Abstract
Objectives:
The incidence of pediatric-onset inflammatory bowel disease (IBD) is increasing worldwide. We used population-based health administrative data to determine national Canadian IBD incidence, prevalence, and trends over time of childhood-onset IBD.
Methods:
We identified children <16 years (y) diagnosed with IBD 1999–2010 from health administrative data in five provinces (Alberta, Manitoba, Nova Scotia, Ontario, Quebec), comprising 79.2% of the Canadian population. Standardized incidence and prevalence were calculated per 100,000 children. Annual percentage change (APC) in incidence and prevalence were determined using Poisson regression analysis. Provincial estimates were meta-analyzed using random-effects models to produce national estimates.
Results:
5,214 incident cases were diagnosed during the study period (3,462 Crohn’s disease, 1,382 ulcerative colitis, 279 type unclassifiable). The incidence in Canada was 9.68 (95% CI 9.11 to 10.25) per 100,000 children. Incidence was similar amongst most provinces, but higher in Nova Scotia. APC in incidence did not significantly change over the study period in the overall cohort (+2.06%, 95% CI −0.64% to +4.76%). However, incidence significantly increased in children aged 0–5y (+7.19%, 95% +2.82% to +11.56%). Prevalence at the end of the study period in Canada was 38.25 (95% CI 35.78 to 40.73) per 100,000 children. Prevalence increased significantly over time, APC +4.56% (95% CI +3.71% to +5.42%).
Conclusions:
Canada has amongst the highest incidence of childhood-onset IBD in the world. Prevalence significantly increased over time. Incidence was not statistically changed with the exception of a rapid increase in incidence in the youngest group of children.
Introduction
The incidence and prevalence of childhood-onset inflammatory bowel disease (IBD) has risen rapidly over the past two decades (1). While previously considered rare in children, IBD has emerged as a global disease in developed and developing nations (2, 3). The increasingly early onset of Crohn’s disease (CD) and ulcerative colitis (UC) raises important questions regarding the role of early-life factors in the pathogenesis of IBD (4). It also represents a significant burden to health care systems (5), because the treatment of childhood-onset IBD is more expensive in the long-term (6).
Canadian health administrative data represent an unprecedented opportunity to assess the trends in epidemiology and health services utilization of children with IBD. Each Canadian province has independent single-payer health systems, so all legal residents of each province are included within health administrative data. Independently, the trends of pediatric IBD have been described in individual Canadian provinces (7, 8, 9, 10, 11, 12). However, application of the same case identification algorithm in different jurisdictions without additional validation can result in misclassification bias (13). Even though the health administrative data from each province are similar in database population and data structure, research using these data has revealed differences in trends. For example, studies from Ontario have consistently reported increasing incidence in children and teenagers (7, 8, 9), with less pronounced increased incidence in adults (9). However, research from Quebec (12) and Nova Scotia (11) has demonstrated stable incidence in children and decreased incidence in adults. These disparities may be due to differences in methodology (13), or due to true variation. In addition, single-province studies may not be powered to demonstrate trends in age groups where IBD is rare, such as those with disease onset prior to the age of 10 years.
The Canadian Gastro-Intestinal Epidemiology Consortium (CanGIEC) was formed to provide national-level information using distributed network analysis of multiple Canadian provincial health administrative data, reducing heterogeneity of methods and improving power for smaller populations or uncommon outcomes. The aim of this study was to determine trends in incidence and prevalence of childhood-onset IBD in Canada to determine the burden of IBD in Canadian children.
Methods
Study design and setting
This study was approved by the research ethics boards of the Children’s Hospital of Eastern Ontario, the Ottawa Hospital, University of Manitoba, Dalhousie University, Conjoint Health Research Ethics Board of the University of Calgary, and the Montreal Jewish General Hospital. In addition, this study was reviewed for privacy concerns by Manitoba Health’s Health Information Privacy Committee, the Privacy Officer of the Institute for Clinical Evaluative Sciences (ICES), and the Comission d’acces a l’information du Quebec (CAI). We conducted a retrospective cohort study using the provincial health administrative and population databases from five Canadian provinces: Alberta, Manitoba, Nova Scotia, Ontario, and Quebec. These databases contain records of all legal residents of each province, representing a source population of 26,530,074 people, 79.2% of the Canadian population (14).
CanGIEC conducted a distributed network analysis in which the same methodology was applied to each Canadian province’s health administrative data to produce standardized estimates of incidence and prevalence in each province. In addition, we determined trends over time by age group: overall (6 months to 15.9 years (y) at diagnosis), and in sub-groups: 6 months to 4y, 5–9y, 10–13y, and 14–15.9y at diagnosis or at the time of the point prevalence estimate. Standardized estimates and trends were then combined by meta-analysis using random-effects models analysis to account for heterogeneity. This method has been used previously to conduct observational pharmaco-epidemiology research in Canada and the United Kingdom (15).
Population
To create the cohort, we identified all children <16y at the time of IBD diagnosis in each province. For incident cases, we excluded people with discontinuous health care coverage within the province, such as those who left a province and then returned. Incident cases diagnosed <6 months old were also excluded due to the risk of misclassification with allergic or other non-IBD causes of enterocolitis. We used validated algorithms to identify persons with IBD for the provinces of Alberta, Manitoba, and Ontario (7, 16, 17). These algorithms (see Supplementary Table 1) online consisted of combinations of outpatient, hospitalization and procedural codes to accurately distinguish IBD cases from the rest of the population, and have all been validated as the most accurate identification method for the province in which they were applied. The Ontario algorithm was validated and found to be accurate in a population of children with and without IBD from academic and community practices, treated by pediatric gastroenterologists, adult gastroenterologists, and primary care providers (7). The Manitoba algorithm was also validated using a random sample chart review from across the province. Although most pediatric patients with IBD were treated in a single-academic center in Winnipeg, the algorithm identified 95% of those patients (17). The Alberta algorithm was validated using a reference standard derived from endoscopy reports from the Calgary Health Region and Capital Health Region and included both academic and community practices (16). Of note, Quebec cases were identified using a modified version of the Alberta algorithm (12), and Nova Scotia cases were identified using the Ontario pediatric algorithm (7). To ensure that the lack of validated algorithms in Quebec and Nova Scotia had not altered study conclusions, we conducted a sensitivity analysis without these provinces included.
The date of diagnosis was defined as the date of the first health care contact (either outpatient or hospitalization) within the identification algorithm with associated International Classification of Disease (ICD), ninth-revision (ICD-9) or ICD, tenth revision (ICD-10) for Crohn’s disease (CD) or ulcerative colitis (UC): 555.x, 556.x, K50.x, or K51.x. For the incident estimates in all provinces, we applied validated look-back and look-forward qualification periods. The 3-year look-back period has been demonstrated to successfully differentiate incident from prevalent cases in 95% of Ontario children (7). A look-back period was not applied in cases where full administrative data was available from birth. In order to qualify as an IBD case, we allowed for the combination of codes to occur within a 3-year interval, which has also been validated as adequate in Ontario children (7). Using these look-back and qualification periods, we created study cohorts of incidence and prevalence of childhood IBD from 1999–2010 in Manitoba and Ontario, 1999–2008 for Alberta and Quebec, and 2000–2008 for Nova Scotia. Complete details on data holdings and data availability for this study are presented in Supplementary Table 1.
In children with IBD, validated algorithms were used to classify them as CD, UC, or IBD type unclassifiable (i.e., cases for whom the algorithm could not distinguish CD or UC classification; see Supplementary Table 1). Disease classification was provided for both the initial diagnosis (grouping of diagnostic codes associated with health care contacts immediately after the date of diagnosis) and latest diagnosis (most recent grouping of health care contacts available for each case). For the purposes of incidence and prevalence estimates, the latest diagnosis was used to reflect the treating physicians’ best assessment of disease classification at the end of the study period. Therefore, if a subject’s diagnosis changed from UC to CD, they were counted as CD for the incidence and prevalence estimates and for the calculation of time trends.
