Abstract
The aims of this study were to 1) compare the inflammatory potential of night- and day-shift nurses’ diets in regard to time-of-day and work status, and 2) explore how the timing of food intake during work and off-work is associated with cardiometabolic syndrome (CMS) risk factors between these two groups. Female nurses (N = 17; 8 day-shift and 9 night-shift) reported food intake over 9 days. On a middle day off of work, metabolic parameters were measured after an overnight fast. Energy/macronutrient intake and inflammatory potential of dietary intake (as assessed via the Dietary Inflammatory IndexTM) were calculated for nurses’ workdays, work nights, off-work days, and off-work nights. Work-night total food intake (grams) accounted for a significant amount of variance in CMS risk factors for night-shift nurses only. Increased total gram consumption during night-shift nurses’ work nights was associated with increased lipid levels – independent of the macronutrient composition of the food consumed. Alternatively, for night-shift nurses, work-day intake of several food parameters accounted for a significant proportion of variance in HDL cholesterol levels, with higher intake associated with higher HDL levels. For both day- and night-shift nurses, food intake during the day was more pro-inflammatory regardless of shift-type or work status. Our novel approach of combining time-of-day-specific and work-day-specific analyses of dietary inflammatory factors and macronutrient composition with measurement of CMS risk factors suggests a link between meal timing and cardiometabolic health for shift-working nurses.
Keywords: Dietary Inflammatory Index, dietary patterns, circadian misalignment
Introduction
Shift workers exhibit a greater risk for several cardiovascular and metabolic diseases including heart disease, obesity, hypertension, and type II diabetes (Boivin et al., 2007; Chen et al., 2010; Knutsson et al., 1986; Kroenke et al., 2007). Furthermore, recent studies have revealed a strong association between shift work and development of cardiometabolic syndrome (CMS), a condition characterized by co-occurrence of several cardiometabolic disease risk factors (e.g., glucose intolerance, dyslipidemia, abnormal blood pressure, and visceral obesity) (De Bacquer et al., 2009; Pietroiusti et al., 2010; Ye et al., 2013). Characteristics of shift workers’ dietary habits and nutritional status represent one potential factor contributing to the increased prevalence and incidence of CMS among this population (Boggild & Knutsson, 1999; Lowden et al., 2010; Pan et al., 2011). Specifically, shift workers have been reported to consume more pro-inflammatory diets than day workers (Wirth, Burch, Shivappa, Steck et al., 2014). The Dietary Inflammatory IndexTM (DII) is a novel tool that has been developed to characterize the inflammatory potential of an individual’s diet (Shivappa, Steck, Hurley, Hussey, & Hébert, 2014). The DII has been found to be associated with shiftwork experience, inflammatory cytokines including C-reactive protein and interleukin-6, the glucose intolerance component of metabolic syndrome, numerous cancers, cardiovascular disease, telomere length, mortality, and other adverse health outcomes (Garcia-Calzon et al., 2015; Graffouillere et al., 2016; Shivappa, Bosetti, et al., 2015; Shivappa, Steck, Hurley, Hussey, Ma, et al., 2014; Shivappa, Steck, et al., 2015; Shivappa, Zucchetto, et al., 2015; Wirth, Burch, Shivappa, Steck, et al., 2014; Wirth, Burch, Shivappa, Violanti, et al., 2014; Wirth et al., 2015). However, it has yet to be determined whether the DII varies across the 24-hour day or by work status (work vs. off-work) among night- and day-shift workers.
Another proposed mechanism for the increased risk of metabolic and cardiovascular disease among shift workers is related to circadian misalignment (Scheer et al., 2009), which occurs when the central circadian clock (located in the suprachiasmatic nucleus [SCN] of the hypothalamus) that regulates daily rhythms of sleep/wake behavior, hormones, and metabolism becomes desynchronized with social, sleep/wake, and food intake patterns (Baron & Reid, 2014; Roden et al., 1993; Sack et al., 1992). Acute circadian misalignment of the sleep/wake and feeding cycle (as experimentally induced via a forced 28-hour day outside humans’ range of entrainment) in healthy, non-shift workers is associated with disrupted glucose, insulin, leptin, and cortisol rhythms and is even capable of inducing a pre-diabetic state in as few as three days (Scheer et al., 2009). Observational studies have shown that shift workers exhibit more irregular food intake patterns across the day compared to day workers and exhibit different food intake patterns on work and rest days (Esquirol et al., 2009; Lowden et al., 2010; Reeves et al., 2004). Specifically, re-distribution of food intake from day to night during night shift work has been associated with higher serum lipid levels in male industrial workers in a rotating 3-shift schedule (Lennernas et al., 1994). However, no previous studies have investigated how the timing of food intake both during shift work and on days-off is associated with CMS risk factors among female night shift workers in a hospital setting.
Thus, the primary goal of the present study was to examine how meal timing and dietary inflammatory potential may contribute to CMS indicators. Specifically, we sought to 1) identify differences in DII scores by shift-type (day-shift vs. night-shift nurses), time-of-day (day vs. night), and work status (work vs. off-work), and 2) determine how the timing of food intake on work days and off-work days is associated with CMS risk factors in day- and night-shift nurses. We hypothesized that greater food consumption on work nights would be associated with CMS risk factors for night-shift, but not day-shift, nurses.
