of acute ischemic kidney injury [16, 17]. Elevated urine NGAL levels predict a composite outcome of dialysis initiation or death during hospitalization when used as a risk stratification tool in unselected emergency department patients at the time of admission to the hospital [18]. Cystatin C (CysC), a 13-kDa proteinase inhibitor, can be measured in serum and in urine and, unlike creatinine, is not affected by muscle mass or age [19]. Urine CysC is a marker of tubular dysfunction or damage [20], and elevated levels may be associated with AKI [15]. Tissue inhibitor of metalloproteinases-2 (TIMP-2) is an inducer of G1 cell-cycle arrest, predicts severe AKI, and is associated with mortality in critically ill adult patients [21]. Neutrophil gelatinase-associated lipocalin and CysC are induced during inflammation. These biomarkers have not been evaluated in the context of HIV infection and opportunistic infections. In resource-limited settings where liposomal amphotericin is not available, a urine biomarker that could predict development of AKI early and accurately could be a valuable clinical tool to tailor duration of amphotericin therapy.
We assessed these urine biomarkers together with urine protein and creatinine levels in patients with HIV-associated cryptococcal meningitis treated with amphotericin therapy and investigated association with AKI and mortality.
METHODS
Human immunodeficiency virus–infected, antiretroviral therapy (ART)–naive adults with a new diagnosis of cryptococcal meningitis were prospectively enrolled into the Cryptococcal Optimal ART Timing (COAT) trial during 2010–2012 and treated with amphotericin B, fluconazole, and ART according to the COAT trial protocol [22, 23]. Baseline and follow-up serum creatinine concentrations were measured at regular intervals as per the COAT trial protocol (5–6 time points during amphotericin treatment and at 1 and 2 weeks after completion of amphotericin therapy). Estimated GFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation [24], and AKI was defined as a decrease in GFR to <60 mL/min/1.73 m2 at any point within 3 weeks of cryptococcal diagnosis. Urine output was not routinely monitored and could not be used to define AKI. Ten-week and 12-month mortality was ascertained.
We included 130 participants with stored urine samples, which were collected a median of 4 (interquartile range [IQR] = 4–5) days from diagnosis of cryptococcal meningitis (which is similar to time from amphotericin initiation: median = 4; IQR = 4–5 days), and measured NGAL, CysC, and TIMP-2 by enzyme-linked immunosorbent assay (R&D Systems) and protein and creatinine (Beckman Coulter DXC 800) on stored urine samples. We summarized incidence of AKI and explored risk factors for incident AKI using Cox univariate and multivariable proportional hazards models. Models were estimated with biomarkers on a continuous scale (after log2 transformation, so that the hazard ratio is per doubling of the biomarker). Other models compared the upper quartile versus the lower 3 quartiles (an a priori comparison to assess clinical outliers). Each biomarker was first statistically tested individually and then in a multivariable model adjusted for timing of ART initiation group, age, sex, altered mental status at diagnosis, CD4 cell count, and cerebrospinal fluid quantitative cryptococcal culture at diagnosis. We examined the association of incident AKI with 12-month mortality using time-updated Cox proportional hazards models. With the time-updated models, an indicator for incident AKI was recalculated at the time of each creatinine measurement to assess the impact of developing AKI on mortality. All analyses were completed using SAS 9.3. Relevant ethics and regulatory authority approvals were obtained. Written informed consent was obtained to participate in the COAT trial, as previously described [22].
RESULTS
Incidence and Risk of Acute Kidney Injury
The COAT trial enrolled 177 patients [22], of whom 130 patients had urine samples stored and were included in this study. The median age was 35 years (IQR = 30–40), and 52% (n = 68/130) were men. The median CD4 T-cell count was 21 cells/μL (IQR = 9–74). Forty-four percent (n = 57/130) died within 12 months; 1 withdrew consent and 1 was lost to follow-up. Four persons (3%) had GFR <60 mL/min/1.73 m2 at baseline. Incident AKI occurred in 42% (n = 53/126) at a median of 8 days (IQR = 6–11) after diagnosis of cryptococcal meningitis. Persons developing AKI had higher baseline CD4 T-cell counts (49 vs 14 cells/μL; P < .01), and a higher proportion were women (59% vs 40%; P = .04). Table 1 displays baseline characteristics, urine biomarker values, and mortality.
Table 1.