Data sources
Health administrative data from all provinces consisted of outpatient physician billing records, and hospitalization records. The databases used to identify cases were described in detail in the studies describing validation of the identification algorithms (7, 16, 17). In the case of all five provinces, a record of the full IBD population was available to investigators for analysis. In Ontario and Nova Scotia, the full non-IBD population of the province was available to investigators. However, in Alberta, Manitoba, and Quebec general population estimates from the 2001 and 2006 Canadian censuses were used to determine the denominator of incidence and prevalence rates, and to calculate inter-censal population estimates.
Statistical analysis
Descriptive statistics were reported as medians with interquartile range (IQR), or proportions where appropriate. Sex- and age-standardized annual prevalence and incidence per 100,000 population were determined for each province for the fiscal years (1 April to 31 March) in which data was available, with corresponding 95% confidence intervals (CI) based on Gamma distribution (18). Yearly and overall rates for the age groups (6 months to 4y, 5–9.9y, 10–13.9y, 14–15.9y) were standardized by sex only, with standard populations consisting of at-risk people within the appropriate age group. Trends in incidence over time were reported as an annual percentage change (APC) over the study period by exponentiating the beta coefficient of the sex-adjusted Poisson regression and subtracting it by one (exp(β)-1). This Poisson model was also used to assess statistical significance in trends in incidence over time, stratified by age category with age-appropriate denominator populations. An APC is statistically significant when its 95% CI does not cross zero. Analyses were conducted using SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Meta-analysis using random-effects models was used to produce national estimates of incidence and prevalence. Incidence and prevalence estimates with 95% CIs were reported for each year, and for the overall study period. We assessed trends in incidence and prevalence over time, stratified by age groups, by meta-analyzing the APC for each province using random-effects models. Only standardized estimates were included in meta-analyses (19). Heterogeneity was calculated using the I2 statistic and Cochrane χ2 test (Q-test), which describe the percentage of total variation across incidence estimates due to heterogeneity rather than chance. We conducted a sensitivity analysis to determine APC in incidence and prevalence excluding provinces without a validated algorithm (Quebec and Nova Scotia). Meta-analyses were conducted with STATA statistical software version 11 (STATA Corp, College Station, TX, USA).
Results
Descriptive characteristics
A total of 5,214 incident cases and 6,554 prevalent cases were included in the study population. The study cohorts are described in Table 1. For the incidence cohort, the characteristics of the populations in each province were similar. One exception was the greater predominance of CD in Quebec compared to other provinces for initial diagnosis (86.4 vs. 57.7%). Similar results were seen for latest diagnosis (88.5 vs. 59.1%) The median age at diagnosis (for overall cohort: 12.3y, IQR 10.5–14.1) and sex distribution (females 43.3%) was not significantly different between provinces. The sex distribution was not significantly different between age groups, with a male majority present in all pediatric age groups (Supplementary Table 2).
Table 1. Descriptive characteristics of the study cohort of incident cases of IBD in children <16 in Canada.
| Alberta | Manitoba | Nova Scotia | Ontario | Quebec | Overall | |
|---|---|---|---|---|---|---|
| Incidence cohort (diagnosed with IBD, age <16y, 1999–2010) | ||||||
| Cases (n) | 655 | 221 | 236 | 2,656 | 1,437 | 5,214 |
| Median age in years at diagnosis (IQR) | 12.0 (9.0–14.0) | 12.8 (10.7–14.6) | 12.0 (10.0–14.0) | 12.8 (10.3–14.6) | 13.3 (11.0–14.8) | 12.64 (11.71–13.56) |
| Age at diagnosis | ||||||
| 6 months–4.9y | 82 (12.5%) | NAa | 13 (5.5%) | 136 (5.1%) | 34 (2.4%) | 265 (5.1%) |
| 5–9.9y | 112 (17.1%) | 46 (20.8%)a | 38 (16.1%) | 471 (17.7%) | 223 (15.5%) | 890 (17.1%) |
| 10–13.9y | 245 (37.4%) | 102 (46.2%) | 112 (47.5%) | 1,144 (43.1%) | 595 (41.4%) | 2,200 (42.2%) |
| 14–15.9y | 216 (33.0%) | 73 (33.0%) | 73 (30.9%) | 905 (34.1%) | 585 (40.7%) | 1,852 (35.5%) |
| Sex (F) | 298 (45.5%) | 101 (45.7%) | 101 (42.8%) | 1,125 (42.4%) | 628 (43.7%) | 2,556 (43.3%) |
| Initial diagnosis | ||||||
| CD | 396 (60.5%) | 131 (59.3%) | 151 (64.0%) | 1,497 (56.4%) | 1,242 (86.4%) | 3,421 (65.6%) |
| UC | 187 (28.5%) | 90 (40.7%) | 70 (29.7%) | 926 (34.9%) | 159 (11.1%) | 1,435 (27.5%) |
| IBD type unclassifiable | 72 (11.0%) | NA | 15 (6.4%) | 233 (8.8%) | 36 (2.5%) | 358 (6.9%) |
| Latest Diagnosis | ||||||
| CD | 386 (62.0%) | 129 (58.4%) | 149 (63.1%) | 1,570 (59.1%) | 1,228 (88.5%) | 3,462 (67.6%) |
| UC | 192 (30.8%) | 92 (41.6%) | 73 (30.9%) | 902 (34.0%) | 123 (8.9%) | 1,382 (27.0%) |
| IBD type unclassifiable | 45 (7.2%) | NA | 14 (5.9%) | 184 (6.9%) | 36 (2.6%) | 279 (5.4%) |
| Prevalence cohort (alive and <16y on 1 July 2010 (Manitoba and Ontario) or 2008 (Alberta, Nova Scotia, Quebec) | ||||||
| Cases (n) | 360 | 67 | 99 | 1,025 | 681 | 3,328 |
| Median age in years at diagnosis (IQR) | 12 (9.0–14.0) | 10.7 (8.1–13.3) | 10.0 (7.0–12.0) | 13.4 (10.8–14.9) | 11.4 (8.3–13.5) | 12.1 (11.1–13.1) |
| Sex (F) | 167 (46.4%) | 31 (46.3%) | 43 (43.4%) | 431 (42.0%) | 278 (40.8%) | 1,465 (44.0%) |
| Latest diagnosis | ||||||
| CD | 188 (53.3%) | 42 (62.7%) | 62 (62.6%) | 554 (54.0%) | 580 (89.5%) | 2,360 (70.9%) |
| UC | 110 (31.2%) | 25 (37.3%) | 32 (32.3%) | 380 (37.1%) | 67 (9.8%) | 714 (21.4%) |
| IBD type unclassifiable | 55 (15.6%) | NA | 5 (5.1%) | 91 (8.9%) | 19 (2.8%) | 190 (5.7%) |
CD, Crohn’s disease; IBD, inflammatory bowel disease; IQR, interquartile range; UC, ulcerative colitis; y: years.
Due to small cell size, raw count was suppressed. The count for children diagnosed <10y was combined.
