Abstract
Objectives:
The esophageal mucosal integrity is impaired in eosinophilic esophagitis (EoE) and it has been suggested that the duodenal permeability is increased. The absence of food allergens may restore the integrity. The aims of this study were to assess duodenal permeability in EoE and to evaluate the effect of an elemental diet on the esophageal and duodenal integrity.
Methods:
In this prospective study 17 adult EoE patients and 8 healthy controls (HC) were included. Esophageal biopsy specimens were sampled before and after 4 weeks of elemental diet to measure eosinophil counts and gene expression of tight junction and barrier integrity proteins. Esophageal and duodenal impedance were measured by electrical tissue impedance spectroscopy and Ussing chambers were used to measure transepithelial resistance (TER) and transepithelial molecule flux. Small intestinal permeability was measured using a test, measuring lactulose/mannitol (L/M) ratios.
Results:
In EoE patients, the esophageal but not the duodenal integrity was impaired, compared with HC. We observed no significant difference between L/M ratios of HC and EoE patients. After diet, eosinophil counts decreased significantly, which was paralleled by normalization of esophageal impedance and transepithelial molecule flux. The esophageal TER improved significantly, but did not reach values seen in HC. Esophageal expression of genes encoding for barrier integrity proteins filaggrin and desmoglein-1 was impaired at baseline and restored after diet.
Conclusions:
An elemental diet restores esophageal integrity, suggesting that it is at least partly secondary to allergen exposure. Duodenal integrity seems not to be affected in EoE, and possibly plays a minor role in its pathophysiology.
Introduction
Eosinophilic esophagitis (EoE) is an allergic inflammatory disease of the esophagus, characterized histologically by a mucosal infiltration of eosinophils and symptomatically by dysphagia and food impaction (1). Since EoE was first described as a distinct entity in 1993, the incidence of the disease has increased tremendously and EoE has become the most common cause of dysphagia in adolescents and young adults. Without adequate treatment, continuous inflammation leads to progressive narrowing of the esophagus (2).
Although the pathophysiology of EoE has not been completely elucidated, it seems to align with other atopic diseases such as atopic dermatitis and asthma (3). Ingested food allergens are thought to be the driver of the inflammation and studies have shown that elimination of food allergens can induce disease remission, providing further evidence for the allergic component of the disease (4, 5). However, the exact mechanism of how exposure to food allergens results in EoE is unknown. In patients with active EoE, the esophageal mucosal integrity is impaired and intercellular spaces are dilated (6). The relationship between an impaired epithelial barrier function and allergy has been studied extensively in atopic dermatitis, in which an increased skin permeability is primarily responsible for permeation of allergens and subsequent activation of immune system (7). This increased permeability is thought to be induced by a dysregulation of tight junction proteins: claudin 1 (CLDN1) and zonula occludes 1 (ZO-1) (8). However, it is also possible that the impaired mucosal integrity found in EoE is only secondary to products produced by the inflammatory infiltrate, and thus merely the result of the inflammation and not its cause. Previous studies have shown that the EoE-associated inflammatory cytokine interleukin (IL)-13 and IL-5 have indeed the ability to downregulate desmoglein (DSG1) and filaggrin (FLG), two barrier integrity proteins (9, 10). Interestingly, in atopic dermatitis, barrier dysfunction was not only observed in the skin but also in the small intestine (11). This raises the question whether the esophagus is the sole site of allergen uptake in EoE, or whether allergens also permeate through the small intestine and initiate or aggravate an inflammation in a distant organ—the esophagus. The current literature on duodenal involvement in EoE is contradictory, with indications for increased small intestinal permeability found in one study in adults but not in another study in children (12, 13). Either way, esophageal and/or small intestinal mucosal integrity may be of pathophysiological relevance, and restoration of the mucosal integrity may be essential to accomplish complete histological remission.
Studies with elemental diets, lacking food allergens, showed impressive clinical and pathologic response in EoE (14, 15). However, the effect of absence of food allergens, through an elemental diet, on the mucosal integrity has never been investigated in these patients.
We aimed to investigate the effect of an elemental diet on the esophageal and small intestinal mucosal integrity in adult EoE patients. We hypothesized that in adult EoE patients complete avoidance of food allergens by an elemental (amino acid-based) diet restores the impaired esophageal mucosal integrity and decreases the esophageal inflammation. In addition, we hypothesized that the duodenal mucosal integrity is impaired in patients with active EoE.
Methods
Study patients
In this prospective cohort study patients were included from the outpatient clinic of the Academic Medical Centrum Amsterdam between December 2014 and October 2015. Healthy controls were recruited through advertisement. Adults were eligible for enrolment if EoE was diagnosed according to current guidelines, defined as typical symptoms of EoE (dysphagia and/or food impaction) and the presence of more than 15 eosinophils per high-power field (HPF) during a high dosage of proton pump inhibition or H2 blockers (1). Exclusion criteria were: other esophageal or gastrointestinal diseases, previous surgery of the digestive tract, severe comorbidity scored as ASA class III or higher, and the inability to stop anti-inflammatory drugs (i.e., topical or systemic steroids and leukotriene inhibitors). Healthy controls were recruited exclusively for participation in this trial and underwent endoscopy solely for study purpose. They were pre-screened to ensure they had no atopic predisposition or other severe comorbidities classified as ASA II or higher. Each study subject gave written informed consent, and the study protocol was approved by the Medical Ethics Committee of our institution and prospectively registered in the Dutch trial registry (Trialregister.nl NTR4892).
Study protocol
In this trial, patients were treated with a newly developed amino acid-based formula (Neocate Nutricia, Utrecht, The Netherlands). The clinical results of the dietary trial have been published before (16), the focus of the current study is on the effects on esophageal and duodenal barrier function. In order for patients to meet their nutritional requirements during the elemental diet, patients visited a dietician with expertise in EoE, who calculated the minimal required daily formula consumption based on a patient’s body mass index (BMI) and weekly physical activity. After a washout period of four weeks during which patients were not allowed to use any immunosuppressive drugs, a baseline endoscopy was performed. After four weeks of elemental diet patients underwent a second endoscopy. Healthy controls were not treated with an elemental diet and only underwent the baseline endoscopy. Before each endoscopy, patients and healthy controls were instructed to consume a dual sugar solution and to collect their urine over 5 h. Details regarding this lactulose/mannitol test will be provided below.