Urinary Biomarkers in Human Immunodeficiency Virus–Associated Cryptococcal Meningitis: Baseline Characteristics for the Overall Cohort, Participants Who Developed Acute Kidney Injury, and Those Who Did Not
| Baseline Variables | Overall Cohorta | Incident Acute Kidney Injury | No Acute Kidney Injury | P Valueb |
|---|---|---|---|---|
| No. | 130 | 53 | 73 | … |
| Early ART initiation arm, no. (%) | 63 (49) | 26 (49) | 37 (51) | .92 |
| Age, median (IQR), y | 35 (30–40) | 34 (30–40) | 37 (29–40) | .55 |
| Female sex, no. (%) | 62 (48) | 31 (59) | 29 (40) | .04 |
| Glasgow Coma Scalec < 15, no. (%) | 38 (29) | 17 (32) | 20 (28) | .60 |
| CSF quantitative culture, median (IQR), log10 CFU/mL | 5.0 (3.8–5.4) | 5.1 (3.8–5.5) | 5.0 (4.0–5.6) | .91 |
| CD4 count, median (IQR), cells/μL | 21 (9–74) | 49 (16–87) | 14 (7–54) | <.01 |
| Weightd, median (IQR), kg | 54 (46–60) | 54 (47–57) | 54 (45–60) | .81 |
| Serum creatinine, median (IQR), μmol/L | 70 (50–88) | 69 (50–91) | 70 (50–80) | .36 |
| Serum creatinine, median (IQR), mg/dL | 0.79 (0.57–1.00) | 0.78 (0.57–1.03) | 0.79 (0.57–0.90) | .36 |
| Serum potassium, median (IQR), mEq/L | 3.9 (3.4–4.2) | 3.8 (3.3–4.1) | 3.9 (3.5–4.2) | .37 |
| Amphotericin dosee, mg/kg/d | ||||
| Overall | 0.93 (0.85–1.00) | 0.95 (0.89–1.0) | 0.93 (0.83–1.0) | .62 |
| Men | 0.93 (0.81–1.0) | 0.96 (0.91–1.0) | 0.92 (0.81–1.0) | … |
| Women | 0.95 (0.89–1.00) | 0.94 (0.89–1.0) | 0.95 (0.91–1.0) | … |
| Urine biomarkersf | ||||
| Cystatin C, ng/mL | 2.2 (1.3–3.5) | 2.6 (1.5–3.5) | 2.0 (1.2–3.3) | .32 |
| Neutrophil gelatinase-associated lipocalin, ng/mL | 4.1 (3.0–5.7) | 4.2 (3.1–6.1) | 4.0 (3.0–5.0) | .41 |
| Tissue inhibitor of metalloproteinases-2, ng/mL | 1.8 (1.4–2.5) | 1.9 (1.5–2.7) | 1.6 (1.3–2.1) | <.01 |
| Urinary creatinine, mg/dL | 4.6 (3.6–5.4) | 4.8 (3.3–5.7) | 4.4 (3.7–5.1) | .39 |
| Urinary protein, g/L | 0.21 (0.11–0.39) | 0.29 (0.16–0.46) | 0.16 (0.08–0.30) | <.01 |
| Protein/creatinine ratio | 0.56 (0.36–0.83) | 0.57 (0.30–0.79) | 0.56 (0.38–0.96) | .49 |
| Ten-week mortality, no. (%) | 48 (37) | 27 (51) | 21 (29) | .01 |
| Twelve-month mortality, no. (%) | 57 (44) | 29 (55) | 28 (38) | .07 |
Bolded P values indicate statistically significant difference between patients who developed AKI and those who did not.
Abbreviations: ART, antiretroviral therapy; CFU, colony-forming unit; CSF, cerebrospinal fluid; IQR, interquartile range.
aIncludes 4 people with prevalent estimated glomerular filtration rate <60 mL/min/1.73m2 at cryptococcal meningitis diagnosis.
bKruskall-Wallis or χ2 tests as appropriate, comparing those who develop incident acute kidney injury to those who do not.
cGlasgow Coma Scale <15 denotes altered mental status.
dWeight was measured for n = 111 participants (44 of 53 who later developed incident acute kidney injury; 63 of 73 who did not develop incident acute kidney injury).
eAmphotericin dose (mg/kg/d) is calculated only for those with a weight measurement.