Incidence
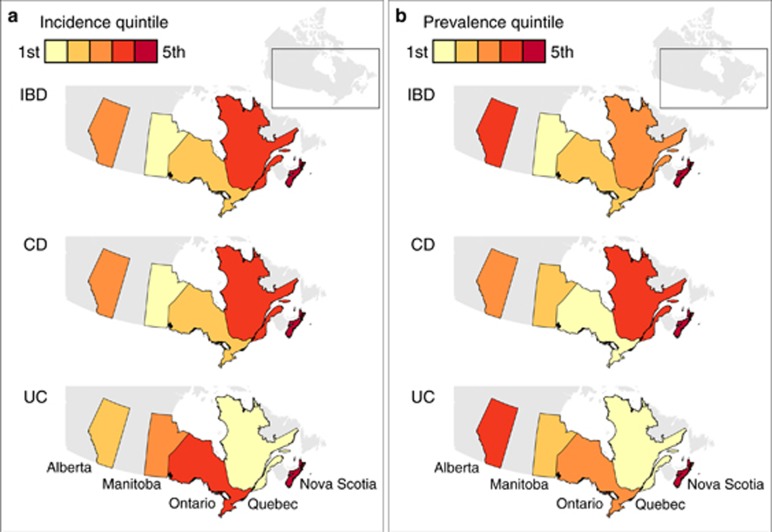
For the full study period, the overall standardized incidence for all provinces was 9.7 (95% CI 9.1–10.2) per 100,000. The incidence by province is demonstrated in Table 2 and Figure 1a. The incidence of IBD in all years was lowest for Manitoba (7.2 per 100,000) and highest in Nova Scotia (15.2 per 100,000), with significant heterogeneity amongst provinces (I2 76.3%, P<0.001). Similar variation across provinces was present for CD (overall incidence 6.5 per 100,000) and UC (overall incidence 2.4 per 100,000). For CD, both Nova Scotia (9.3 per 100,000) and Quebec (8.8 per 100,000) had higher incidence compared to other provinces. Quebec had a lower incidence of UC compared to other provinces (1.0 per 100,000).
Table 2. Standardized incidence and prevalence over the full study period (1999–2010) based on meta-analysis of annual estimatesContinued.
| Alberta | Manitoba | Nova Scotia | Ontario | Quebec | All provinces | I2 (P value) | |
| Alberta | Manitoba | Nova Scotia | Ontario | Quebec | All provinces | I2 (P value) | |
| Incidence | |||||||
| IBD | |||||||
| Number of cases | 655 | 221 | 236 | 2,656 | 1,437 | 5,214 | |
| Incidence per 100,000 (95% CI) | 9.71 (8.81–10.62) | 7.22 (6.18–8.26) | 15.18 (13.1–17.25) | 9.28 (8.22–10.34) | 10.26 (9.50–11.02) | 9.68 (9.11–10.25) | 76.3% (P<0.001) |
| Age group | |||||||
| 6 months–4.9y | 3.60 (2.68–4.53) | a | a | 1.54 (1.11–1.97) | a | 2.06 (1.56–2.55) | 60.2% (P<0.001) |
| 5–9.9y | 4.88 (3.79–5.98) | 3.32 (1.91–4.73) | a | 5.04 (4.12–5.96) | 3.70 (3.04–4.37) | 4.41 (3.91–4.91) | 54.5% (P<0.001) |
| 10–13.9y | 13.62 (11.84–15.40) | 11.49 (8.88–14.11) | 24.67 (18.67–30.67) | 14.26 (12.66–15.87) | 13.98 (12.64–15.32) | 14.13 (13.23–15.04) | 50.4% (P<0.001) |
| 14–15.9y | 22.31 (18.29–26.32) | 15.77 (11.39–20.16) | 31.68 (23.44–39.92) | 21.88 (19.07–24.69) | 28.20 (25.35–31.05) | 23.61 (21.72–25.49) | 62.9% (P<0.001) |
| CD | |||||||
| Number of cases | 386 | 129 | 149 | 1,570 | 1,228 | 3,462 | |
| Incidence per 100,000 (95% CI) | 5.89 (5.30–6.48) | 4.17 (3.36–4.97) | 9.34 (7.69–11.00) | 5.48 (4.91–6.04) | 8.80 (8.15–9.45) | 6.47 (5.91–7.04) | 85.2% (P<0.001) |
| Age group | |||||||
| 6 months–4.9y | 1.62 (0.93–2.32) | a | a | 0.40 (0.22–0.57) | a | 0.51 (0.32–0.71) | 13.9% (P=0.28) |
| 5–9.9y | 1.94 (1.25–2.63) | 2.01 (0.77–3.24) | a | 2.66 (2.15–3.18) | 3.07 (2.51–3.62) | 2.65 (2.35–2.96) | 30.9% (P=0.03) |
| 10–13.9y | 9.52 (8.01–11.02) | 6.60 (4.26–8.93) | 16.41 (11.35–21.47) | 9.21 (8.06–10.36) | 12.21 (11.01–13.37) | 10.13 (9.26–11.01) | 62.2% (P<0.001) |
| 14–15.9y | 14.40 (11.30–17.49) | 8.39 (4.87–11.91) | 15.98 (9.83–22.12) | 12.84 (11.06–14.62) | 24.11 (21.67–26.55) | 16.11 (14.10–18.11) | 79.0% (P<0.001) |
| UC | |||||||
| Number of cases | 192 | 92 | 73 | 902 | 123 | 1,382 | |
| Incidence per 100,000 (95% CI) | 2.67 (2.13–3.21) | 2.77 (2.02–3.51) | 4.22 (3.03–5.41) | 3.11 (2.66–3.56) | 0.99 (0.92–1.06) | 2.35 (2.09–2.61) | 89.6% (P<0.001) |
| Age group | |||||||
| 6 months–4.9y | 1.02 (0.41–1.64) | a | a | 0.81 (0.59–1.03) | a | 0.83 (0.62–1.04) | 0.0% (P=0.71) |