Materials and Methods
Participants
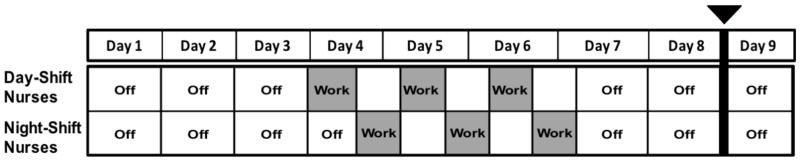
This manuscript explored eating behaviors and their relationship to CMS risk factors among healthy, female shift workers for the purpose of identifying potentially modifiable risk behaviors prior to disease onset. Participants were female, day-shift nurses (n = 8) and night-shift nurses (n = 9) recruited from the University of Alabama at Birmingham (UAB) Hospital who were employed full-time (>26 hours per week) and worked a schedule from either 7:00am – 7:00pm (i.e., day shift) or 7:00pm – 7:00am (i.e., night shift). To be eligible for study participation, nurses were required to: a) have a shift assignment of three consecutive 12-hour work shifts followed by three days off during the study period (Figure 1), and have worked the schedule for at least three weeks prior to study enrollment; b) have ≥1-year experience on the current shift (day or night); and c) be in a generally healthy medical condition (see exclusions below). During the study period, two of the night-shift nurses were called off their third shift, and therefore worked only two shifts. Night-shift nurses had the additional criteria of sleeping primarily during the night while off-work. More specifically, eligible nurses were those who utilized a sleep strategy type defined in Gamble et al. (2011) and Petrov et al. (2014) as a Switch Sleeper, which is characterized by switching from nights to days by increasing sleep duration on the first day of night-shift work or the first day off. This strategy is the most prevalent (approximately 50% of nurses) and one of the more adaptive of the sleep strategies identified (Gamble et al., 2011; Petrov et al., 2014). To determine sleep strategy, nurses were asked to indicate 30-min time blocks in which they would typically be sleeping during a 3-shift work week.
Figure 1.
9-day study protocol for day- and night-shift nurses. Food diaries were kept starting at the beginning of day 1 through the end of day 8. Day- and night-shift nurses worked 3 consecutive 12-hour shifts (greyed out). Blood collection after an overnight fast and blood pressure were taken during the morning of day 9 (indicated by bolded line).
Both day- and night-shift nurses were excluded from participation if they: 1) presented with an Axis I disorder as assessed by the Mini-International Neuropsychiatric Interview (M.I.N.I.); 2) were currently working multiple jobs; 3) reported currently smoking, excessive alcohol use (more than 15 units per week), or use of illegal drugs; 4) reported use of potentially sedating medications or tranquilizers in the last 30 days; or 5) reported use of medications for treatment of hypertension, diabetes, obesity, or hyperlipidemia. The study was approved by the University of Alabama at Birmingham Institutional Review Board (IRB) (F120305016), and all participants gave their informed consent prior to participation in the study.
Dietary Intake and Nutrient Content Analysis
Participants self-reported daily food intake for 8 days (3 work days and 5 off-work days), indicating the type, quantity, and timing of each eating event via a daily, paper-based food diary. The nutrient content of each meal was analyzed using Nutrition Data System for Research (NDSR), a computer-based software application developed at the University of Minnesota Nutrition Coordinating Center (NCC) (Feskanich et al., 1989).
For this study, eating events were divided into one of four bins corresponding to the time of day (day vs. night) and work status (work vs. off-work). Daytime eating events were defined as those taking place between 9 AM and 9 PM, and nighttime eating events were defined as those taking place between 9 PM and 9 AM. For day-shift nurses, work period eating events were defined as those occurring between 7 AM on the first work day and 7 PM on the final work day. For night-shift nurses, work period eating events were defined as those occurring between 7 PM on the first work day and 7 AM on the final work day. Any eating events falling outside this defined work period time frame, when the participant was not working, were defined as off-work eating events.
Dietary Inflammatory Index (DII) Analysis
Calculation of the DII scores based on the 24-hour intakes of specific micro- and macronutrients and specific foods (i.e., DII food parameters) was done according to the protocol by Shivappa, Steck, Hurley, Hussey, & Hébert (2014). Additionally, a similar process was used to create DII values in 12-hour bins, a novel technique that allowed for direct comparison of the inflammatory quality of the nurses’ diets during the day and night, irrespective of the differences in energy intake during these different times of day. All food parameter values from 9AM to 9PM and then from 9PM to 9AM were summed across days and standardized according to that individual’s average daily energy intake. In other words, this process allowed for computation of a “daily” DII score based on consumption of only food items consumed during that specific 12-hour bin. In addition to characterizing the DII by these various time period levels (i.e., 12-hour bin-level and average daily-level) among all nurses and days of sampling, DII values also were characterized by shift-type (i.e., day- vs. night-shift nurses) and work status (i.e., work vs. off-work).
Measurement of Anthropomorphic and Metabolic Parameters
On the morning of day 9 of the study period (a middle day off from work), blood samples (4 mL) were taken after an overnight fast (≥10 hours) to obtain a lipid profile including fasting triglycerides and cholesterol (mg/dL) for each participant. Fasting blood glucose (mg/dL) was measured via a commercial glucose meter, and blood pressure measurements (mmHg) were taken using a standard sphygmomanometer. Weight, height, and waist circumference were measured by study personnel. Demographic information was collected via a self-report questionnaire.