fSamples collected at median of 4 days on amphotericin B therapy and stored at −80°C
Urine Biomarker Associations With Acute Kidney Injury
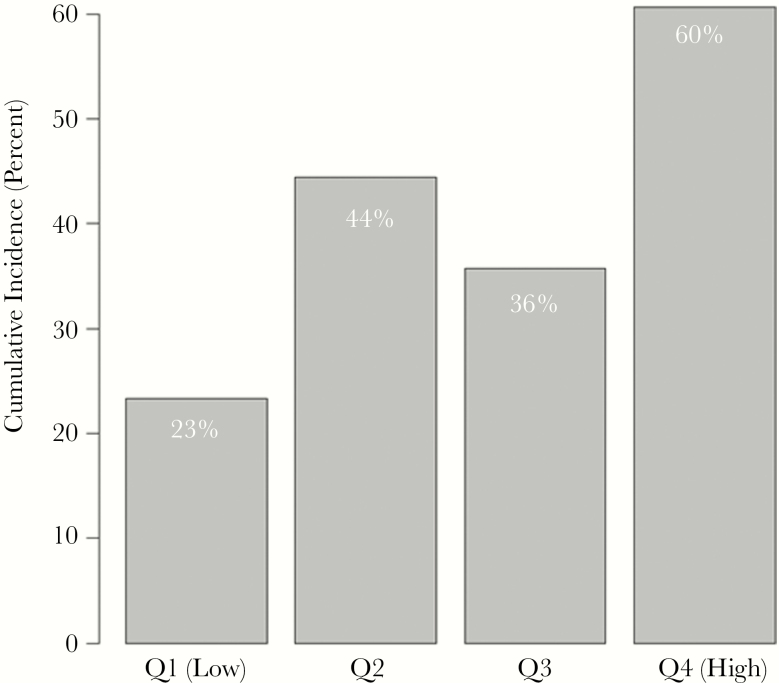
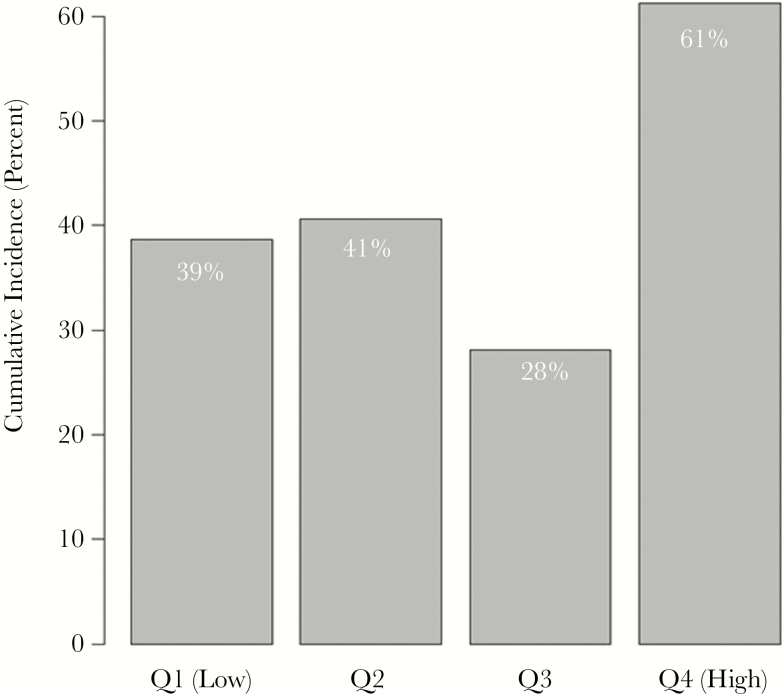
On a continuous scale, there was increased risk of AKI per doubling of urine TIMP-2 and protein levels (hazard ratio [HR] = 1.47, 95% confidence interval [CI] = 1.08–2.00, P = .02; HR = 2.28, 95% CI = 1.05–4.94, P = .04, respectively), but the association became nonsignificant after adjustment (adjusted HR [aHR] = 1.34, 95% CI = 0.95–1.88, P = .09; aHR = 2.23, 95% CI = 0.92–5.41, P = .07). In univariate models, those with NGAL, TIMP-2, and protein levels in the upper quartile had an increased risk of AKI compared with those in the lower quartiles. The risk remained significant for NGAL and protein in multivariable models. Sixty percent of those with values in the highest quartile of protein (>0.30 g/L protein) developed AKI as compared with an average of 35% of those in the lower 3 quartiles (aHR = 1.64; 95% CI = 1.02–2.62; P = .04) (Figure 1 and Table 2). Similarly, of those in the upper quartile of NGAL (>47.7 ng/mL), 61% developed AKI as compared with an average of 36% of those in the lower 3 quartiles (aHR = 1.65; 95% CI = 1.01–2.69) (Figure 2 and Table 2).