| 5–9.9y | 1.42 (0.80–2.05) | 1.99 (0.76–3.23) | a | 1.82 (1.39–2.25) | 0.13 (0.09–0.17) | 0.54 (0.40–0.68) | 75.7% (P<0.001) |
| 10–13.9y | 3.13 (2.07–4.19) | 4.10 (2.32–5.87) | 5.62 (2.87–8.37) | 3.98 (3.33–4.62) | 1.16 (1.03–1.30) | 2.55 (2.18–2.93) | 75.2% (P<0.001) |
| 14–15.9y | 5.66 (3.92–7.41) | 6.73 (3.62–9.85) | 8.33 (3.57–13.09) | 7.43 (6.21–8.65) | 2.89 (2.60–3.19) | 4.91 (4.26–5.56) | 64.6% (P<0.001) |
| Prevalence | |||||||
| IBD | |||||||
| Number of cases (in final year of study period) | 360 | 67 | 99 | 1,025 | 681 | 2,228 | |
| Prevalence per 100,000 (95% CI) | 44.48 (40.87–48.08) | 28.33 (21.95–35.95) | 53.79 (49.63–57.95) | 31.80 (29.18–34.42) | 44.06 (39.00–49.12) | 38.25 (35.78–40.73) | 96.2% (P<0.001) |
| Age group | |||||||
| 6 months–4.9y | 11.15 (9.60–12.69) | a | a | 2.68 (2.14–3.23) | 3.76 (2.89–4.62) | 4.45 (3.71–5.19) | 82.4% (P<0.001) |
| 5–9.9y | 24.85 (20.23–29.48) | 8.12 (2.98–17.64) | 15.26 (13.05–17.46) | a | 11.02 (10.23–11.80) | 14.10 (12.62–15.59) | 86.1% (P<0.001) |
| 10–13.9y | 65.11 (60.35–69.87) | 36.32 (23.02–54.48) | 86.13 (76.84–95.42) | 49.41 (45.06–53.76) | 36.86 (34.61–39.11) | 50.63 (47.30–53.97) | 89.2% (P<0.001) |
| 14–15.9y | 113.71 (105.52–121.90) | 104.70 (73.31–144.9) | 141.35 (123.98–158.73) | 100.79 (94.34–107.21) | 59.63 (53.68–65.58) | 94.15 (86.77–101.52) | 92.7% (P<0.001) |
| CD | |||||||
| Number of cases (in final year of study period) | 185 | 42 | 62 | 554 | 595 | 1,435 | |
| Prevalence per 100,000 (95% CI) | 26.18 (24.97–27.39) | 17.85 (12.87–24.1) | 37.85 (34.66–41.05) | 17.67 (16.27–19.07) | 37.50 (33.19–41.81) | 25.47 (22.85–28.09) | 98.0% (P<0.001) |
| Age group | |||||||
| 6 months–4.9y | 4.30 (3.31–5.30) | a | a | 0.70 (0.48–0.92) | 3.24 (2.49–3.99) | 2.23 (1.74–2.72) | 83.1% (P<0.001) |
| 5–9.9y | 9.56 (8.04–11.08) | 2.71 (0.33–9.72) | a | 6.05 (5.34–6.76) | 8.76 (8.13–9.40) | 7.06 (6.32–7.80) | 70.4% (P<0.001) |
| 10–13.9y | 40.86 (37.90–43.82) | 22.13 (12.1–37.11) | 63.81 (55.78–71.84) | 28.61 (25.73–31.49) | 3 (30.69–34.67) | 32.82 (30.70–34.94) | 81.3% (P<0.001) |
| 14–15.9y | 77.59 (71.74–83.45) | 75.64 (49.41–110.8) | 102.55 (85.53–119.57) | 60.56 (56.30–64.82) | 50.75 (45.70–55.80) | 60.97 (57.03––64.90) | 81.0% (P<0.001) |
| UC | |||||||
| Number of cases (in final year of study period) | 110 | 25 | 32 | 380 | 67 | 613 | |
| Prevalence per 100,000 (95% CI) | 12.92 (10.86–14.97) | 10.47 (6.78–15.43) | 13.59 (10.63–16.55) | 12.04 (11.04–13.03) | 6.22 (5.49–6.95) | 10.70 (9.84–11.56) | 95.9% (P<0.001) |
| Age group | |||||||
| 6 months–4.9y | 2.54 (1.75–3.33) | a | a | 1.56 (1.27–1.85) | 0.70 (0.49–0.90) | 1.33 (1.07–1.59) | 50.2% (P=0.001) |
| 5–9.9y | 9.64 (7.71–11.57) | 5.42 (1.48–13.83) | a | 7.80 (6.46–9.15) | 1.56 (1.38–1.74) | 5.31 (4.56–6.06) | 92.2% (P<0.001) |
| 10–13.9y | 18.54 (14.31–22.77) | 14.19 (6.49–26.93) | 19.34 (13.34–25.33) | 17.54 (16.10–18.99) | 3.90 (3.65–4.16) | 13.46 (12.06–14.85) | 95.0% (P<0.001) |
| 14–15.9y | 27.08 (22.82–31.35) | 29.03 (13.92–53.41) | 30.94 (22.80–39.09) | 34.10 (31.92–36.27) | 6.97 (6.30–7.64) | 24.02 (21.56–26.48) | 95.0% (P<0.001) |