Statistical Analysis
Day- and night-shift nurses were compared on relevant demographic and metabolic measures using either a two-sided independent samples t-test or independent samples Mann-Whitney U nonparametric test as appropriate. For comparisons of 24-hour food/macronutrient intake, values were adjusted for body weight using a residual adjustment method. A three-factor ANOVA with repeated measures was used to investigate differences in DII values by shift-type (day-shift vs. night-shift), work status (work vs. off-work), and time-of-day (day vs. night).
For each participant, average total food consumption (in total grams and total kilocalories) and macronutrient intake (total protein, fat, and carbohydrates) were calculated for work days, work nights, off-work days, and off-work nights using the time-binning procedure described in the previous section (Dietary Intake and Nutrient Content Analysis). In models fit separately for day-shift nurses and night-shift nurses, a two-step hierarchical regression was conducted using fasting glucose, triglycerides, and cholesterol levels and blood pressure as the dependent variables. The control variable of body weight (in kilograms) was entered at step one, and each time- and work-specific food parameter was entered at step 2. The regression assumption of normality of residual distribution was ascertained using the Shapiro-Wilk test performed on the standardized residuals. For analyses in which the normality assumption was not met, the dependent and/or independent variables were transformed using a reciprocal transformation to achieve normality of the residual distribution. Post-hoc hierarchical regression analyses also were performed to examine the relationship between work-night total gram intake and night-shift nurses’ lipid ratios (i.e., triglycerides : HDL ratio, LDL : HDL ratio, and total cholesterol : HDL ratio), controlling for body weight.
For all tests, significance was set at α = .05. Analyses were performed using SPSS®, Version 22 (IBM Corp., Armonk, NY).
Results
Participants
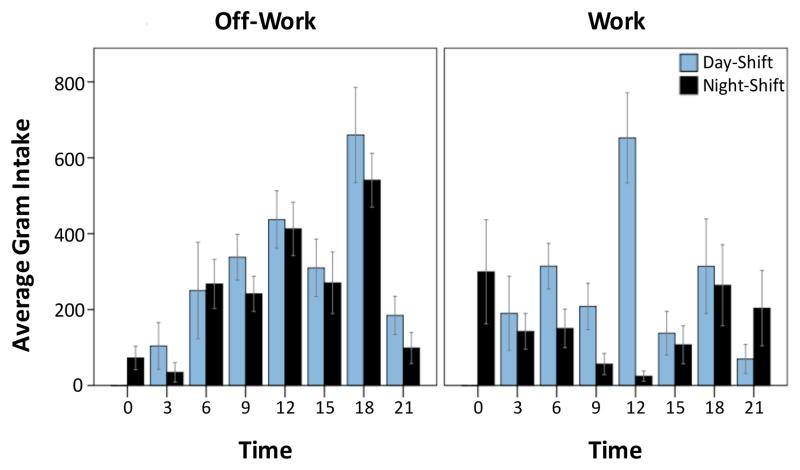
Both day-shift and night-shift participants were predominantly White (87.5% and 88.9%, respectively). There were no statistically significant differences between day- and night-shift nurses for any of the collected demographic variables or metabolic parameters (fasting glucose, triglycerides, cholesterol, and blood pressure) (Table 1). Similarly, there were no differences between day- and night-shift nurses in terms of average 24-hour DII or the food consumption variables of body weight-adjusted, 24-hour intake of total grams, energy, protein, fats or carbohydrates (Table 1). However, while off-work eating patterns were similar for day- and night-shift nurses – with average food intake peaking during dinner time – eating patterns during the work period were notably different between these two groups (Figure 2). On average, during work, day-shift nurses maintained high food consumption during the day (9AM to 9PM), while night-shift nurses redistributed their food intake to the nighttime.
Table 1.
Univariate comparisons between day- and night-shift nurses on participant characteristics.