Esophagogastroduodenoscopy
The endoscopies were performed by a single gastroenterologist per standardized protocol. After routine inspection of the esophagus and stomach, electrical tissue impedance spectroscopy (ETIS) measurements were performed in the esophagus and proximal duodenum, as described below. Subsequently four biopsy specimens were taken in the duodenum for histopathology and two for gene expression profiling. In the same region, another four biopsy specimens were obtained with a larger biopsy forceps (diameter, 3.7 mm) for in vitro analysis of mucosal integrity in Ussing chambers. These measurements were also performed in the mid esophagus. In total, 10 biopsy specimens were taken in the duodenum and 10 in the esophagus.
Histopathologic analysis
Duodenal and esophageal biopsy specimens were directly fixed in formalin and subsequently embedded in paraffin. The biopsy specimens were sectioned at 5 microns and stained with hematoxylin and eosin (H&E) and tryptase. In a low-power view setting the area of most densely populated eosinophilia in the esophageal biopsy specimen was determined. Subsequently a × 400 magnification was used in order to determine the peak eosinophil count per HPF (an area of 0.24 mm2). According to previous literature, complete histologic response was defined as <15 eosinophils/HPF with a decline of more than 50% of pre-diet eosinophil count (17). Partial response was defined as a decline of more than 50% of pre-diet peak eosinophil count but with ≥15 eosinophils/HPF after diet. A gastrointestinal pathologist with extensive expertise in EoE, who was blinded to the patients’ treatment status, scored the biopsy specimens. Other analyzed histologic features included: (i) basal hyperplasia, graded on a scale of 0–3 (absent to severe hyperplasia in two upper third of total epithelial thickness) and (ii) spongiosis, which was scored analogous to basal hyperplasia (18). Duodenal biopsy specimens were evaluated according to the modified Marsh-Oberhuber classification, which describes mucosal architectural and inflammatory changes, villous atrophy, crypt hyperplasia and increased intraepithelial lymphocytes (IELs) (19). All analyses were performed using an Olympus BX41 microscope (Olympus Europe, Hamburg, Germany).
Esophageal and duodenal mucosal integrity assessment in vivo
ETIS measurements were performed as previously described (20). In brief, impedance is the measure of the degree to which an electric circuit resists to an alternating electric current when impressed through tissue (21). Mucosal impedance reflects how electricity travels through intestinal tissue (22). During endoscopy a flexible ETIS probe with a diameter of 3.2 mm was advanced through the working channel of the endoscope and placed against the esophageal and duodenal mucosa. The probe consists of four electrodes, two inject an alternating current and the other two electrodes measure the potential difference between them. In each patient, measurements were performed mid esophageal and in the proximal duodenum, in four quadrants, and since the impedance measurements were directly visualized on the data acquisition unit only stable measurements were obtained. All measurements were performed by the same gastroenterologist with expertise in ETIS.
Esophageal and duodenal mucosal integrity assessment in vitro
In vitro analysis of mucosal integrity was performed according to previously described methods (20). Briefly, biopsy specimens were directly transferred to Ussing chambers, consisting of two baths filled with Meyler solution and gassed with oxygen. The biopsy specimens were mounted in between the baths, separating the two solutions. A voltage electrode measured the ion transport across the membrane, and two electrodes were injecting a constant current. According to Ohm’s law, the transepithelial resistance (TER) was calculated. Simultaneously, transepithelial flux of two fluorescent molecules, fluorescein (molecular weight 0.3 kDa) and rhodamine (40 kDa; size similar to that of common food allergens), was measured. On the luminal side, the Meyler solution was replaced for the same solution with addition of the fluorescing molecules. A sample of the solution was taken from the opposite side, and the concentration of the two molecules was measured, which reflects the permeability of the mucosa in vitro.
Lactulose/mannitol test
The dual sugar test measuring the L/M ratio is one of the most commonly used test for the analysis of small intestinal permeability (23). It consists of a simultaneous oral administration of the disaccharide lactulose and the monosaccharide mannitol and their urinary excretion over period of time. Lactulose is a large non-metabolizable molecule; of which only a small proportion permeates through the small intestinal mucosa, and is excreted in the urine. If the mucosal integrity is impaired the urinary excretion of lactulose will increase. In contrast, mannitol, the smaller molecule, can always pass through the membrane freely, and the percentage that is excreted in the urine is higher. Intestinal permeability is expressed as the ratio of urinary excretion of lactulose vs. mannitol; the higher the ratio the higher the permeability of the small intestine. Patients and healthy controls drank 100 ml of water with 2 g of lactulose and 5 g of mannitol. The subjects consumed the solution within the period of fasting that preceded the endoscopic procedure. Subsequently, urine was collected for 5 h after ingestion, which is a validated timeframe for small intestinal permeability (24). An aliquot of the total urine volume was preserved in a −20° freezer until analysis. L/M ratios were calculated by high-performance liquid chromatography (HPLC). The upper limit of a normal L/M ratio was defined as 0.03, as previously described (25).
Quantitative real-time PCR
Expression of genes encoding for barrier integrity and tight junction proteins was measured by qPCR as previously described (16). Briefly, esophageal and duodenal biopsy specimens were immediately immersed in RNA stabilization reagent (RNAlater, Qiagen, Hilden, Germany). RNA was extracted from homogenized biopsy specimens according to the manufacturer’s instructions of RNeasy Micro Kit (Qiagen). A Nanodrop Spectrophotometer (Nanodrop Technologies, Wilmington, DE) calculated the concentration of RNA, and subsequently cDNA was synthesized by making use of a reverse transcriptase reaction which was performed according to the MBI Fermentas cDNA synthesis kit (Fermentas, Vilnius, Lithuania), using both the Oligo (dT) 18 and the D (N) 6 primers. Quantitative real-time RTPCR was performed on a LightCycler 480 (Roche Diagnostic, Almere, The Netherlands) using SYBR Green PCR Master Mix (Roche Diagnostic) and primers from Invitrogen. For quantitative real-time PCR, samples were normalized for the mean of the two most stable housekeeping genes Cyclophilin and Hypoxanthine-Guanine Phosphoribosyl Transferase (HGPRT). Transcript values of zonula occludes 1(ZO-1), desmoglein-1 (DSG1), claudin-1 (CLDN1), and filaggrin (FLG) were determined in duplicate. Primer sequences are applied as Supplementary Table 1 online.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (version 22.0) (SPSS, Chicago, USA). Descriptive statistics were used to summarize findings. Categorical variables were described as percentages and continuous variables were expressed as median with interquartile ranges. Before and after treatment, binary data were compared using McNemar’s test and ordinal data were compared using the Wilcoxon’s signed rank test for non-parametric variables. Unpaired non-parametric data were compared using the Mann–Whitney U-test for ordinal data and a χ2-test for categorical data. Correlations were calculated using the Spearman correlation coefficient. A P-value <0.05 was considered significant.