CD, Crohn’s disease; CI, confidence intervals; IBD, inflammatory bowel disease; UC, ulcerative colitis; y, years.
Due to small cell size, standardized incidence model did not converge.
Figure 1.
(a) Incidence of IBD (1999–2010) and (b) prevalence of IBD in the final year of available data in each province of Canada.
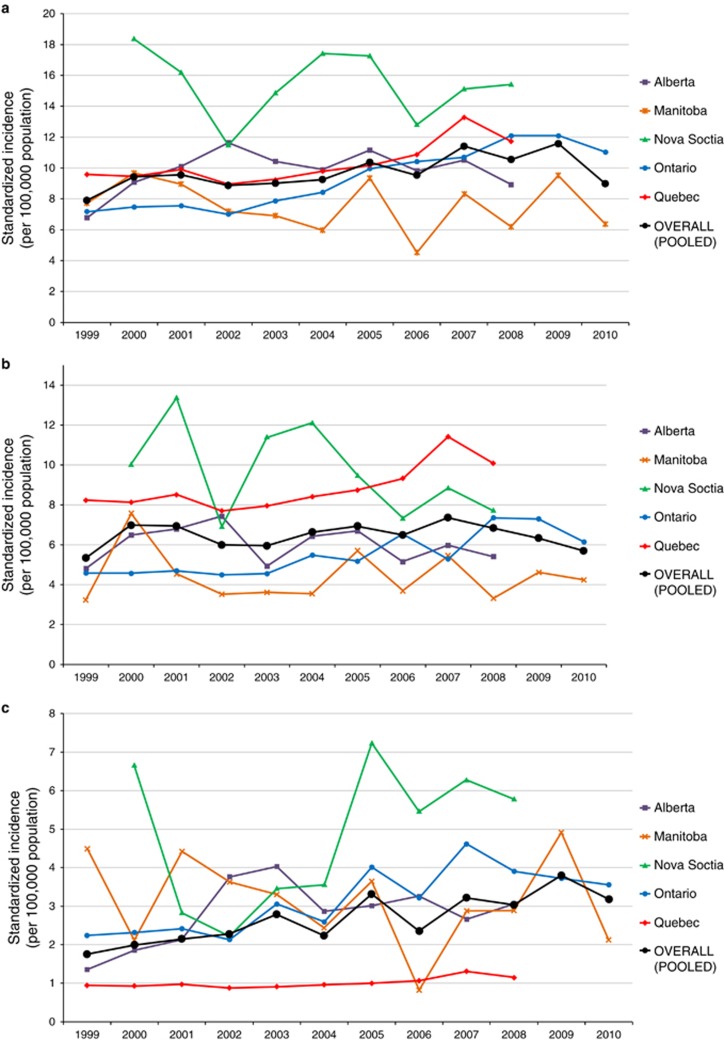
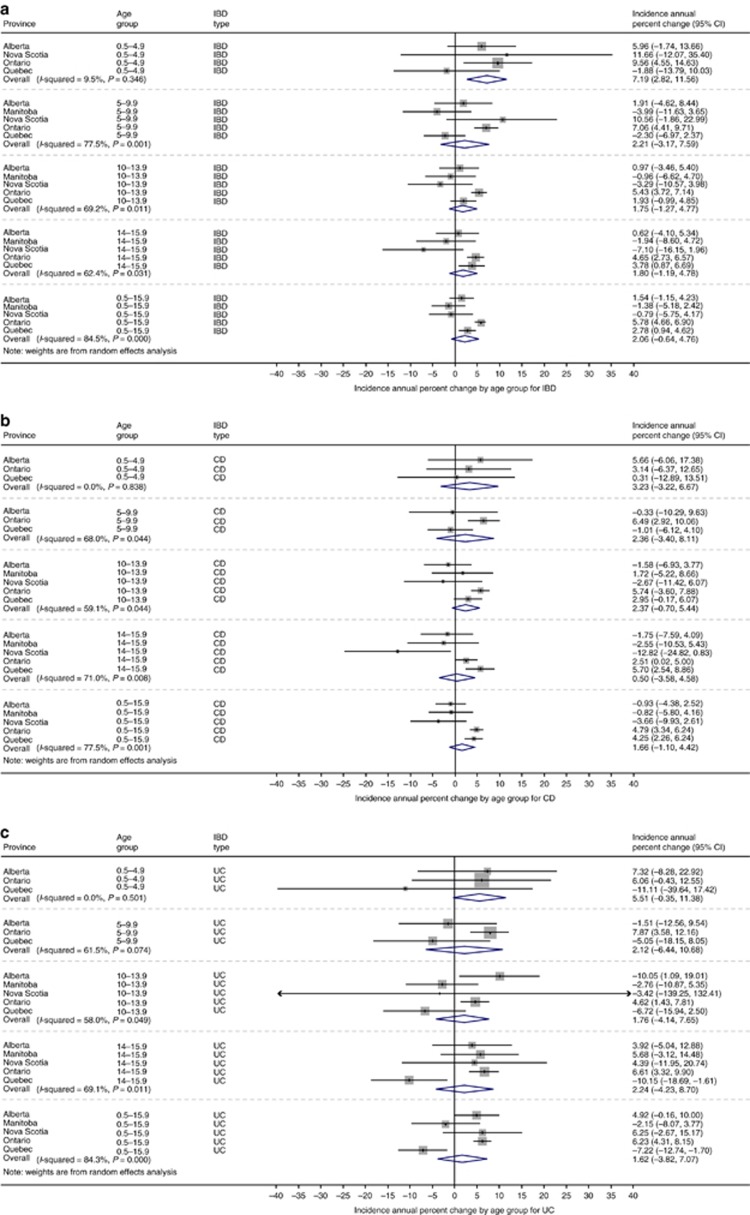
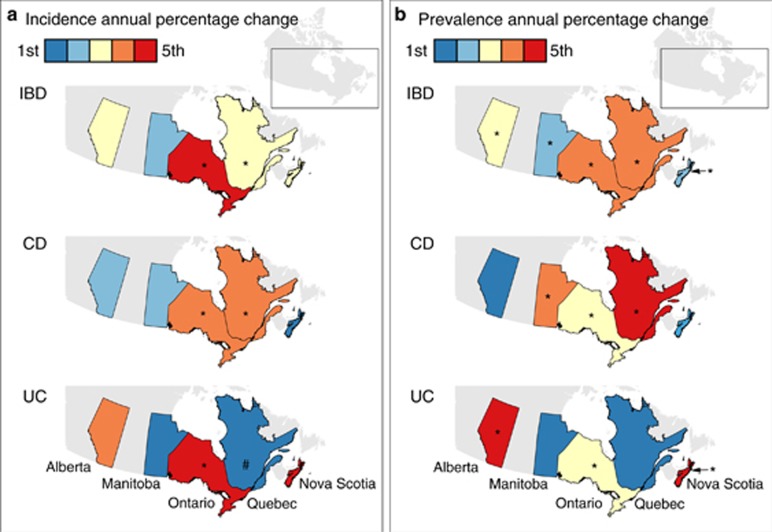
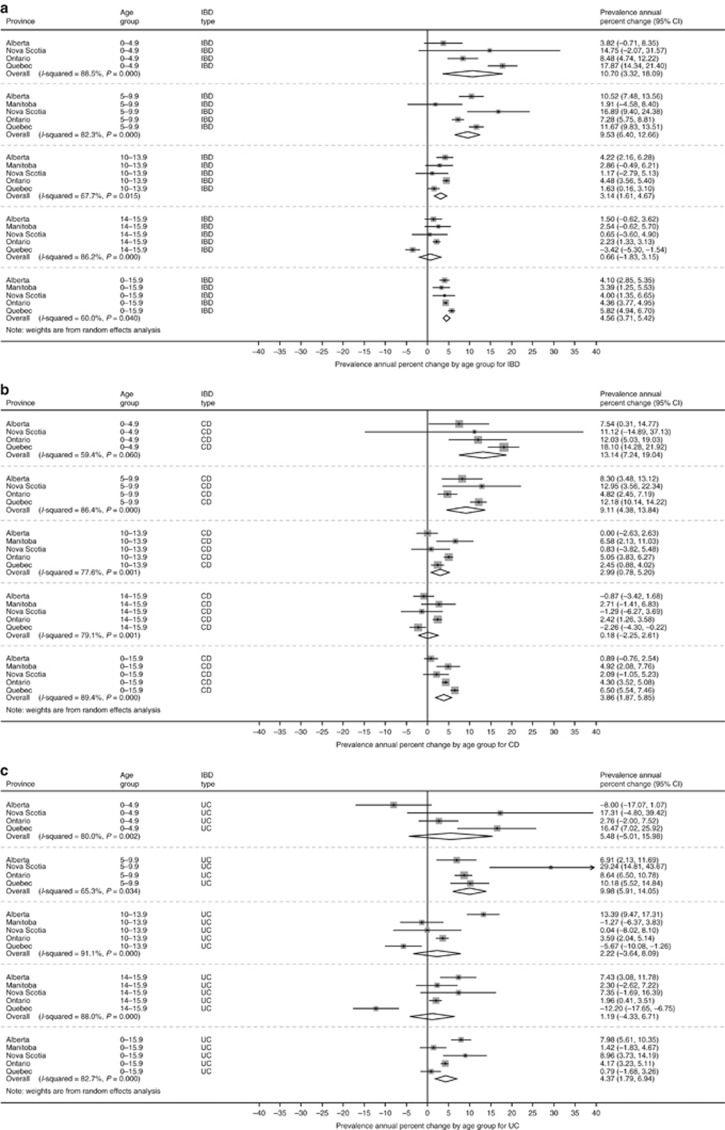
The incidence of IBD over the study period is demonstrated in Figure 2. Overall incidence per 100,000 children changed from 7.9 (95% CI 6.4 to 9.4) in 1999 to 10.6 (95% CI 8.5 to 12.6) in 2008, the final year that all provinces could contribute data. In 2010, when only Ontario and Manitoba could provide data, the combined incidence was 9.0 (95% CI 4.4 to 13.5) per 100,000. Change in incidence over time is demonstrated in Figure 3, with graphical representation on a map in Figure 4a. For children <16y, the incidence increased over time in Ontario (APC +5.8%, 95% CI +4.6 to +7.0%) and Quebec (APC +2.8% 95% CI +0.9 to +4.7%). Incidence did not change over time in Alberta (APC +1.5%, 95% CI −1.2 to +4.2%), Manitoba (APC −1.4%, 95% CI −5.2 to +2.4%), or Nova Scotia (APC −0.8%, 95% CI −5.8 to +4.2%). Overall, the combined incidence rate was stable (APC +2.1% 95% CI −0.06 to +4.8%). Significant heterogeneity was observed between provinces in APCs over time (I2 84.3%, P<0.001). Incidence rates were also stable over time when IBD was stratified into CD (APC +1.7% 95% CI −1.0 to +4.4%) and UC (APC +1.7% −3.8% to +7.1%). The only age group with statistically significant increased incidence was children 6 month to 5y (APC +7.2% 95% CI +2.8 to 11.6%). In this youngest group, incidence did not rise significantly when stratified by CD (APC +3.2% 95% CI −3.2 to +9.7%) and UC (APC +5.5% 95% CI −0.3% to +11.4%). Of note, significant heterogeneity was not present amongst provinces for this age group (IBD: I2 9.5%, P=0.35; CD: I2 0.0%, P=0.84; UC: I2 0.0%, P=0.50). Due to the rarity of cases of children <5y, the rate change models did not converge for Manitoba in calculation of IBD trends, nor for Manitoba and Nova Scotia in calculation of CD and UC trends.
Figure 2.
Annual standardized incidence (per 100,000 population) of (a) IBD, (b) Crohn’s disease, and (c) ulcerative colitis in Canada (1999–2010).
Figure 3.
Annual percentage change in incidence of (a) IBD, (b) CD, and (c) UC by age group. Meta-analysis was conducted to combine provincial estimates. I2 was calculated to determine heterogeneity in provincial results.
Figure 4.
Annual percentage change in (a) incidence and (b) prevalence represented on a map of Canada. *P<0.05 for increased incidence over time; #P<0.05 for decreased incidence over time.
Results of the sensitivity analysis excluding Nova Scotia and Quebec are presented in Supplementary Figure 1. Excluding provinces without validated identification algorithms did not significantly alter APC of incidence.