| Day Shift (N = 8) | Night Shift (N = 9) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean | SD | Median | IQR | Mean | SD | Median | IQR | t / U | p | |
| Age (years) | 34.9 | 9.4 | 33.0 | 15.5 | 29.9 | 12.1 | 26.0 | 7.5 | 18.5 | .09a |
| Weight (lbs.) | 174.8 | 57.7 | 171.5 | 101.0 | 167.7 | 34.7 | 160.0 | 22.8 | 34.0 | .88a |
| (kg) | 79.3 | 26.2 | 77.8 | 45.8 | 76.1 | 15.7 | 72.8 | 10.3 | 34.0 | .88a |
| Height (in.) | 65.5 | 3.1 | 65.0 | 4.8 | 65.3 | 1.8 | 65.0 | 2.5 | 0.1 | .89 |
| Waist (in.) | 34.3 | 8.5 | 33.0 | 15.4 | 36.5 | 11.4 | 32.9 | 12.3 | 38.5 | .82a |
| BMI | 28.5 | 8.4 | 28.6 | 16.7 | 27.7 | 5.7 | 25.7 | 6.1 | 0.2 | .81 |
|
| ||||||||||
| Metabolic Parameters | ||||||||||
| Fasting Glucose (mg/dL) | 88.4 | 8.0 | 89.0 | 12.0 | 89.2 | 11.0 | 84.0 | 17.0 | − 0.2 | .86 |
| Fasting Triglycerides (mg/dL) | 99.3 | 50.8 | 78.0 | 99.0 | 73.4 | 61.0 | 46.0 | 58.0 | 17.0 | .07a |
| Total Cholesterol (mg/dL) | 170.9 | 42.1 | 175.5 | 63.3 | 170.3 | 36.5 | 157.0 | 41.0 | 30.5 | .61a |
| HDL (mg/dL) | 45.3 | 10.3 | 44.0 | 18.8 | 55.8 | 12.0 | 52.0 | 17.0 | − 1.9 | .07 |
| LDL (mg/dL) | 109.3 | 35.0 | 114.0 | 55.0 | 97.9 | 38.1 | 82.0 | 58.5 | 29.0 | .54a |
| BP Systolic (mmHg) | 112.9 | 9.78 | 112.5 | 10.50 | 122.3 | 10.0 | 120.0 | 18.0 | − 2.0 | .07 |
| BP Diastolic (mmHg) | 79.1 | 7.9 | 79.5 | 8.8 | 77.9 | 9.2 | 76.0 | 14.5 | 0.3 | .77 |
|
| ||||||||||
| 24-hr Dietary Intakeb | ||||||||||
| Total Grams | 2126.1 | 875.6 | 2034.2 | 1162.9 | 1687.5 | 434.4 | 1541.3 | 566.3 | 1.3 | .23 |
| Total Energy (kcal) | 1703.5 | 395.1 | 1674.8 | 425.7 | 1646.5 | 326.6 | 1741.1 | 590.6 | 0.3 | .75 |
| Total Fat | 60.8 | 12.3 | 61.6 | 20.7 | 63.3 | 12.3 | 61.7 | 21.2 | − 0.4 | .68 |
| Total Protein | 58.8 | 14.6 | 60.5 | 7.0 | 66.3 | 12.8 | 63.7 | 21.1 | 43.0 | .54a |
| Total Carbohydrates | 228.2 | 67.8 | 231.1 | 72.5 | 201.3 | 43.1 | 218.7 | 85.9 | 1.0 | .34 |
| 24-hr DII | 2.6 | 2.1 | 2.6 | 2.4 | 2.9 | 2.3 | 3.0 | 3.9 | 0.2 | .83 |
BP: blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein; DII: dietary inflammatory index
Mann-Whitney U test
Adjusted for kilograms (kg) bodyweight; averaged across work and off-work period
Figure 2.
Comparison of day- and night-shift nurses’ average food consumption patterns by time and work status. Daily pattern of mean ± SEM total gram consumption for day-shift nurses (light blue; n = 8) and night-shift nurses (black; n = 9) per 3-hour bins during work and off-work.
Dietary Inflammatory Index (DII)
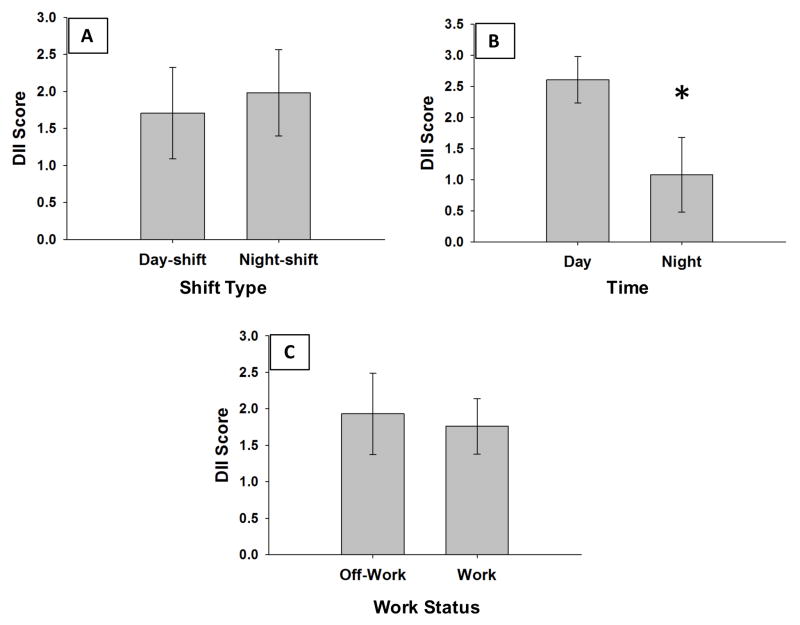
The first aim of this study was to identify differences in DII scores by shift-type, time-of-day, and work status. Three-factor, repeated measures ANOVA revealed a significant main effect of time of food consumption on DII scores (day vs. night, F (1,15) = 8.0, p < .05). For all nurses, daytime DII scores were higher (more pro-inflammatory) than nighttime DII scores (Figure 3, B). There was no significant effect of shift-type (Figure 3, A; day-shift nurses vs. night-shift nurses, F(1, 15) = 0.10, p = 0.75) or work status (Figure 3, C; work vs. off-work, F(1, 15) = 0.16, p = 0.70) on DII scores. There were no significant interactions between shift-type, time-of-day, and work status.
Figure 3.
DII scores differ by time-of-day, but not shift-type or work status. Mean ± SEM DII scores by A) shift-type (day-shift vs. night-shift nurses), B) time-of-day (day vs. night), and C) work status (work vs. off-work).