Results
Twenty-five patients were eligible for participation. After baseline endoscopy, four patients were excluded because of spontaneous remission with a peak eosinophil counts <15 eos/HPF. Seventeen of the 21 patients, who started the elemental diet, completed the trial according to the protocol guidelines. Eight age-matched healthy controls were included and underwent the baseline endoscopy. Baseline characteristics of the included EoE patients and healthy controls are listed in Table 1.
Table 1. Baseline characteristics.
| EoE patients (N=17) | Healthy controls (N=8) | |
|---|---|---|
| Gender, male | 12 (71%) | 4 (50%) |
| Age, median (IQR) years | 46 (30–51) | 45 (28–51) |
| Race, Caucasian | 13 (77%) | 8 (100%) |
| Atopic diathesis | 12 (71%) | 0 (0%) |
| Food allergy | 8 (47%) | |
| Asthma | 9 (53%) | |
| Allergic rhinitis | 10 (59%) | |
| Atopic dermatitis | 3 (18%) | |
| Presence of endoscopic signsa | ||
| Rings | 13 (76%) | 1 (13%) |
| Furrows | 14 (82%) | 0 (0%) |
| White exudates | 13 (76%) | 0 (0%) |
| Edema | 16 (94%) | 2 (25%) |
| Stricture | 7 (41%) | 0 (0%) |
| Crepe paper | 2 (11%) | 0 (0%) |
| Histology | ||
| Peak eosinophil count, median (IQR) | 43 (29–80) | 0 (0–0) |
| Abnormal Marsh classification | 0 (0%) | 0 (0%) |
| Spongiosis | ||
| None | 0 (0%) | 8 (100%) |
| Mild | 4 (24%) | 0 (0%) |
| Moderate | 8 (47%) | 0 (0%) |
| Severe | 5 (29%) | 0 (0%) |
| Basal hyperplasia | ||
| None | 0 (0%) | 7 (87%) |
| Mild | 4 (24%) | 1 (13%) |
| Moderate | 9 (52%) | 0 (0%) |
| Severe | 4 (24%) | 0 (0%) |
EoE, eosinophilic esophagitis; IQR, interquartile range.
Scored as present or absent.
Histopathological response
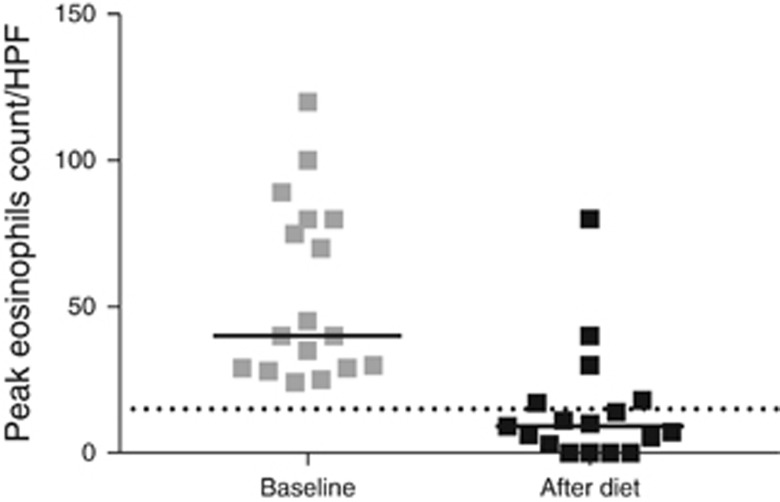
After four weeks of elemental diet the peak eosinophil count in the esophageal mucosa had decreased significantly, from 43 (29–80) per HPF to 9 (2–18) per HPF (median, interquartile range) (P<0.001; Figure 1). Twelve of the 17 (71%) patients achieved complete histological response and 4 (24%) patients achieved partial response. One patient was classified as a non-responder.
Figure 1.
Peak eosinophil counts before and after the diet. The black horizontal lines reflect the group medians and the dotted line indicates the threshold of active disease (15 eosinophils/HPF).
The proportion of patients with moderate to severe spongiosis in their esophageal biopsies was significantly higher before the diet, and decreased from 13 (76%) to 5 (30%) after the diet (P=0.015). In a minority of patients spongiosis had completely disappeared after the diet (6%). Likewise, the diet had an effect on basal cell hyperplasia: 13 (76%) patients had moderate to severe basal cell hyperplasia before the diet, compared with 2 (12%) patients after the diet (P=0.006).
In the duodenal biopsy specimens of EoE patients no abnormalities were observed before or after elemental diet and all biopsy specimens were classified as Marsh 0. In the healthy controls, no abnormalities were observed in the esophageal and duodenal biopsy specimens.