Figure 1.
Urinary biomarkers in human immunodeficiency virus–associated cryptococcal meningitis: acute kidney injury by quartiles of urine protein values. χ2P value comparing the 4 groups = .03. Abbreviation: Q, quartile.
Table 2.
Urinary Biomarkers in Human Immunodeficiency Virus–Associated Cryptococcal Meningitis: Urinary Biomarkers Associated With Incident Acute Kidney Injury
| Urine Biomarker | Association Between Urinary Biomarkers (per doubling of value) and Acute Kidney Injury | |||
|---|---|---|---|---|
| Univariate Hazard Ratioa (95% CI) |
P Value | Multivariable Hazard Ratioa,c (95% CI) |
P Value | |
| CysC | 1.04 (0.88–1.23) | .62 | 1.05 (0.88–1.25) | .61 |
| NGAL | 1.06 (0.91–1.24) | .46 | 1.01 (0.85–1.19) | .91 |
| TIMP-2 | 1.47 (1.08–2.00) | .02 | 1.34 (0.95–1.88) | .09 |
| Protein | 2.28 (1.05–4.94) | .04 | 2.23 (0.92–5.41) | .07 |
| Creatinine | 1.04 (0.82–1.32) | .74 | 1.07 (0.83–1.38) | .59 |
| Protein/creatinine ratio | 0.83 (0.38–1.80) | .63 | 0.63 (0.28–1.45) | .28 |
| Association of Upper Quartile of Urinary Biomarkers With Acute Kidney Injury: Fourth Quartile of Biomarkers Compared With Quartiles 1–3 | ||||
| Hazard Ratiob (95% CI) | P Value | Hazard Ratioc (95% CI) | P Value | |
| CysC | 1.05 (0.70–1.59) | .81 | 1.05 (0.68–1.63) | .82 |
| NGAL | 1.92 (1.25–2.94) | <.01 | 1.65 (1.01–2.69) | .04 |
| TIMP-2 | 1.70 (1.10–2.61) | .02 | 1.46 (0.91–2.33) | .12 |
| Protein | 1.73 (1.12–2.68) | .01 | 1.64 (1.02–2.62) | .04 |
| Creatinine | 1.41 (0.92–2.16) | .12 | 1.48 (0.94–2.32) | .09 |
| Protein/creatinine ratio | 0.92 (0.60–1.42) | .71 | 0.84 (0.53–1.34) | .47 |
Bolded P values indicate statistically significant difference between patients who developed AKI and those who did not.
Abbreviations: CI, confidence interval; CysC, cystatin C; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2.
aProportional hazards regression analyses using log2 transformed biomarker data. The hazard ratio presents the risk per doubling of the biomarker for developing incident acute kidney injury (GFR < 60 mL/min/1.73 m2) within 3 weeks of diagnosis of cryptococcal meningitis.
bFourth quartile versus quartile 1–3. The hazard ratio presents the risk of developing acute kidney injury if the urine biomarker level falls within the highest quartile compared with the lower 3 quartiles.
cAdjusted for antiretroviral treatment (ART) group (early ART or deferred ART group), age, sex, decreased level of consciousness at diagnosis, CD4 cell count, and cerebrospinal fluid quantitative cryptococcal culture at diagnosis (but not for other biomarkers).
Figure 2.
Urinary biomarkers in human immunodeficiency virus–associated cryptococcal meningitis: acute kidney injury by quartiles of urine neutrophil gelatinase-associated lipocalin (NGAL) values. χ2P value comparing the 4 groups = .06. Abbreviation: Q, quartile.