Prevalence
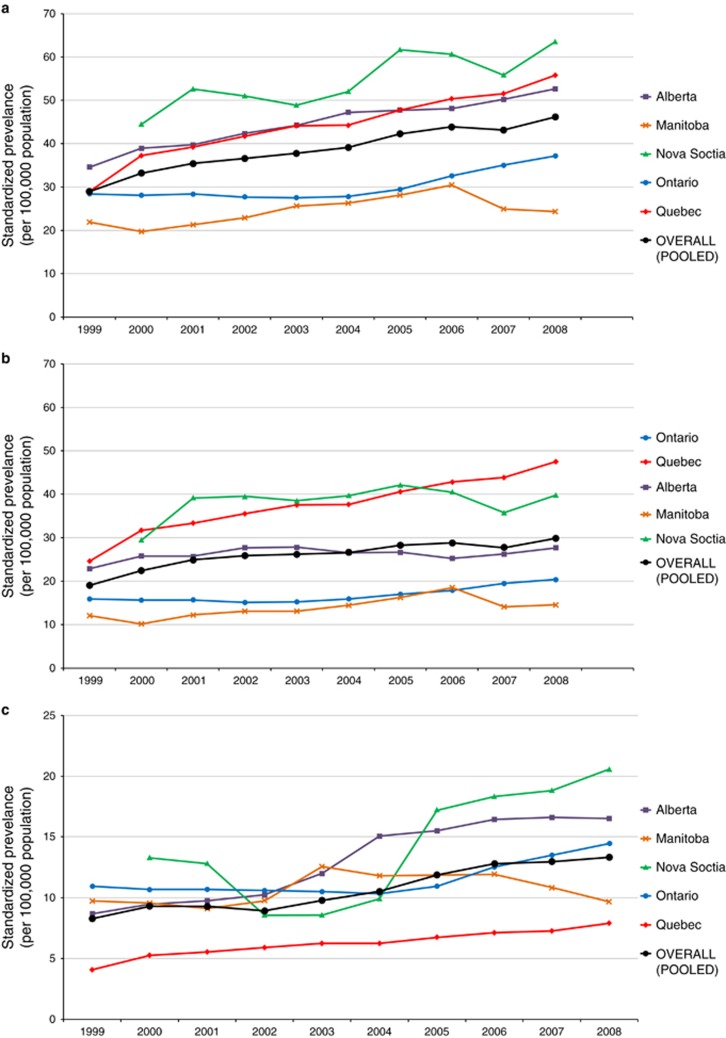
Based on meta-analysis, the standardized period prevalence for all provinces was 38.2 (95% CI 35.8 to 40.7) per 100,000 children. The prevalence by province is demonstrated in Table 2 and Figure 1b. In 2008, the final year in which all provinces reported data, the national point prevalence was 46.2 (95% CI 34.6 to 57.8) per 100,000, significantly increased from the prevalence in 2000 of 33.2 (95% CI 26.6 to 39.8) per 100,000. Overall, the prevalence for CD was 25.5 (95% CI 22.8 to 28.1) per 100,000. For UC, prevalence was 10.7 (95% CI 9.8 to 11.6) per 100,000. Figure 5 demonstrates prevalence by province between 1999 and 2008. Time trends analysis demonstrated statistically significantly increased prevalence in all age groups, except adolescents aged 14–15.9y (Figure 6). Overall, IBD prevalence increased by 4.6% (95% CI 3.7 to 5.4%) per year, CD prevalence increased by 3.9% (95% CI 1.9 to 5.9%) per year, and UC prevalence increased by 4.4% (95% CI 1.8 to 6.9%) per year (Figure 4b). Results of the sensitivity analysis excluding Nova Scotia and Quebec are presented in Supplementary Figure 2. Excluding provinces without validated identification algorithms did not significantly alter APC of prevalence.
Figure 5.
Annual standardized prevalence (per 100,000 population) of (a) IBD, (b) Crohn’s disease, and (c) ulcerative colitis in Canada on July 1 of the final year of the provincial cohort (2008 for Nova Scotia and Quebec, 2010 for Alberta, Manitoba, and Ontario).
Figure 6.
Annual percentage change in prevalence of IBD by age group. Meta-analysis was conducted to combine provincial estimates. I2 was calculated to determine heterogeneity in provincial results.
Discussion
Using a distributed network methodology applied to five Canadian population-based health administrative databases, we confirmed that Canada has amongst the highest incidence and prevalence of childhood-onset IBD in the world. As previously reported, the predominant form of pediatric-onset IBD is CD, with more boys than girls affected. The incidence of childhood-onset IBD was stable during the first decade of the twenty-first century, with the exception of rising incidence among children diagnosed under the age of 5y. Finally, we noted significant heterogeneity in both incidence and trends over time amongst provinces despite use of standardized analysis, indicating significant regional variation. Extrapolating our results to the entire country, we estimate that approximately 600–650 children are diagnosed with IBD every year, and 2,695 children under 16y were living with IBD in Canada in 2008.
The high incidence and prevalence of pediatric IBD in Canada has been demonstrated in previous single-province studies (9, 12, 20). In our study, the incidence of IBD in children was amongst the highest in the world which is consistent with the only previous multi-province Canadian study (21). A previous systematic review which included studies published before 2010 demonstrated that only Norway had similar incidence, 10.6 per 100,000 children <16y (22). More recently, a study from Stockholm County, Sweden reported an incidence of 12.8 per 100,000 in children <16y between 2002–2007, significantly increased from previous reports from the same region (23). By contrast, incidence estimates from other regions of Europe and the United States were significantly lower (1). The reasons for the higher rates in children from Canada and Northern Europe are not known. Lack of sunlight exposure and high rates of vitamin D deficiency, antibiotic use, diet, and migration patterns have been hypothesized as contributors (4, 24). The interaction between environmental risk factors, the intestinal microbiome, and genetic predisposition are important areas under investigation. This trend of increased incidence in the northern latitude has also been demonstrated in the United States (25). Even amongst northern states, incidence may be increasing. A recent population-based study from Rhode Island demonstrated a much higher incidence of IBD in children and adults (26) compared with older estimates from Wisconsin (27) and Minnesota (28). It remains to be seen whether American states at northern latitudes will develop a similarly high incidence of pediatric IBD noted in Canada over the past two decades.
The incidence of pediatric IBD was stable during the study period, with the exception of incidence in children under 5 years old. The rise in the incidence of very early onset IBD (VEO-IBD) (29) in our study population was consistent across all provinces as evidence by the lack of statistical heterogeneity between provinces. The rapid increase in VEO-IBD was previously noted in Ontario (8). This multi-province study demonstrated that incidence was rising by ~7% per year in that age group, with little heterogeneity amongst provinces. This finding may also explain the observation of increased prevalence of children <16y living with IBD, since these VEO-IBD cases would remain in the cohort for longer. However, the reason for the increased incidence of VEO-IBD cases in Canada is unexplained. While IBD diagnosed in children <2y is more likely due to monogenic factors (30), this represented a very small proportion of our VEO-IBD cohort. Therefore, most patients with a diagnosis <6y were not likely to have the monogenic forms of IBD, and early-life environmental factors may have played a role in the observed increased incidence (4). The association between early-life environmental factors and childhood-onset IBD was particularly noted in studies demonstrating increased risk due to early-life antibiotic (31) and air pollution exposure (32). However, changes in health services factors may also have resulted in increased recognition in young children. Whereas all studies relying on administrative database are subject to misclassification errors on the study population, this bias is less likely in children under the age of 5 years because a previous validation study showed that only 1% of children diagnosed <6y did not have any health services contacts with associated IBD diagnoses at the end of a mean 9y follow-up period (8). This means that most children initially labeled IBD continued to be treated as IBD years later.