Esophageal mucosal integrity
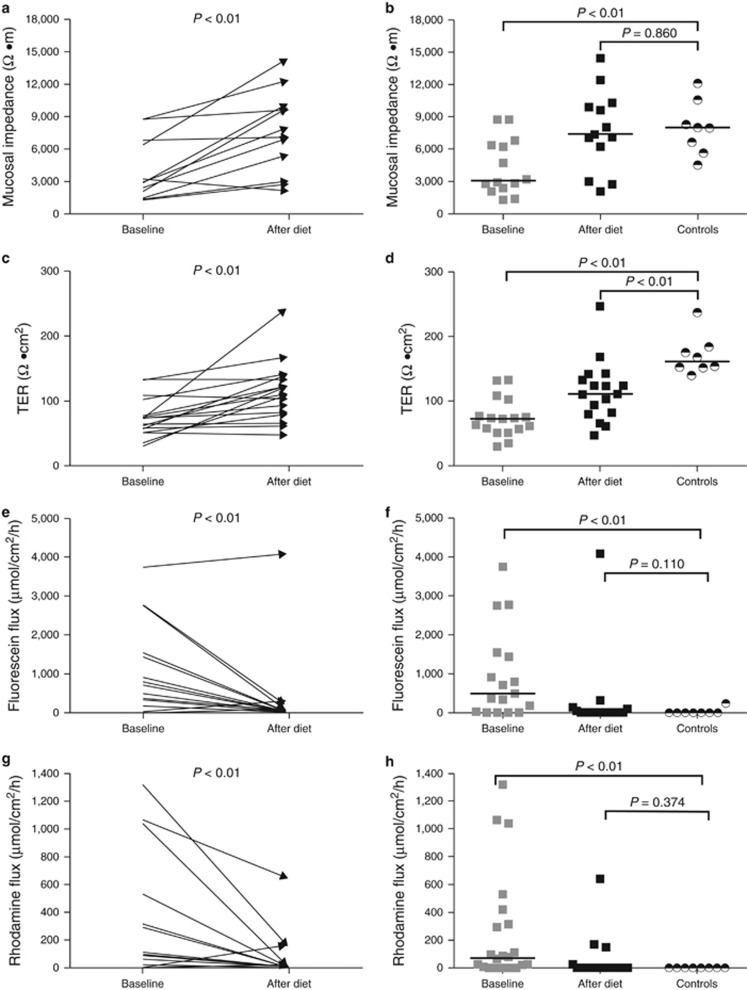
At baseline, patients had a significantly lower esophageal mucosal impedance (ETIS) than after the diet and as compared with healthy controls. After the diet, esophageal mucosal impedance values in both groups were no longer significantly different. Accordingly, the esophageal TER had significantly improved in patients after diet when compared with baseline TER. However, despite this improvement, the esophageal TER in EoE patients after diet remained significantly lower than the TER in healthy controls (Table 2 and Figure 2). Although not statistically significantly different, after treatment the TER in the complete responders was considerably higher than the TER in the partial/non-responder (124 Ω cm2 vs. 80 Ω cm2 (MEDIANS)). Subsequently, the esophageal mucosal permeability, measured by the transepithelial flux of 0.3 kDa fluorescein molecules and 40 kDa rhodamine molecules, had decreased significantly after the diet. After the diet, esophageal fluorescein and rhodamine flux in patients were reduced to healthy control levels (Table 2 and Figure 2). In the compete responders the mean transepithelial fluorescein and rhodamine flux were lower (MEDIAN 43 μmol/cm2/h and 15 μmol/cm2/h) compared with the mean flux in partial/non-responders (MEDIAN 768 μmol/cm2/h and 162 μmol/cm2/h), although statistical significance was not reached.
Table 2. Esophageal mucosal integrity in EoE patients (N=17) at baseline and after diet compared with healthy controls (N=8).
| Baseline, median(IQR) | Diet, median(IQR) | HC, median(IQR) | P valuea | P valueb | |
|---|---|---|---|---|---|
| Esophagus | |||||
| ETIS Ω m | 2,905 (1,940–6,500) | 7,580 (4,239–10,109) | 7,996 (5,913–10,016) | 0.003 | 0.860 |
| TER Ω cm2 | 73 (54–90) | 111 (81–137) | 161 (152–182) | 0.002 | 0.001 |
| Fluorescein fluxc | 531 (13–1492) | 0 (0–75) | 0 (0–0) | 0.004 | 0.110 |
| Rhodamine fluxc | 65 (0–423) | 0 (0–13) | 0 (0–0) | 0.011 | 0.374 |
| Duodenum | |||||
| ETIS Ω m | 1,397 (1,177–1,860) | 1,540 (1,249–1,997) | 1,314 (1,075–2,148) | 0.110 | 0.743 |
| TER Ω cm2 | 69 (63–74) | 66 (60–72) | 77 (74–80) | 0.552 | 0.07 |
| Fluorescein fluxc | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.180 | 1.0 |
| Rhodamine fluxc | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.180 | 1.0 |
EoE, eosinophilic esophagitis; ETIS, electrical tissue impedance spectroscopy; HC, healthy controls; IQR, interquartile range; TER, transepithelial resistance.
P-values baseline vs. after diet (Wilcoxon’s signed rank test).
P-value after diet vs. healthy controls (Mann–Whitney U-test).
Expressed as μmol/cm2/h.
Figure 2.
Complete absence of food allergens by an elemental diet significantly increased the esophageal mucosal impedance and TER (a, c) and decreased the esophageal transepithelial flux of fluorescein (0.3 kDa) and rhodamine molecules (40 kDa) (e, g). The esophageal mucosal impedance and the fluorescein and rhodamine flux reached values comparable to those in healthy controls (b, f, h). The esophageal TER remained impaired after the elemental diet (d). Black horizontal lines indicate group medians.
Duodenal mucosal integrity
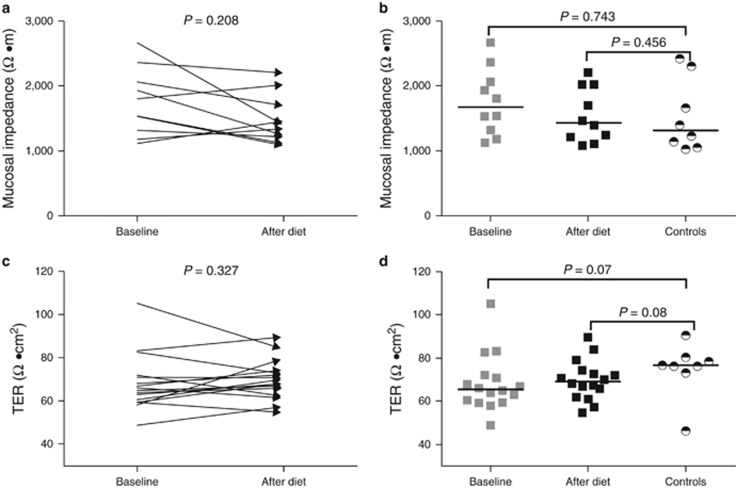
The duodenal mucosal impedance and TER values in EoE patients were similar to those found in healthy controls and were not affected by the diet (Table 2 and Figure 3). Accordingly, the duodenal mucosa in patients before and after diet and in healthy controls was completely impermeable for fluorescein and rhodamine molecules (Table 2).