Associations with 12-Month Mortality
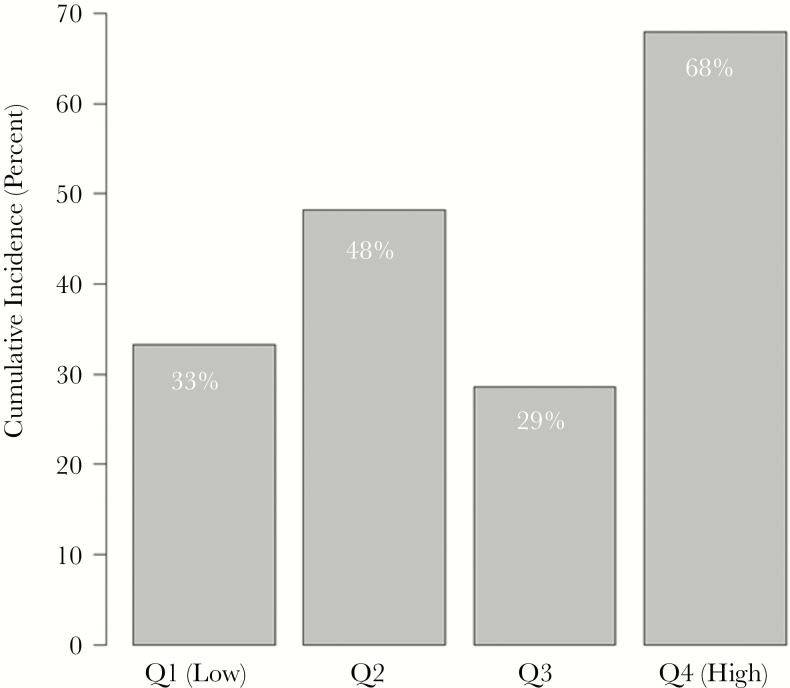
Elevated urine NGAL and protein-to-creatinine ratio, respectively, were associated with mortality in univariate analysis (HR = 1.16, 95% CI = 1.01–1.34, P = .04; HR = 1.86, 95% CI = 1.01–3.41, P = .05) but not in multivariable analysis (aHR = 1.14, 95% CI = 0.98–1.34, P = .09; aHR = 1.53, 95% CI = 0.73–3.20, P = .26) when evaluated on a continuous log2 scale. Urine protein levels in the upper quartile independently predicted 12-month mortality, and 68% of patients with urine protein levels in the highest quartile died within 12 months as compared with 23%, 44%, and 36% of those in the lower quartiles, respectively (aHR = 2.13; 95% CI = 1.15–3.96) (Figure 3 and Table 3).
Figure 3.
Urinary biomarkers in human immunodeficiency virus–associated cryptococcal meningitis: 12-month mortality by quartiles of urine protein values. χ2P value comparing the 4 groups = .01. Abbreviation: Q, quartile.
Table 3.
Urinary Biomarkers in Human Immunodeficiency Virus–Associated Cryptococcal Meningitis: Analyses of Urinary Biomarkers and Acute Kidney Injury With 12-Month Mortality
| Urine Biomarker | Association Between Urinary Biomarkers (per doubling of value) and 12-Month Mortality | |||
|---|---|---|---|---|
| Univariate Hazard Ratioa (95% CI) |
P Value | Multivariable Hazard Ratioa,c (95% CI) |
P Value | |
| CysC | 1.15 (0.99–1.34) | .06 | 1.14 (0.98–1.33) | .10 |
| NGAL | 1.16 (1.01–1.34) | .04 | 1.14 (0.98–1.34) | .09 |
| TIMP-2 | 1.25 (0.91–1.73) | .17 | 1.23 (0.87–1.75) | .24 |
| Protein | 1.92 (0.90–4.11) | .09 | 1.94 (0.83–4.57) | .12 |
| Creatinine | 1.08 (0.88–1.33) | .45 | 1.16 (0.92–1.47) | .21 |
| Protein/creatinine ratio | 1.86 (1.01–3.41) | .05 | 1.53 (0.73–3.20) | .26 |
| Acute kidney injuryd (GFR < 60) | 3.27 (1.87–5.73) | <.001 | 2.82 (1.55–5.17) | <.001 |
| Severe acute kidney injuryd (GFR < 30) | 12.17 (4.98–29.73) | <.001 | 8.11 (3.19–20.60) | <.001 |
| Association of Upper Quartile of Urinary Biomarkers with 12-Month Mortality: Fourth Quartile of Biomarkers Compared With Quartiles 1–3 | ||||
| Hazard Ratiob (95% CI) | P Value | Hazard Ratioc (95% CI) | P Value | |
| CysC | 1.04 (0.57–1.87) | .91 | 1.18 (0.63–2.19) | .61 |
| NGAL | 1.12 (0.62–2.02) | .71 | 0.99 (0.52–1.88) | .97 |
| TIMP-2 | 1.56 (0.85–2.85) | .15 | 1.66 (0.87–3.17) | .12 |
| Protein | 2.30 (1.30–4.08) | <.01 | 2.13 (1.15–3.96) | .02 |
| Creatinine | 1.11 (0.59–2.08) | .75 | 1.20 (0.61–2.35) | .59 |
| Protein/creatinine ratio | 2.03 (1.14–3.62) | .02 | 1.53 (0.80–2.93) | .20 |
Bolded P values indicate statistically significant difference between patients who died within 12 months after diagnosis of cryptococcal meningitis and those who did not.