Timing of Food Intake and CMS Risk Factors
We next sought to investigate how the timing of food parameters is associated with CMS risk factors in day- and night-shift nurses on work days and off-work days. For each participant, average total food consumption and macronutrient intake were calculated for work days, work nights, off-work days, and off-work nights. Significant results of the R2 change in the two-step hierarchical regression of each time- and work-specific food parameter and metabolic risk factor are reported in Table 2 (night-shift nurses) and Table 3 (day-shift nurses).
Table 2.
Significant results from the hierarchical regression analyses of time- and work-specific food parameters and CMS risk factors in night-shift nurses.
| Night Shift Nurses (n = 9) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CMS Risk Factor | Model F | p | β | r | R2 | R2 Δ | p | |
| Work Days (WD) | ||||||||
|
| ||||||||
| HDL | Step 1 | 1.3 | .30 | .15 | ||||
| KG | − 0.39 | |||||||
| Step 2 | 8.5 | .02* | .74 | .59 | .01* | |||
| KG | − 0.13 | − .39 | ||||||
| WD Kcal | 0.81 | .85 | ||||||
|
| ||||||||
| HDL | Step 1 | 1.3 | .30 | .15 | ||||
| KG | − 0.39 | |||||||
| Step 2 | 5.5 | .04* | .65 | .50 | .03* | |||
| KG | − 0.14 | − .39 | ||||||
| WD Protein | 0.75 | .79 | ||||||
|
| ||||||||
| HDL | Step 1 | 1.3 | .30 | .15 | ||||
| KG | − 0.39 | |||||||
| Step 2 | 6.8 | .03* | .69 | .54 | .02* | |||
| KG | − 0.13 | −.39 | ||||||
| WD Carbs | 0.78 | .82 | ||||||
|
| ||||||||
| Work Nights (WN) | ||||||||
|
| ||||||||
| Total Cholesterol | Step 1 | 1.6 | .25 | .18 | ||||
| KG | 0.43 | |||||||
| Step 2 | 13.3 | .01* | .82 | .63 | .01* | |||
| KG | 0.04 | .43 | ||||||
| WN Grams | 0.88 | .90 | ||||||
|
| ||||||||
| Triglycerides | Step 1 | 3.5 | .11 | .33 | ||||
| KG | 0.57 | |||||||
| Step 2 | 9.8 | .01* | .77 | .44 | .02* | |||
| KG | 0.26 | .57 | ||||||
| WN Grams | 0.73 | .84 | ||||||
|
| ||||||||
| Reciprocal LDL | Step 1 | 2.3 | .18 | .25 | ||||
| KG | − 0.50 | |||||||
| Step 2 | 5.7 | .04* | .65 | .41 | .04* | |||
| KG | − 0.19 | − .50 | ||||||
| WN Grams | − 0.71 | − .80 | ||||||
|
| ||||||||
| Off Nights (ON) | ||||||||
|
| ||||||||
| Reciprocal Triglycerides | Step 1 | 4.4 | .08 | .39 | ||||
| KG | − 0.62 | |||||||
| Step 2 | 9.3 | .02* | .76 | .37 | .02* | |||
| KG | 0.04 | − .62 | ||||||
| ON KCal | − 0.90 | − .87 | ||||||
|
| ||||||||
| Work Nights (WN) | ||||||||
|
| ||||||||
| Triglycerides : HDL Ratio | Step 1 | 5.2 | .06 | .42 | ||||
| KG | 0.65 | |||||||
| Step 2 | 11.5 | .01* | .79 | .37 | .02* | |||
| KG | 0.36 | .65 | ||||||
| WN Grams | 0.67 | .83 | ||||||
|
| ||||||||
| LDL : HDL Ratio | Step 1 | 4.4 | .07 | .39 | ||||
| KG | 0.62 | |||||||
| Step 2 | 10.0 | .01* | .77 | .38 | .02* | |||
| KG | 0.32 | 0.62 | ||||||
| WN Grams | 0.69 | 0.83 | ||||||
|
| ||||||||
| Total Cholesterol : HDL Ratio | Step 1 | 3.8 | .09 | .35 | ||||
| KG | 0.60 | |||||||
| Step 2 | 10.3 | .01* | .77 | .42 | .02* | |||
| KG | 0.28 | .60 | ||||||
| WN Grams | 0.72 | .84 | ||||||
HDL: high-density lipoprotein; LDL: low-density lipoprotein; KG: kilograms; WD: work days; WN: work nights; ON: off-work nights
p < .05
Table 3.