Figure 3.
Complete absence of food allergens by an elemental diet did not affect the duodenal mucosal impedance and TER (a, c). The duodenal mucosal impedance and TER at baseline and after the elemental diet were not significantly different from values seen in healthy controls (b, d). Black horizontal lines indicate group medians.
Lactulose mannitol test
The L/M ratios of patients before the diet (median (interquartile range) 0.018 (0.007–0.047)), after the diet (median (interquartile range) 0.022 (0.012–0.061)) and in healthy controls (median (interquartile range) 0.020 (0.013–0.024) were all within the reference range and fell below the upper limit of normal permeability values (0.03). There was no statistically significant difference between groups (P>0.05).
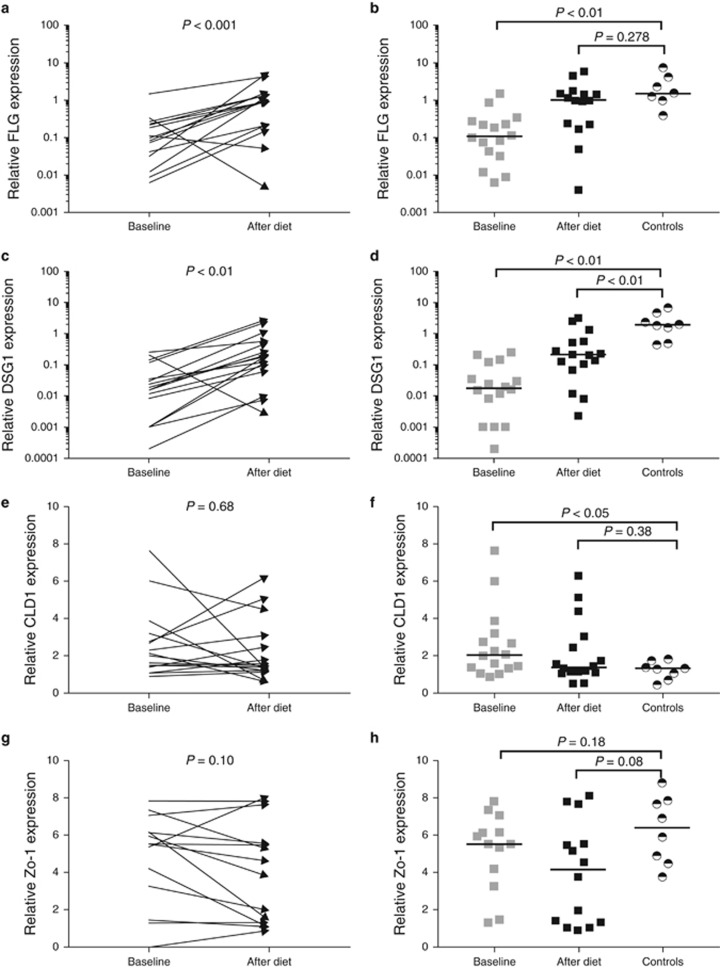
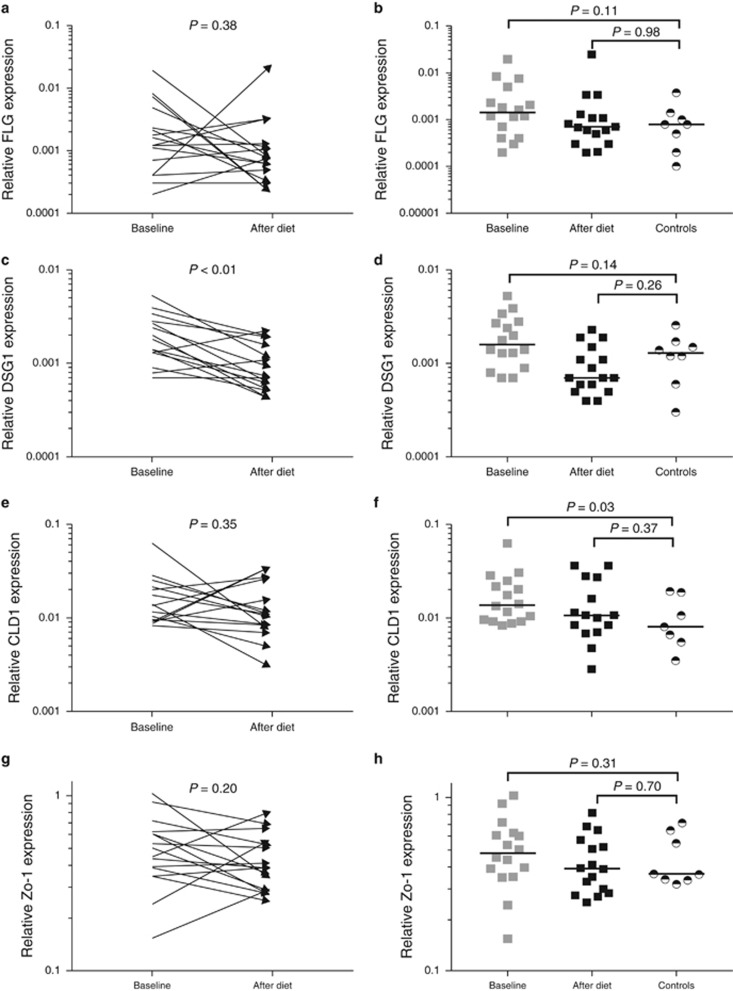
Esophageal expression of transcripts for barrier integrity and tight junction proteins
The expression of the transcripts for tight junction and barrier integrity proteins FLG and DSG1, measured in the esophageal biopsy specimens of EoE patients at the onset of the diet, were significantly lower when compared with healthy controls. The expression of the transcripts for both proteins was increased significantly after the diet. The gene expression of the transcripts for FLG reached values similar to those in healthy controls. Despite the increased expression of the transcripts for DSG1 after the diet, expression levels were still lower than in healthy controls (Figure 4). Comparison of the baseline DSG1 and FLG expression in partial/non-responders to complete responders showed that the expression in partial/non-responders seems to be below those of responders, although it did not reach statistical significance (FLG 4 × 10−2(1 × 10−2–2 × 10−2) vs. 19 × 10−2(10 × 10−2–32 × 10−2), P=0.06 and DSG1 8 × 10−3(.6 × 10−3–22 × 10−3) vs. 30 × 10−3(10 × 10−3–140 × 10−3) P=0.08). This potential difference in FLG and DSG1 gene expression between responders and partial/non-responders persisted after the elemental diet.