There was significant heterogeneity in estimates of incidence, prevalence, and trends over time for those diagnosed above 5y old, indicating variation between provinces. In particular, we noted that Nova Scotia demonstrated significantly higher incidence and prevalence of IBD compared with other provinces. In addition, Quebec demonstrated a much greater proportion of IBD patients being classified as having CD compared with UC. Both of these findings were demonstrated in other adult-focused cohort studies, and remain unexplained (12, 21). Different genetic backgrounds may play a role. Quebec is primarily comprised of a unique French Canadian (Quebecois) population with a distinct phenotype (33). Nova Scotia is comprised of 31% of people of Scottish origin and 28% of people from other British Isles (compared to 15 and 20%, respectively, in all Canadians) (34), and Scotland has been noted to have a higher incidence of pediatric IBD than the rest of the United Kingdom (35). Finally, Ontario and Quebec have larger immigrant populations than other provinces, and Manitoba has a higher First Nations population than other provinces. Immigrants and First Nations people have been demonstrated to have lower rates of IBD than other Canadians (36, 37, 38). Finally, the differences in incidence observed amongst provinces may be related to access to health services. If families from Nova Scotia are more likely to seek medical attention for their children, this would explain the higher incidence observed. Of note, pediatric asthma prevalence was also reported to be higher in Nova Scotia than other provinces (39). Future research will examine the issue of access and variation of care of children with IBD in Canada.
The rising incidence of VEO-IBD, and particularly in children under 5 years has important clinical implications. Children with onset of disease at younger ages have been demonstrated to have lower rates of hospitalization, emergency department utilization and surgical resection (8, 40, 41, 42). However, these patients will live longer with the disease and research regarding long-term outcomes is lacking. In addition, young children present more frequently with isolated colonic disease, with a change to a more ileo-colonic phenotype in children presenting between 6–9 years old (40, 42). Therefore, it is much more difficult to determine IBD sub-type in children under 6 years presenting with colitis, which may impact medication choice, and the decision on whether to proceed to colectomy in patients failing medical management. Finally, the psycho-social impact on the family of caring for a young child with a chronic illness is significant. This may be reflected in the need for age-appropriate educational material, decisions regarding medical or dietary therapy, when to proceed to surgery, frequent school absences for the child, or parental absences from work. These issues may be best addressed in centers offering multi-disciplinary care such as support from a psychologist, social worker, dietitian, and specialized nurse. In light of these unique challenges in very young children with IBD, we recommend that these patients be treated by specialized pediatricians with expertise in multi-disciplinary IBD care. In addition, we would suggest that the Paris classification of pediatric IBD be modified to sub-categorized VEO-IBD (diagnosis under 10-years old) between those diagnosed <6 years and those diagnosed between 6 and 10 years. This modification would acknowledge the rising incidence and the uniqueness of this cohort, and encourage further research into etiology, natural history and therapeutic options in young children.
Limitations
Misclassification bias can always affect research using routinely collected health data (43), and the heterogeneity observed amongst provincial results may have been due to the use of different algorithms to identify IBD patients in each province. We used the most accurate identification and classification algorithms for each specific province to reduce the risk of misclassification error, and the algorithms used were validated in the provinces to which they were applied (for Alberta, Manitoba, and Ontario). In contrast, Nova Scotia and Quebec applied non-validated coding algorithms and were the provinces with the most inconsistent findings. A sensitivity analysis that included only provinces that identified patients with locally validated algorithms did not demonstrate different findings. Nonetheless, no administrative data algorithm is completely accurate, and differential accuracies may have resulted in the appearance of heterogeneity. Future work will continue to develop and validate algorithms to identify patients with IBD, and to identify the causes of the variation seen across Canadian provinces.
This study was also limited by the time periods of data availability, and particularly by the requirement for look-back and look-forward periods to differentiate incident from prevalent cases. While use of these validated periods ensured accurate estimates of incidence, we were unable to determine trends in incidence prior to or after the study period. In addition, our analysis was able to determine overall ecological trends in IBD epidemiology, but not the reasons for the observed changes. Future research should determine whether changing environmental risk factors, new gene-environment interactions and/or alterations to the health system resulted in the changing rates.
Conclusions
In summary, we have demonstrated that Canada has amongst the highest rates of childhood-onset IBD in the world. While the incidence of IBD has stabilized in children over the age of 5 years, the incidence is rising rapidly in children under 5 years old. These findings have important implications on the children, their families, and the health care system, because these children will live longer with the disease, have a more extensive disease phenotype, and result in higher direct costs for IBD care compared with adults (6, 44). In addition, the increasingly early age of onset implies early-life environmental triggers in at-risk patients. Since young children are exposed to fewer environmental triggers in the course of their short lives, future research should focus on identification of triggers in children with IBD, understanding the biology behind changes resulting in disease, and intervention to prevent the occurrence of IBD in this vulnerable age group.
Study Highlights
Acknowledgments
We would like to thank Danielle Birman (program manager for CanGIEC) and Fox Underwood (health geographer who created the color maps). We would also like to thank Aida Fernandes (Crohn’s and Colitis Canada) and Michele Hepburn (IBD Foundation) for providing input on the design, conduct, results and interpretation of study findings. This study is based in part on data provided by Alberta Health and Manitoba Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Governments of Alberta and Manitoba. Neither the Government of Alberta nor Alberta Health expressed any opinion in relation to this study. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Eric I. Benchimol, MD, PhD, FRCPC.
Specific author contributions: Eric I. Benchimol: study conception and design; analysis and interpretation of data; drafting of manuscript; statistical analysis; obtained funding. Charles N. Bernstein: study conception and design; analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Alain Bitton: study conception and design; analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Matthew W. Carroll: study conception and design; analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Harminder Singh: study conception and design; analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Anthony R. Otley: study conception and design; analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Maria Vutcovici: analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Wael El-Matary: analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Geoffrey C. Nguyen: study conception and design; analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding. Anne M. Griffiths: analysis and interpretation of data; critical revision of the manuscript; obtained funding. David R. Mack: Analysis and interpretation of data; critical revision of the manuscript; obtained funding. Kevan Jacobson: analysis and interpretation of data; critical revision of the manuscript; obtained funding. Nassim Mojaverian: analysis and interpretation of results; statistical analysis; technical support; critical revision of the manuscript for important Divine Tanyingoh: analysis and interpretation of results; statistical analysis; technical support; critical revision of the manuscript for important. Yunsong Cui: analysis and interpretation of results; statistical analysis; technical support; critical revision of the manuscript for important. Zoann Nugent: analysis and interpretation of results; statistical analysis; technical support; critical revision of the manuscript for important. Janie Coulombe: analysis and interpretation of results; statistical analysis; technical support; critical revision of the manuscript for important. Laura E. Targownik: analysis and interpretation of data; critical revision of the manuscript; obtained funding. Jennifer L. Jones: analysis and interpretation of data; critical revision of the manuscript; obtained funding. Desmond Leddin: analysis and interpretation of data; critical revision of the manuscript; obtained funding. Sanjay K. Murthy: analysis and interpretation of data; critical revision of the manuscript; obtained funding. Gilaad G. Kaplan: study conception and design; analysis and interpretation of data; critical revision of the manuscript; statistical analysis; obtained funding.
Financial Support: This research was funded by operating grants from the Crohn’s and Colitis Canada and the Ontario Research Fund: Early Researcher Awards. CanGIEC is funded by the Canadian Institutes of Health Research (CIHR) Foundation Scheme. This study also received financial support from the Canadian Children IBD Network: A Joint Partnership of CIHR and the CH.I.L.D. Foundation. Eric Benchimol and Geoffrey Nguyen were supported by New Investigator Awards from CIHR, Crohn’s and Colitis Canada, and the Canadian Association of Gastroenterology. Charles Bernstein is supported in part by the Bingham Chair in Gastroenterology.
Potential competing interests: The following investigators are also investigators or collaborators in the Canadian Children IBD Network: A Joint Partnership of CIHR and the CH.I.L.D. Foundation, which provided funding for this study: Eric I. Benchimol, Matthew W. Carroll, Anthony R. Otley, Wael El-Matary, Anne M. Griffiths, David R. Mack, Kevan Jacobson, Gilaad G. Kaplan. The following authors served on the Scientific and Medical Advisory Council of Crohn’s and Colitis Canada, which provided funding for this study: Eric I. Benchimol, Charles N. Bernstein, Anthony R. Otley, Gilaad G. Kaplan.