Abbreviations: CI, confidence interval; CysC, cystatin C; GFR, estimated glomerular filtration rate using the Modification of Diet in Renal Disease study equation; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2.
aProportional hazards regression analyses using log2-transformed biomarker data. The hazard ratio presents the 12-month mortality risk per doubling of the biomarker.
bFourth quartile versus quartile 1–3. The hazard ratio presents the 12-month mortality risk if the urine biomarker level falls within the highest quartile compared to the lower three quartiles.
cAdjusted for antiretroviral treatment (ART) group (early ART or deferred ART group), age, sex, decreased level of consciousness at diagnosis, CD4 cell count, and cerebrospinal fluid quantitative cryptococcal culture at diagnosis (but not for other biomarkers).
dFrom time-updated models with indicators for GFR <30 or <60 mL/min/1.73m2 at each visit.
Incident AKI was independently associated with 12-month mortality (time-updated aHR = 2.82; 95% CI = 1.55–5.17; P < .001). Patients developed AKI at a median of 8 days after diagnosis of cryptococcal meningitis (IQR = 6–11 days) and died a median of 8 days (IQR = 4–23 days) after AKI developed. All 9 patients who developed severe AKI (estimated GFR of <30 mL/min/1.73 m2) died.
Ten-Week Outcomes in Acute Kidney Injury Patients
The outcome of abnormal renal function was assessed in survivors at 10 weeks after diagnosis of cryptococcal meningitis. Among 53 patients who developed AKI, 51% (n = 27) died before 10 weeks, and renal function recovered to eGFR >60 mL/min/1.73 m2 in the remaining 49% (n = 26/53) by 8 weeks. All 4 patients with abnormal renal function at baseline survived and recovered renal function to eGFR >60 mL/min/1.73 m2 by 10 weeks.
DISCUSSION
To our knowledge, this is the first study to evaluate urine biomarkers in patients with HIV-associated cryptococcal meningitis treated with amphotericin. Acute kidney injury was a common complication and associated with high mortality. Urine protein level in the highest quartile independently predicted AKI and 12-month mortality, whereas the other biomarkers tested showed no strong associations. Urine biomarkers are being increasingly applied in clinical practice, particularly in settings where AKI is common and can potentially be mitigated by amending management strategies, such as intensive care units. Urine biomarkers have not been studied in the context of HIV-associated cryptococcosis where AKI is a common complication of amphotericin therapy and mortality is high. Urine protein can also be measured by urine dipstick, which is a cheap and readily available test in resource-limited settings. This could be a useful marker to investigate in similar settings as an adjunctive tool to aid clinical decision making. Elevated urine protein levels at initiation of therapy could prompt more intensive fluid replacement, closer monitoring of renal function, and possible decreased duration of amphotericin, but this needs to be evaluated in further studies.
Acute kidney injury, as defined in this study, developed in 42% of patients in this cohort. The Kidney Disease: Improving Global Outcomes (KDIGO) published case definition of AKI consists of (1) increase in serum creatinine within 48 hours of ≥26.5 μmol/L (0.3 mg/dL) or (2) increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred in the prior 7 days, or urine volume of <0.5 mL/kg/h for 6 hours [11]. We did not measure urine output in this cohort, and using serial serum creatinine values, 80% of our patients met creatinine-based KDIGO AKI case definition criteria. We used an amended case definition, which is equal to a grade 3 (severe) adverse event [25] and has clinical relevance in this critically ill cohort who required treatment with a nephrotoxic drug. Amphotericin therapy is generally interrupted or stopped when a patient’s GFR declines to <60 mL/min/1.73 m2.