Figure 4.
Complete absence of food allergens by an elemental diet significantly increased the expression of genes encoding mucosal barrier proteins filaggrin (FLG) and desmoglein-1 (DSG1) (a, c), and did not affect the expression of genes encoding tight junction proteins claudin-1 (CLDN1) and zonula occludes-1 (ZO-1) (e, g) in the esophageal biopsy specimens. FLG, CLDN1 and ZO-1 expression reached values that were not significantly different compared with values seen in healthy controls (b, f, h), whereas DSG1 expression remained significantly impaired after the elemental diet (d). CLDN1 expression at baseline in EoE patients was significantly higher compared with values seen in healthy controls (f). Logarithmic y axes were used to visualize the skewed data of FLG and DSG1 expression (a–d). Black horizontal lines indicate group medians.
The baseline gene expression levels of the tight junction proteins ZO-1 and CLDN1 were not significantly different compared with those found after the diet. In addition, the expression of ZO-1 and CLDN1 after diet did not significantly differ from values seen in healthy controls. Interestingly, the baseline expression of CLDN1 was significantly higher in EoE patients compared with healthy controls (Figure 4).
Duodenal expression of transcripts for barrier integrity and tight junction proteins
The expression of genes encoding FLG, ZO-1 and CLDN1 in the duodenal biopsy specimens was not affected by the diet and values before and after the diet were not significantly different. Notably, the baseline gene expression of DSG1 was significantly higher compared with the expression values after diet. Comparison of the gene expression of FLG, DSG1, and ZO-1 in patients at baseline and after the diet to the gene expression in healthy controls showed no significant differences. However, the baseline CLDN1 expression in EoE patients was significantly higher than that in healthy controls (Figure 5).
Figure 5.
Complete absence of food allergens by an elemental diet did not affect the expression of genes encoding barrier protein filaggrin (FLG) or tight junction proteins claudin-1 (CLDN1) and zonula occludes-1 (ZO-1) in the duodenal biopsy specimens (a, e, g). The expression of desmoglein-1 (DSG1) after diet was significantly lower when compared with baseline expression (c). There were no significant differences in the expression of FLG, DSG1 and ZO-1 between EoE patients and healthy controls (b, d, h). The baseline gene expression of CLDN1 in EoE patients was significantly higher when compared with values seen in healthy controls, whereas the expression of CLDN1 after diet was no longer significantly different from values seen in healthy controls (f). Logarithmic y axes were used to visualize the skewed data. Black horizontal lines indicate group medians.
Correlations between mucosal integrity, peak eosinophil count and FLG and DSG1 expression
Spearman’s correlation analysis demonstrated a negative correlation between peak eosinophil counts and esophageal mucosal impedance and TER (r=−0.48 and r=−0.68 both <0.05). A positive correlation was found between peak eosinophil counts and esophageal mucosa permeability, expressed as the transepithelial flux of fluorescein and rhodamine (r=0.43 and r=0.49, both P<0.05). Consequently, a negative correlation was found between peak eosinophil counts and the esophageal gene expression of the transcripts for the barrier integrity proteins FLG and DSG1 (r=−0.56 and r=−0.57, both P<0.05).
Esophageal TER was positively correlated to the gene expression of FLG (r=0.44, P<0.05) and DSG1 (r=0.61, P<0.05). In addition, esophageal mucosal impedance correlated positively with the gene expression of the transcripts for the barrier integrity proteins FLG (r=0.46, P<0.05) and DSG1 (r=0.54, P<0.05).
Discussion
This prospective study was the first study to demonstrate that, in EoE, induction of remission, by excluding dietary allergen with use of an elemental diet, leads to recovery of the esophageal mucosal integrity. To our knowledge, the small intestinal permeability of EoE patients has never been investigated prospectively before and after treatment in the same cohort. Our study gives a comprehensive overview of the esophageal and duodenal mucosal integrity in EoE by studying five different measures.
We demonstrate that complete avoidance of dietary allergens, achieved by an elemental diet, leads to restoration of the mucosal impedance and disturbed permeability of the esophageal mucosa. After 4 weeks of elemental diet, the esophageal mucosal impedance and permeability corresponded with the values observed in healthy controls. Although the elemental diet significantly improved the esophageal TER, values did not completely normalize when compared with healthy controls. Besides restoration of the esophageal mucosal integrity, the reduced baseline gene expression of the transcripts for barrier integrity protein FLG and DSG1 in the esophageal biopsy specimens is increased significantly by an elemental diet. Comparison of esophageal TER and FLG and DSG1 expression of complete responders to partial/non-responders showed that responders had a greater improvement after diet, suggesting that improvement paralleled histologic response. In contrast to the convincing effects of the elemental diet on the esophageal mucosal integrity, the duodenal mucosal integrity seemed not be affected and the duodenal mucosal integrity was not impaired compared with healthy controls.
The small intestinal permeability of EoE patients has been investigated twice before. Our results are in keeping with the conclusion of Leung et al., that an alteration in small intestinal permeability does not play a role in EoE (26). In contrast, the group of Katzka et al. demonstrated an increased small intestinal permeability in patients with active EoE (12). Intestinal permeability depends on numerous factors, and potentially plays a role in atopic dermatitis, food allergy and asthma (27). EoE patients often present with concurrent allergic diatheses, and although Katzka et al. found an equal distribution of concurrent allergic diatheses among groups, the disease severity and activity could have differed between groups. This may have been an explanation for the observed increased intestinal permeability. Additionally, the measurements were not performed in the same patients before and after treatment. Furthermore, in a majority of Katzka’s patients disease remission was achieved upon topical steroid treatment, which could have had a beneficial effect on other atopic diatheses, which are associated with increased intestinal permeability.