Supplementary Material
References
- Benchimol EI, Fortinsky KJ, Gozdyra P et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011;17:423–39. [DOI] [PubMed] [Google Scholar]
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- Molodecky NA, Soon IS, Rabi DM et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54, e42. [DOI] [PubMed] [Google Scholar]
- Aujnarain A, Mack DR, Benchimol EI. The role of the environment in the development of pediatric inflammatory bowel disease. Curr Gastroenterol Rep 2013;15:326. [DOI] [PubMed] [Google Scholar]
- Rocchi A, Benchimol EI, Bernstein CN et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol 2012;26:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelman MD, Rifas-Shiman SL, Porter CQ et al. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology 2008;135:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchimol EI, Guttmann A, Griffiths AM et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut 2009;58:1490–7. [DOI] [PubMed] [Google Scholar]
- Benchimol EI, Mack DR, Nguyen GC et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 2014;147:803–13.e7 quiz e14–5. [DOI] [PubMed] [Google Scholar]
- Benchimol EI, Manuel DG, Guttmann A et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis 2014;20:1761–9. [DOI] [PubMed] [Google Scholar]
- El-Matary W, Moroz SP, Bernstein CN. Inflammatory bowel disease in children of Manitoba: 30 years' experience of a tertiary center. J Pediatr Gastroenterol Nutr 2014;59:763–6. [DOI] [PubMed] [Google Scholar]
- Leddin D, Tamim H, Levy AR. Decreasing incidence of inflammatory bowel disease in eastern Canada: a population database study. BMC Gastroenterol 2014;14:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton A, Vutcovici M, Patenaude V et al. Epidemiology of inflammatory bowel disease in Quebec: recent trends. Inflamm Bowel Dis 2014;20:1770–6. [DOI] [PubMed] [Google Scholar]
- Kaplan GG. Pitfalls and perils of using administrative databases to evaluate the incidence of inflammatory bowel disease overtime. Inflamm Bowel Dis 2014;20:1777–9. [DOI] [PubMed] [Google Scholar]
- Statistics Canada Population and dwelling counts, for Canada, provinces and territories, 2011 and 2006 censuses, 2012. Available at: http://www12.statcan.gc.ca/census-recensement/2011/dp-pd/hlt-fst/pd-pl/Table-Tableau.cfm?LANG=Eng&T=101&S=50&O=A Accessed 8 January 2016.
- Suissa S, Henry D, Caetano P et al. CNODES: the Canadian Network for Observational Drug Effect Studies. Open Med 2012;6:e134–40. [PMC free article] [PubMed] [Google Scholar]
- Rezaie A, Quan H, Fedorak RN et al. Development and validation of an administrative case definition for inflammatory bowel diseases. Can J Gastroenterol 2012;26:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Blanchard JF, Rawsthorne P et al. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol 1999;149:916–24. [DOI] [PubMed] [Google Scholar]
- Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997;16:791–801. [DOI] [PubMed] [Google Scholar]
- Peters J, Mengersen K. Selective reporting of adjusted estimates in observational epidemiology studies: reasons and implications for meta-analyses. Eval Health Prof 2008;31:370–89. [DOI] [PubMed] [Google Scholar]
- El-Matary W, Moroz S, Bernstein C. The epidemiology of inflammatory bowel disease in children of manitoba: 30 years' experience. Can J Gastroenterol 2014;28:110A. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Wajda A, Svenson LW et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol 2006;101:1559–68. [DOI] [PubMed] [Google Scholar]
- Perminow G, Brackmann S, Lyckander LG et al. A characterization in childhood inflammatory bowel disease, a new population-based inception cohort from South-Eastern Norway, 2005-07, showing increased incidence in Crohn's disease. Scand J Gastroenterol 2009;44:446–56. [DOI] [PubMed] [Google Scholar]
- Malmborg P, Grahnquist L, Lindholm J et al. Increasing incidence of paediatric inflammatory bowel disease in northern Stockholm County, 2002-2007. J Pediatr Gastroenterol Nutr 2013;57:29–34. [DOI] [PubMed] [Google Scholar]
- Frolkis A, Dieleman LA, Barkema HW et al. Environment and the inflammatory bowel diseases. Can J Gastroenterol 2013;27:e18–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili H, Huang ES, Ananthakrishnan AN et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012;61:1686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JM, Zoega H, Shah SA et al. Incidence of Crohn's disease and ulcerative colitis in Rhode Island: report from the Ocean State Crohn's and Colitis Area Registry. Inflamm Bowel Dis 2016;22:1456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugathasan S, Judd RH, Hoffmann RG et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr 2003;143:525–31. [DOI] [PubMed] [Google Scholar]
- Loftus CG, Loftus EV Jr, Harmsen WS et al. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis 2007;13:254–61. [DOI] [PubMed] [Google Scholar]
- Levine A, Griffiths A, Markowitz J et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- Muise AM, Snapper SB, Kugathasan S. The age of gene discovery in very early onset inflammatory bowel disease. Gastroenterology 2012;143:285–8. [DOI] [PubMed] [Google Scholar]
- Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn's disease and ulcerative colitis. Am J Gastroenterol 2011;106:2133–42. [DOI] [PubMed] [Google Scholar]
- Kaplan GG, Hubbard J, Korzenik J et al. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol 2010;105:2412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M, Nguyen GC, Pare P et al. Phenotypic and genotypic characteristics of inflammatory bowel disease in French Canadians: comparison with a large North American repository. Am J Gastroenterol 2009;104:2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population by selected ethnic origins, by province and territory (2006 Census) (Canada), 28 July 2009. Available at http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo26a-eng.htmAccessed 5 May 2016.
- Sawczenko A, Sandhu BK, Logan RF et al. Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet 2001;357:1093–4. [DOI] [PubMed] [Google Scholar]
- Blanchard JF, Bernstein CN, Wajda A et al. Small-area variations and sociodemographic correlates for the incidence of Crohn's disease and ulcerative colitis. Am J Epidemiol 2001;154:328–35. [DOI] [PubMed] [Google Scholar]
- Green C, Elliott L, Beaudoin C et al. A population-based ecologic study of inflammatory bowel disease: searching for etiologic clues. Am J Epidemiol 2006;164:615–23 discussion 624–8. [DOI] [PubMed] [Google Scholar]
- Benchimol EI, Mack DR, Guttmann A et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol 2015;110:553–63. [DOI] [PubMed] [Google Scholar]
- Dales RE, Raizenne M, el-Saadany S et al. Prevalence of childhood asthma across Canada. Int J Epidemiol 1994;23:775–81. [DOI] [PubMed] [Google Scholar]
- Henderson P, Rogers P, Wilson DC A 17-year prospective cohort study of paediatric inflammatory bowel disease patients diagnosed less than 10 years of age (Paris A1a). In: British Society for Pediatric Gastroenterology, Hepatology and Nutrition 2015. Bridgefoot, Stratford-upon-Avon, UK; 2015.
- Schaefer ME, Machan JT, Kawatu D et al. Factors that determine risk for surgery in pediatric patients with Crohn's disease. Clin Gastroenterol Hepatol 2010;8:789–94. [DOI] [PubMed] [Google Scholar]
- Oliva-Hemker M, Hutfless S, Al Kazzi ES et al. Clinical presentation and five-year therapeutic management of very early-onset inflammatory bowel disease in a large north American cohort. J Pediatr 2015;167:527–32.e1-3. [DOI] [PubMed] [Google Scholar]
- Benchimol EI, Smeeth L, Guttmann A et al. The REporting of studies conducted using observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Limbergen J, Russell RK, Drummond HE et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.