People with higher CD4 T-cell counts developed AKI more commonly in our study. The reason for this is unclear. It is possible that patients with very low CD4 T-cell counts did not survive to develop AKI, and there is thus a competing risk of death; however, CD4 count was not associated with survival in this cryptococcal cohort [22]. More women developed AKI in our study, which is consistent with the literature, which lists women as more susceptible to AKI [26].
Tissue inhibitor of metalloproteinases-2 and urine protein levels were associated with development of AKI in univariate analysis but not after adjusting for potential confounders on a continuous scale. Urine TIMP-2 combined with insulin-like growth factor-binding protein 7 (IGFBP7) has been shown to predict development of severe AKI in acutely ill patients within 12 hours of collection of urine sample [27]. Higher urine TIMP-2 has also been associated with mortality in acutely ill patients [21] but not in our study. Our urine samples were collected at a median of 4 days after diagnosis of cryptococcal meningitis, and AKI developed at a median of 8 days on therapy, which is a different scenario to the previous studies investigating the predictive value of TIMP-2.
Higher urine CysC was not associated with development of AKI or mortality in our study when evaluated on a continuous log2 scale or comparing the upper quartile with lower quartiles. Cystatin C is an early marker of tubular dysfunction or damage, and it is possible that (1) the mechanism of development of AKI in our cohort was different or (2) that we measured CysC levels too long before AKI events to detect elevated levels.
We found AKI to be independently associated with mortality. Patients in this study were severely immune suppressed, and although it is possible that AKI secondary to amphotericin therapy could have played a role in mortality, there are other factors such as nosocomial sepsis, opportunistic infections, and decreased level of consciousness with decreased oral intake that likely contributed to both AKI and mortality.
A limitation of our study is that we used a nonstandard case definition of AKI. This limits comparability across studies, and importantly, patients who had normal renal function at baseline would have had to experience a much larger proportional decline in renal function before being classified as an AKI case compared with patients who entered the study with a GFR close to the threshold of 60 mL/min/1.73 m2. Furthermore, urine was collected after initiation of amphotericin therapy, and urine biomarker findings may reflect early subclinical amphotericin-related AKI and not purely the theoretical potential to develop AKI. The timing of best measurement of urine biomarkers is unknown, and further elucidation of the change in biomarkers over time would be of interest. Ultimately, the clinical utility of urine biomarkers would be optimal if future AKI could be predicted accurately early after diagnosis to guide dosing and duration of amphotericin therapy. There is considerable interest in shortening amphotericin regimens for treatment of cryptococcal meningitis in resource-limited settings [28, 29] and a urine biomarker that could predict risk of AKI accurately and early could be valuable to guide duration of amphotericin treatment. Not all patients from the COAT trial were included because not all patients had stored urine specimens. Patients who were enrolled in the COAT trial and not included in this study had no difference in baseline CD4, HIV load, age, sex, COAT treatment arm, and cerebrospinal fluid quantitative culture (data not shown) compared with this cohort.
In conclusion, AKI occurred in 42% of HIV-infected patients treated with amphotericin B deoxycholate for cryptococcal meningitis, and AKI was associated with mortality. However, AKI was transient among survivors. Urine protein levels are easy to measure and may be useful for antecedent prediction of amphotericin-associated AKI. The utility of measuring urine protein levels for aiding decisions regarding duration of amphotericin therapy needs further evaluation.
Acknowledgments
The COAT Trial team worked on this trial in Uganda and South Africa from 2010 to 2012. The enzyme-linked immunosorbent assays on Cape Town samples were performed in the laboratory of Professor Robert J. Wilkinson at the University of Cape Town. Dr Nicola Wearne at the University of Cape Town, Department of Medicine, Renal Unit provided input on the study during the planning phase.
Financial support. This work was supported by provided by the National Institute of Allergy and Infectious Diseases (U01AI089244 and T32AI055433), Wellcome Trust (081667 and 098316 to G. M.), a Fogarty International Center South Africa TB/AIDS Training Award (NIH/FIC 1U2RTW007373- 01A1 and U2RTW007373 ICORTA to G. M. and C. S.). The South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (64787 to G. M.), NRF incentive funding (UID: 85858 to G. M.) and the South African Medical Research Council through its TB and HIV Collaborating Centres Programme with funds received from the National Department of Health (RFA no. SAMRC-RFA-CC: TB/HIV/AIDS-01-2014 to G. M.). The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone.