Significant results from hierarchical regression analyses of time- and work-specific food parameters and CMS risk factors in day-shift nurses.
| Day Shift Nurses (n = 8) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CMS Risk Factor | F | p | β | r | R2 | R2 Δ | p | |
| Work Days (WD) | ||||||||
|
| ||||||||
| HDL | Step 1 | 0.4 | .53 | .07 | ||||
| KG | − 0.26 | |||||||
| Step 2 | 8.4 | .03* | .77 | .70 | .01* | |||
| KG | − 0.27 | − .26 | ||||||
| WD Carbs | − 0.84 | − .84 | ||||||
|
| ||||||||
| Off Days (OD) | ||||||||
|
| ||||||||
| Diastolic BP | Step 1 | 3.4 | .11 | .36 | ||||
| KG | 0.60 | |||||||
| Step 2 | 12.8 | .01* | .84 | .47 | .01* | |||
| KG | 1.83 | .60 | ||||||
| OD Fat | − 1.41 | .19 | ||||||
BP: blood pressure; HDL: high-density lipoprotein; KG: kilograms; WD: work days; OD: off-work days
p < .05
For night-shift nurses only, greater work day energy, protein, and carbohydrate intake were each significantly associated with higher HDL levels. Also among night-shift nurses, greater work-night total gram intake predicted higher levels of total cholesterol, triglycerides, and LDL cholesterol as well as the lipid ratios of triglycerides : HDL, LDL : HDL, and total cholesterol: HDL. Greater off-work night-time energy intake also predicted higher triglyceride levels for night-shift nurses. Similarly, off-work nighttime fat intake accounted for an additional 33% of the variance in night-shift nurses’ fasting triglyceride levels above and beyond body weight; however, the R2 change failed to reach significance (p = 0.05, β = 0.76; data not shown).
For day-shift nurses, greater work-day carbohydrate intake predicted lower HDL cholesterol levels. Alternatively, greater off-work daytime fat intake predicted lower diastolic blood pressure; however, this relationship should be interpreted with caution due to moderate correlation between the two predictor variables. Off-work daytime fat intake accounted for 34% additional variance in day-shift nurses’ systolic blood pressure; however, the R2 change for this predictor failed to reach significance (p = 0.05, β = −1.19; data not shown).
A summary of the significant time- and work-specific food parameters (predictors) and their effect on the associated metabolic parameter is provided in Table 4.
Table 4.
Summary of significant time- and work-specific food parameters identified using the two-step hierarchical regression.
| CMS Indicator | Day Shift Nurses | Night Shift Nurses | ||||||
|---|---|---|---|---|---|---|---|---|
| Off-Work | Work | Off-Work | Work | |||||
| 9am–9pm Day |
9pm–9am Night |
9am–9pm Day |
9pm–9am Night |
9am–9pm Day |
9pm–9am Night |
9am–9pm Day |
9pm–9am Night |
|
| Fasting Glucose | ||||||||
| Fasting Triglycerides | Kcal | Grams | ||||||
| Total Cholesterol | Grams | |||||||
| LDL | Grams | |||||||
| HDL | Carbs | Kcal Carbs Protein |
||||||
| Diastolic BP | Fat | |||||||
| Systolic BP | ||||||||
BP: blood pressure; HDL: high-density lipoprotein; Kcal: kilocalories; KG: kilograms; LDL: low-density lipoprotein
Note. Cases where increased intake of the food parameter was associated with unfavorable levels of the metabolic parameter are shown in red. Cases where increased intake of the food parameter was associated with favorable levels of the metabolic parameter are shown in blue.
Discussion
Although numerous studies have demonstrated an association between shift work and greater prevalence and incidence of metabolic syndrome and cardiovascular disease (Boggild & Knutsson, 1999; Pietroiusti et al., 2010), the contributing role of diet, and specifically, the timing of food intake, has remained relatively uncertain. In this paper, we aimed to 1) compare the inflammatory quality of night- and day-shift nurses’ diets in regard to time-of-day and shift-status, and 2) explore how the timing of food intake during work and off-work is associated with CMS risk factors between these two groups. First, we showed that there was no significant difference in average, daily DII score between night- and day-shift nurses; however, food intake during the day was scored as more pro-inflammatory regardless of shift-type or work status. Second, this study is the first to show that the timing of food consumption, with regard to both time-of-day and work status, is differentially associated with CMS risk factors in night- and day-shift nurses. Specifically, our results indicate that work-night total food intake accounted for a significant amount of variance in CMS risk factors for night-shift nurses only. Increased total gram consumption during night-shift nurses’ work nights was associated with increased lipid levels – independent of the macronutrient composition of the food consumed. Alternatively, for night-shift nurses, work-day intake of several food parameters was shown to account for a significant amount of variance in HDL cholesterol levels, with higher intake associated with higher HDL levels.
A previous study of a large cohort of shift workers showed that females working night or evening shifts had significantly higher DII scores compared to day-shift workers (Wirth, Burch, Shivappa, Steck, et al., 2014). Surprisingly, in the current study, we did not observe a statistically significant difference in DII scores between night- and day-shift nurses based on either the 24-hour DII algorithm or the 12-hour DII scaling algorithm. In addition to differences in shift-type definitions and specific worker populations (12-hour permanent night- or day-shift nurses in the present study), our sample likely did not have sufficient power to detect the ~20% difference in DII scores previously reported by Wirth and colleagues (2014). Nonetheless, the present study did demonstrate a proof-of-principle in using the DII to determine day-night differences in dietary inflammatory potential. In fact, our results showed a significant effect of time-of-day such that both day- and night-shift nurses consumed food with greater pro-inflammatory parameters (higher DII scores) during the day compared to during the night. Ultimately, further in-depth, meal-level analyses of DII should be undertaken in the future to better isolate individual food parameters that may be driving the day-night difference in DII reported here.