The present study indicates that in EoE patients the inflammation and mucosal integrity disruption seem to be restricted to the esophagus. A possible explanation is that EoE is initiated and regulated by a local immune response. Indeed, several studies suggested local esophageal B and T cell activation, including class cell recombination to IgE in the esophagus, local IgE production and IgE-bearing mast cells in the esophageal mucosa (28, 29). Moreover, it has been shown that esophageal epithelial cells are able to process and present antigens, possibly stimulating a Th-2 immune response (30).
Given the paucity of data in the literature on the effect of dietary treatment on the esophageal mucosal integrity, a direct comparison between previous studies is difficult. However, we previously demonstrated that, in PPI-REE patients, histological response to PPI treatment was associated with improvement of the esophageal integrity (31). Subsequently, it was shown that successful topical steroid treatment restored the esophageal integrity in EoE patients (6). An elemental diet counteracts the esophageal inflammation in EoE by elimination of food allergens in sensitized patients, whereas topical steroids attack the inflammatory cells or modify signaling pathways (32). Despite different approaches, the esophageal mucosal integrity values of patients who responded successfully to topical steroid treatment were also comparable with values in healthy controls (6). These findings imply that histologic response to dietary or immunosuppressant approaches result in a comparable complete restoration of the esophageal mucosal integrity, suggesting that the disruption is at least partly secondary to esophageal inflammatory damage. Nevertheless our study was not specifically designed to answer this question and further mechanistic research is warranted to confirm that the barrier function defect is indeed secondary to inflammatory injury.
A major question relevant for EoE is how food allergens initiate the esophageal inflammation and disrupt the esophageal mucosal integrity. We have previously shown that Thymic Stromal Lymphopoietin (TSLP), a cytokine which drives the inflammatory response in multiple Th2-type associated allergic diatheses, was overexpressed in esophageal biopsy specimens of patients with active EoE, and normalized by complete absence of food allergens (16, 33). Recently, in an experimental study, it was shown that in vitro food allergen stimulation of esophageal epithelial cells induced TSLP secretion, which in turn may activate esophageal dendritic cells leading to a Th-2 immune response (33). In addition, in vitro incubation of monocytes with specific allergens led to elevations of the EoE-associated inflammatory cytokines IL-5 and IL-13, showing that food allergens can directly activate the immune system. Our current study follows previous work in which we have demonstrated that the overexpression of the transcripts for IL-5 and IL-13 in active EoE was reversed by absence of food allergens through an elemental diet (16). Considering the ability of IL-13 to downregulate DSG1 and FLG, we assume that in our study the upregulation of IL-13 is at least partly responsible for the impaired gene expression of the transcripts for FLG and DSG1 (9, 10). Although the gene expression of FLG was normalized, gene expression of DSG1 was only partly restored upon elemental dietary treatment. DSG1 dysfunction induces segregation of epithelial cells which is histologically described as diluted intercellular spacing (DIS) or spongiosis (10). Remarkably, spongiosis could not completely be resolved by elemental dietary treatment or by topical steroid treatment (6); these findings may support the hypothesis that the expression of DSG1 recovers slower than the 4-week follow-up of our study (6, 14).
An alternative explanation is that this resultant impairment of the mucosal barrier in the absence of food allergens is preexisting and may facilitate initial transepithelial allergen passage and thus initiate inflammation. It has recently been shown that antigen penetration in active EoE might indeed be facilitated by impairment of epithelial integrity (34). These findings would argue against impaired mucosal integrity as a pure consequence of the inflammation, and suggest it is a primary defect predisposing to EoE. Given that the relationship between an impaired epithelial barrier function and allergy has been studied extensively in atopic dermatitis, and since the pathophysiological mechanisms seem to align, we hypothesized that tight junction proteins CLDN1 and ZO-1, both dysregulated in atopic dermatitis, would play a role in the pathogenesis of EoE (8). However, in this study we found no decreased expression of genes encoding CLDN1 and ZO-1. These results are in keeping with the conclusion for previous studies (10). Altogether, these data suggest that the impaired mucosal integrity in EoE is not affected by an alteration of tight junctions.
The limitations of this study must be pointed out. The first of these is the relatively small sample size, which is, however, comparable with that of other prospective mechanistic EoE trials. The sample size was large enough to find strong significant differences. Second, the ETIS has not been validated for duodenal tissue. However, the values of healthy controls served as a valuable reference standard and ETIS has successfully been used to detect the electrical characteristics of many different tissues, and ETIS measurements correlated with the other parameters of duodenal mucosal integrity (35). Third, we have evaluated gene expressions, which is a prediction of protein expression. It would have provided additional information if we would have measured the actual changes in the encoded proteins.
In conclusion, complete dietary allergen avoidance, using an elemental diet, restores the impaired esophageal mucosal integrity. This study confirms that dysregulation of filaggrin and desmoglein-1 plays a role in the impaired esophageal barrier function rather than alterations in tight junction proteins. Finally, our study provides further evidence that impaired duodenal mucosal integrity is not involved in the pathogenesis of EoE.
Study Highlights
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Marijn J. Warners, MD.
Specific author contributions: Conception and design; M.J.W., B.J.V.-B., B.D.v.R., M.T.J.V.A., L.F.H. and A.J.B. Analysis and interpretation of the data; M.J.W., P.H.P.v.H., B.J.V.-B., J.V., W.J.J. and A.J.B. Drafting of the article: M.J.W. Critical revision of the article for important intellectual content; M.J.W., B.J.V.-B., J.V., B.D.v.R., M.T.J.V.A., L.F.H., W.J.J., A.J.P.M.S. and A.J.B. Final approval of the article; M.J.W., B.J.V.-B., P.H.P.v.H., J.V., B.D.v.R., M.T.A., L.F.H., W.J.J., A.J.P.M.S. and A.J.B.