Potential conflict of interest. All authors: No reported conflicts of interest.All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Park BJ, Wannemuehler KA, Marston BJ et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. [DOI] [PubMed] [Google Scholar]
- 2. Pyrgos V, Seitz AE, Steiner CA et al. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One. 2013;8:e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liechty CA, Solberg P, Were W et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35. [DOI] [PubMed] [Google Scholar]
- 4. French N, Gray K, Watera C et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–8. [DOI] [PubMed] [Google Scholar]
- 5. Bagnis CI, Deray G. Amphotericin B nephrotoxicity. Saudi J Kidney Dis Transpl. 2002;13:481–91. [PubMed] [Google Scholar]
- 6. Burgess JL, Birchall R. Nephrotoxicity of amphotericin B, with emphasis on changes in tubular function. Am J Med. 1972;53:77–84. [PubMed] [Google Scholar]
- 7. Girmenia C, Cimino G, Di Cristofano F et al. Effects of hydration with salt repletion on renal toxicity of conventional amphotericin B empirical therapy: a prospective study in patients with hematological malignancies. Support Care Cancer. 2005;13:987–92. [DOI] [PubMed] [Google Scholar]
- 8. Anderson CM. Sodium chloride treatment of amphotericin B nephrotoxicity. Standard of care? West J Med. 1995;162:313–7. [PMC free article] [PubMed] [Google Scholar]
- 9. Kagan S, Ickowicz D, Shmuel M et al. Toxicity mechanisms of amphotericin B and its neutralization by conjugation with arabinogalactan. Antimicrob Agents Chemother. 2012;56:5603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falk R, Hacham M, Nyska A et al. Induction of interleukin-1beta, tumour necrosis factor-alpha and apoptosis in mouse organs by amphotericin B is neutralized by conjugation with arabinogalactan. J Antimicrob Chemother. 2005;55:713–20. [DOI] [PubMed] [Google Scholar]
- 11. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Suppl. 2012;2:1–138. [Google Scholar]
- 12. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Shlipak MG, Grunfeld C, Choi AI. Incidence and risk factors for acute kidney injury in HIV Infection. Am J Nephrol. 2012;35:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franceschini N, Napravnik S, Eron JJ Jr et al. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005;67:1526–31. [DOI] [PubMed] [Google Scholar]
- 15. Tsigou E, Psallida V, Demponeras C et al. Role of new biomarkers: functional and structural damage. Crit Care Res Pract. 2013;2013:361078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bennett M, Dent CL, Ma Q et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singer E, Markó L, Paragas N et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf). 2013;207: 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nickolas TL, Schmidt-Ott KM, Canetta P et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59: 246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Post FA, Wyatt CM, Mocroft A. Biomarkers of impaired renal function. Curr Opin HIV AIDS. 2010;5:524–30. [DOI] [PubMed] [Google Scholar]
- 20. Conti M, Moutereau S, Zater M et al. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med. 2006;44:288–91. [DOI] [PubMed] [Google Scholar]
- 21. Yamashita T, Doi K, Hamasaki Y et al. Evaluation of urinary tissue inhibitor of metalloproteinase-2 in acute kidney injury: a prospective observational study. Crit Care. 2014;18:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boulware DR, Meya DB, Muzoora C et al. ; COAT Trial Team. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bahr NC, Rolfes MA, Musubire A et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource-limited settings. Open Forum Infect Dis. 2014;1:ofu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 25. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0 November 2014. Available at: https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf?sfvrsn=8. Accessed 15 February 2017. [Google Scholar]
- 26. Bienholz A, Wilde B, Kribben A. From the nephrologist’s point of view: diversity of causes and clinical features of acute kidney injury. Clin Kidney J. 2015;8:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kashani K, Al-Khafaji A, Ardiles T et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson AT, Nussbaum JC, Phulusa J et al. A phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitis. AIDS. 2012;26:1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muzoora CK, Kabanda T, Ortu G et al. Short course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitis. J Infect. 2012;64:76–81. [DOI] [PubMed] [Google Scholar]