Coinciding with previous research showing that the temporal distribution and frequency of food intake (but not the 24-hour energy intake or nutritional quality) is disrupted by night shift work (Bonham et al., 2016; Esquirol et al., 2009; Lennernas et al., 1994; Reeves et al., 2004), we found no differences in total, body weight-adjusted 24-hour food consumption between day- and night-shift hospital nurses in terms of total energy, grams, or macronutrient intake. However, the distribution of food intake (in average total grams) was notably different during work and off-work days for night-shift nurses only. It is interesting to note that there was an increase in food intake from breakfast, to lunch, to dinner on off-work days for all nurses. High consumption of calories (i.e., greater than 30% of daily calories) in the evening or later has been associated with outcomes such as weight gain and breast cancer recurrence (Garaulet & Gomez-Abellan, 2014; Marinac et al., 2016). Although this concept is not the main focus of this manuscript, more work is needed to understand how the timing of food consumption affects health, especially among shift-working populations.
Our results revealed that total gram intake on work nights accounted for a significant amount of variance in night-shift nurses’ total cholesterol, triglycerides, and LDL cholesterol above and beyond the variance accounted for by body weight alone. Work night gram intake was found to account for approximately 40% additional variance in night shift nurses’ triglycerides and LDL cholesterol levels and 60% additional variance in total cholesterol levels. Furthermore, total gram intake on work nights also explained a significant amount of variance in night-shift nurses’ lipid ratios, which have been suggested to have better predictive value than independent lipid levels alone (Millán et al., 2009). Work-night gram intake was found to account for approximately 40% of the variance in each of these lipid ratios when added to the regression model with body weight. Increased work-night food consumption was associated with increased lipid levels and lipid ratios – signifying an unfavorable relationship with regard to cardiometabolic health. This finding suggests that, for night-shift nurses, eating during the night on work days is associated with higher levels of these CMS risk factors, regardless of the macronutrient composition of the food consumed.
A potential explanation for this detrimental effect of nighttime food intake among shift workers is the potency of nutrient intake as an entraining signal for peripheral circadian clocks involved in metabolism. Coupled with the relative insensitivity of the central clock to food entrainment (Damiola et al., 2000), the shift work environment is likely to produce central-peripheral clock misalignment. Support for this explanation comes primarily from animal studies, in which food consumption is restricted to a rodent’s normal rest phase. As a result, peripheral tissues readily synchronize to the timing of feeding, which induces desynchrony among the body’s central circadian pacemaker and peripheral metabolic clocks of the liver, heart, and pancreas (Bray et al., 2013; Damiola et al., 2000; Gamble & Young, 2013; Pezuk et al., 2010). Alternatively, a rodent model of shift work supports that restricting food intake to the normal active period may be capable of rescuing the metabolic dysfunction induced by shift work (Salgado-Delgado et al., 2010). Indeed, our results showed that work-day intake of several food parameters (energy, protein, and carbohydrates) each accounted for a significant amount of variance in night-shift nurses’ HDL levels, with higher intake associated with higher HDL levels for these nurses. Further research is needed to explore whether eating large meals during the work day, instead of the work night, may be particularly beneficial for night-shift nurses’ cardiometabolic health.
With regard to our findings, several considerations warrant mention. First, blood samples were taken from nurses on a middle-day off from work in order to minimize any bias attributable to shift-related differences in the timing of blood collection. Secondly, all nurses in the sample worked standardized 7-to-7 shifts during the study period and were recruited from the same hospital, thereby reducing variability within the groups attributable to work schedule and environment. Lastly, night-shift nurses utilized the same behavioral sleep strategy – a consideration that has been shown to influence adaptation to shift work. The primary limitation of this study is the small sample size, which limited statistical power and the feasibility of correcting for the multiple comparisons performed for the regression analyses. Other limitations include the self-report nature of nurses’ dietary food intake and the cross-sectional, observational design.
Based on the findings presented here, we speculate that restricting food intake on work nights may be a beneficial intervention strategy to reduce CMS risk factors among night-shift nurses. However, additional investigation of the associations presented in this study must be undertaken to include a larger, more diverse sample before any definitive conclusions can be drawn. Nevertheless, this study represents an important first step toward the development of empirically-based dietary guidelines for night-shift workers, which will hopefully serve to alleviate the excess burden of cardiometabolic disease among this population.
Footnotes
H.E.M and K.L.G were responsible for conception and design of the study, analysis and interpretation of the data, and initial drafting of the article. M.D.W., J.B., N.S. and J.R.H. were responsible for the analysis and interpretation of the data related specifically to the Dietary Inflammatory Index. R.L.J. contributed to collection and analysis of the data.
Declaration of Interests statement
This research was supported by the UAB Center for Clinical and Translational Science (CCTS) Grant Number ULITR000165, the UAB Department of Vision Sciences, the UAB Comprehensive Diabetes Center, and the UAB Department of Psychiatry Office of Clinical Research. We would like to thank the following individuals for making this study possible: the participants who gave their time, Betty Darnell and the UAB Center for Clinical and Translational Science Bionutrition Core, Sherer Thomson, and Brittny White.
Drs. Wirth, Shivappa, and Hébert were supported by grant number R44DK103377 from NIH’s National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Drs. Michael Wirth and Nitin Shivappa are employees of CHI.