Financial support: A.J.B. received research funding from Endostim and Danone and received speaker and/or consulting fees from MMS, Astellas, AstraZeneca, Almirall and Allergan. W.J.J. (VIDI grant) is supported by the Netherlands Organization for Scientific Research (NWO). W.J.J. received research funding from Glaxo Smith Kline Research, and Mead Johnson Nutrition. M.T.A. and L.F.H. are employees of Nutricia Research—results of the current study do not affect salary. This study was an investigator-initiated study, partly funded by Nutricia Research.
Potential competing interests: None.
Supplementary Material
References
- Dellon ES, Gonsalves N, Hirano I et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–692. [DOI] [PubMed] [Google Scholar]
- Schoepfer AM, Safroneeva E, Bussmann C et al. Delay in Diagnosis of Eosinophilic Esophagitis Increases Risk for Stricture Formation in a Time-Dependent Manner. Gastroenterology 2013;145:1230–6.e1-2. [DOI] [PubMed] [Google Scholar]
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008;6:531–535. [DOI] [PubMed] [Google Scholar]
- Arias A, González-Cervera J, Tenias JM et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014;146:1639–1648. [DOI] [PubMed] [Google Scholar]
- Aceves SS. Food allergy testing in eosinophilic esophagitis: what the gastroenterologist needs to know. Clin Gastroenterol Hepatol 2014;12:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rhijn BD, Verheij J, van den Bergh Weerman MA et al. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol 2015;110:1289–1297. [DOI] [PubMed] [Google Scholar]
- Agrawal R, Woodfolk JA. Skin barrier defects in atopic dermatitis. Curr Allergy Asthma Rep 2014;14:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki T, Tobiishi M, Kusaka-Kikushima A et al. Impaired tight junctions in atopic dermatitis skin and in a skin-equivalent model treated with interleukin-17. PLoS ONE 2016;11:e0161759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard C, Stucke EM, Burwinkel K et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol 2010;184:4033–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill JD, Kc K, Wu D et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol 2014;7:718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Goolamali SK, Levi AJ et al. Intestinal permeability in patients with atopic eczema. Br J Dermatol 1985;112:291–297. [DOI] [PubMed] [Google Scholar]
- Katzka DA, Geno DM, Blair HE et al. Small intestinal permeability in patients with eosinophilic oesophagitis during active phase and remission. Gut 2015;64:538–543. [DOI] [PubMed] [Google Scholar]
- Leung AJT, Persad S, Slae M et al. Intestinal and gastric permeability in children with eosinophilic esophagitis and reflux esophagitis. J Pediatr Gastroenterol Nutr 2015;60:236–239. [DOI] [PubMed] [Google Scholar]
- Peterson Ka, Byrne KR, Vinson LA et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013;108:759–766. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Lazenby AJ, Rowe PC et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995;109:1503–1512. [DOI] [PubMed] [Google Scholar]
- Warners MJ, Vlieg-Boerstra BJ, Verheij J et al. Elemental (amino acid-based) diet effectively decreases eosinophilic inflammation and improves symptoms in adult eosinophilic esophagitis patients. Aliment Pharmacol Ther 2017;45:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf WA, Cotton CC, Green DJ et al. Evaluation of histologic cutpoints for treatment response in eosinophilic esophagitis. J Gastroenterol Hepatol Res 2015;4:1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Neureiter D, Aigner T et al. Comparison of histological parameters for the diagnosis of eosinophilic oesophagitis versus gastro-oesophageal reflux disease on oesophageal biopsy material. Histopathology 2008;53:676–684. [DOI] [PubMed] [Google Scholar]
- Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11:1185–1194. [DOI] [PubMed] [Google Scholar]
- Weijenborg PW, Rohof WOA, Akkermans LMA et al. Electrical tissue impedance spectroscopy: A novel device to measure esophageal mucosal integrity changes during endoscopy. Neurogastroenterol Motil 2013;25:574–578 e457-8. [DOI] [PubMed] [Google Scholar]
- Ravi K, Katzka DA. Esophageal impedance monitoring: clinical pearls and pitfalls. Am J Gastroenterol 2016;111:1245–1256. [DOI] [PubMed] [Google Scholar]
- Jones DM, Smallwood RH, Hose DR et al. Modelling of epithelial tissue impedance measured using three different designs of probe. Physiol Meas 2003;24:605–623. [DOI] [PubMed] [Google Scholar]
- Mishra A, Makharia GK. Techniques of functional and motility test: how to perform and interpret intestinal permeability. J Neurogastroenterol Motil 2012;18:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AS, Camilleri M, Eckert DJ et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol 2011;301:G919–G928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leo V, D’Incà R, Diaz-Granado N et al. Lactulose/mannitol test has high efficacy for excluding organic causes of chronic diarrhea. Am J Gastroenterol 2003;98:2245–2252. [DOI] [PubMed] [Google Scholar]
- Leung AJT, Persad S, Slae M et al. Intestinal and gastric permeability in children with eosinophilic esophagitis and reflux esophagitis. J Pediatr Gastroenterol Nutr 2015;60:236–239. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Barbara G, Buurman W et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario M, Blanchard C, Stringer KF et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut 2010;59:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straumann A, Bauer M, Fischer B et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol 2001;108:954–961. [DOI] [PubMed] [Google Scholar]
- Mulder DJ, Pooni A, Mak N et al. Antigen presentation and MHC class II expression by human esophageal epithelial cells: role in eosinophilic esophagitis. Am J Pathol 2011;178:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rhijn BD, Weijenborg PW, Verheij J et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014;12:1815–23.e2. [DOI] [PubMed] [Google Scholar]
- Caldwell JM, Blanchard C, Collins MH et al. Glucocorticoid-regulated genes in eosinophilic esophagitis: a role for FKBP51. J Allergy Clin Immunol 2010;125:879–888.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouleeswaran PM, Shen D, Lee AJ et al. Preferential secretion of thymic stromal lymphopoietin (TSLP) by terminally differentiated esophageal epithelial cells: relevance to eosinophilic esophagitis (EoE). PLoS ONE 2016;11:e0150968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marietta EV, Geno DM, Smyrk TC et al. Presence of intraepithelial food antigen in patients with active eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;45:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera TK. Bioelectrical impedance methods for noninvasive health monitoring: a review. J Med Eng 2014;2014:381251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.