References
- Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26:139–54. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggild H, Knutsson A. Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health. 1999;25:85–99. doi: 10.5271/sjweh.410. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Tremblay GM, James FO. Working on atypical schedules. Sleep Med. 2007;8:578–89. doi: 10.1016/j.sleep.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Bonham MP, Bonnell EK, Huggins CE. Energy intake of shift workers compared to fixed day workers: A systematic review and meta-analysis. Chronobiol Int. 2016;33:1086–100. doi: 10.1080/07420528.2016.1192188. [DOI] [PubMed] [Google Scholar]
- Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J of Obes. 2013;37:843–52. doi: 10.1038/ijo.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JD, Lin YC, Hsiao ST. Obesity and high blood pressure of 12-hour night shift female clean-room workers. Chronobiol Int. 2010;27:334–44. doi: 10.3109/07420520903502242. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemakers in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: A prospective study. Int J Epidemiol. 2009;38:848–54. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B. Shift work and metabolic syndrome: Respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009;26:544–59. doi: 10.1080/07420520902821176. [DOI] [PubMed] [Google Scholar]
- Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30:47–57. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Motsinger-Reif AA, Hida A, Borsetti HM, Servick SV, Ciarleglio CM, Robbins S, Hicks J, Carver K, Hamilton N, Wells N, Summar ML, McMahon DG, Johnson CH. Shift work in nurses: Contribution of phenotypes and genotypes to adaptation. PloS One. 2011;6:e18395. doi: 10.1371/journal.pone.0018395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Young ME. Metabolism as an integral cog in the mammalian circadian clockwork. Critical reviews in biochemistry and molecular biology. Crit Rev Biochem Mol Biol. 2013;48:317–31. doi: 10.3109/10409238.2013.786672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: A novel association. Physiol Behav. 2014;134:44–50. doi: 10.1016/j.physbeh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- García-Calzón S, Zalba G, Ruiz-Canela M, Shivappa N, Hébert JR, Martínez JA, Fitó M, Gómez-Gracia E, Martínez-González MA, Marti A. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: Cross-sectional and longitudinal analyses over 5 y. Am J Clin Nutr. 2015;102:897–904. doi: 10.3945/ajcn.115.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffouillère L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hébert JR, Wirth MD, Latino-Martel P, Hercberg S, Galan P, Julia C, Kesse-Guyot E, Touvier M. Prospective association between the Dietary Inflammatory Index and mortality: modulation by antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Am J Clin Nutr. 2016;103:878–85. doi: 10.3945/ajcn.115.126243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–83. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- Lennernas M, Akerstedt T, Hambraeus L. Nocturnal eating and serum cholesterol of three-shift workers. Scand J Work Environ Health. 1994;20:401–6. doi: 10.5271/sjweh.1381. [DOI] [PubMed] [Google Scholar]
- Lowden A, Moreno C, Holmbäck U, Lennernäs M, Tucker P. Eating and shift work: Effects on habits, metabolism and performance. Scand J Work Environ Health. 2010;36:150–62. doi: 10.5271/sjweh.2898. [DOI] [PubMed] [Google Scholar]
- Marinac CR, Nelson SH, Breen CI, Hartman SJ, Natarajan L, Pierce JP, Flatt SW, Sears DD, Patterson RE. Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncol. 2016;2:1049–55. doi: 10.1001/jamaoncol.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF, Masana L, Mangas A, Hernández-Mijares A, González-Santos P, Ascaso JF, Pedro-Botet J. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov ME, Clark CB, Molzof HE, Johnson RL, Jr, Cropsey K, Gamble KL. Sleep strategies of night shift nurses on days off: which ones are most adaptive? Front Neurol. 2014;5:277. doi: 10.3389/fneur.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010;25:432–41. doi: 10.1177/0748730410385204. [DOI] [PubMed] [Google Scholar]
- Pietroiusti A, Neri A, Somma G, Coppeta L, Iavicoli I, Bergamaschi A, Magrini A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67:54–7. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- Reeves SL, Newling-Ward E, Gissane C. The effect of shift-work on food intake and eating habits. J Nutr Food Sci. 2004;34:216–21. [Google Scholar]
- Roden M, Koller M, Pirich K, Vierhapper H, Waldhauser F. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol. 1993;265:R261–7. doi: 10.1152/ajpregu.1993.265.1.R261. [DOI] [PubMed] [Google Scholar]
- Sack RL, Blood ML, Lewy AJ. Melatonin rhythms in night shift workers. Sleep. 1992;15:434–41. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–29. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hébert JR. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr. 2015;113:278–83. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hébert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutr. 2014;17:1825–33. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hussey JR, Ma Y, Hébert JR. Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in National Health and Nutrition Examination Survey III Study. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hébert JR. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer Causes Control. 2015;26:1439–47. doi: 10.1007/s10552-015-0636-y. [DOI] [PubMed] [Google Scholar]
- Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, Hébert JR. Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. J Occup Environ Med. 2014;56:145–8. doi: 10.1097/JOM.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hébert JR. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56:986–9. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MD, Shivappa N, Steck SE, Hurley TG, Hébert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br J Nutr. 2015;113:1819–27. doi: 10.1017/S000711451500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye HH, Jeong JU, Jeon MJ, Sakong J. The association between shift work and the metabolic syndrome in female workers. Ann Occup Environ Med. 2013;25:33. doi: 10.1186/2052-4374-25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]