Abstract
Background
Cochlear implantation is an effective habilitation modality for adults with significant hearing loss. However, post-implant performance is variable. A portion of this variance in outcome can be attributed to clinical factors. Recent physiological studies suggest that the health of the spiral ganglion also impacts post-operative cochlear implant outcomes. The goal of this study was to determine whether genetic factors affecting spiral ganglion neurons may be associated with cochlear implant performance.
Methods
Adults with post-lingual deafness who underwent cochlear implantation at the University of Iowa were studied. Pre-implantation evaluation included comprehensive genetic testing for genetic diagnosis. A novel score of genetic variants affecting genes with functional effects in the spiral ganglion was calculated. A Z-scored average of up to three post-operative speech perception tests (CNC, HINT, and AzBio) was used to assess outcome.
Results
Genetically determined spiral ganglion health affects cochlear implant outcomes, and when considered in conjunction with clinically determined etiology of deafness, accounts for 18.3% of the variance in postoperative speech recognition outcomes. Cochlear implant recipients with deleterious genetic variants that affect the cochlear sensory organ perform significantly better on tests of speech perception than recipients with deleterious genetic variants that affect the spiral ganglion.
Conclusion
Etiological diagnosis of deafness including genetic testing is the single largest predictor of postoperative speech outcomes in adult cochlear implant recipients. A detailed understanding of the genetic underpinning of hearing loss will better inform pre-implant counseling. The method presented here should serve as a guide for further research into the molecular physiology of the peripheral auditory system and cochlear implants.
Keywords: Cochlear implant, deafness, genetics, genomics
Introduction
The cochlear implant (CI) has proven to be a remarkably successful treatment for persons with severe-to-profound sensorineural hearing loss by significantly improving hearing levels, speech understanding and several measures of quality of life including communication, relationships, feelings of isolation, and burden to others (Mo et al., 2005). The majority of CI recipients have a successful outcome and, over the last two decades, mean post-operative performance levels on tests of speech perception in quiet and noise have improved steadily with improvements in technology (Wilson and Dorman, 2008). Outcome variance, however, is still wide and a significant number of CI recipients struggle to understand speech even in quiet listening situations. Some enjoy little or no benefit from the device, and up to 7% of pediatric CI patients become non-users (Raine et al., 2008).
Identifying factors that contribute to CI performance has been a scientific goal for decades for our group (Gantz et al., 1993) and many others, but the large number of clinical variables associated with outcome makes tightly controlled studies difficult to complete. For adults who are postlingually deafened, factors that contribute to CI outcome include the amount of residual hearing, duration of deafness, neurocognitive functioning, device implanted, method of implantation, surgeon experience, postoperative complications and environmental variables such as socioeconomic status (Moberly et al., 2016). However, two recent studies on a cohort of 2,251 patients conducted using two different statistical modeling methods found that only 10.5% and 22% of variance in postoperative speech perception testing could be accounted for by considering up to 9 different clinical factors (Blamey et al., 2013; Lazard et al., 2012). The goal of this proof-of-principle study was to determine whether we could improve predictability of CI performance by considering genetic variables.
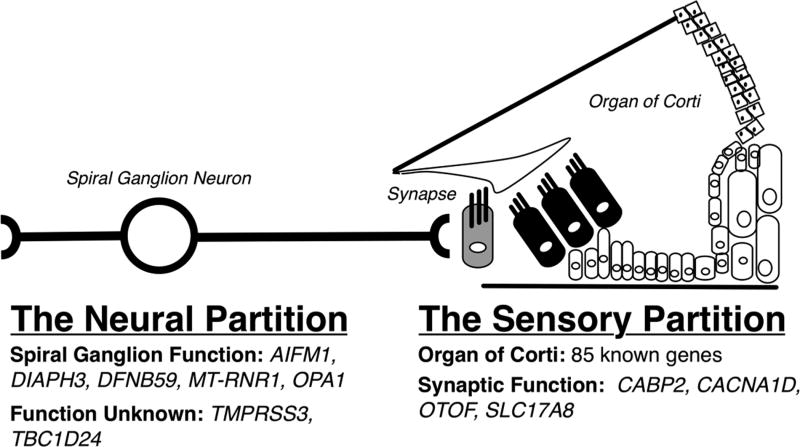
The peripheral auditory sensory system is a serial integration of: (1) the sensory portion – the organ of Corti and supporting cells, which enable mechanotransduction of sound into an ionic gradient; (2) the synapse between hair cells and the spiral ganglion afferent neuron, which converts the ionic gradient to a neural action potential; and (3) the bipolar spiral ganglion neurons, which transmit the neural signal to the central auditory system. In the context of how a CI functions, there are only two partitions – the sensory partition, which includes the organ of Corti and the synapse and therefore is bypassed by the CI, and the neural partition (Figure 1). CIs stimulate spiral ganglion cells and recent studies have shown that the integrity of these cells impacts CI performance. For example, McClellan and colleagues used intraoperative electrocochleography to assess the health of spiral ganglion. These physiologic measures were found to be predictive of postoperative audiometric outcome and could account for 40% of the variance in outcome in 32 adult CI recipients (McClellan et al., 2014). In addition, CI recipients with auditory neuropathy spectrum disorder generally have relatively poor CI outcomes (Blamey et al., 2013; Rance and Starr, 2015). These data implicate the health of the spiral ganglion as an important factor in CI outcomes.
Figure 1.
Overview of division of peripheral auditory system based on function and known effect of genes causing deafness at these sites. A cochlear implant bypasses the sensory partition (including the organ of Corti and the synapse).
A few studies have explored this relationship from a genetic perspective however these studies have been limited by sample size and heterogeneity of data collection making conclusions difficult. One study that included 81 subjects with post-lingual hearing loss showed that CI users with genetic mutations in the sensory partition performed well after cochlear implantation, but no statistical analysis was performed (Miyagawa et al., 2016). A case-control study examined pediatric patients with good performance (n=30) and poor performance (n=12) and showed that genetic mutations in the sensory partition were more common in the high performing group, and that a specific mutation in spiral ganglion neurons (DFNB59 p.Gly292Arg) was significantly more common in the poor performing group (Wu et al., 2015). Our goal was to further examine this relationship using a large number of subjects and comprehensive genetic testing which incorporates examination of causative genetic mutations affecting the spiral ganglion but also deleterious genetic variants that may independently affect this structure.
In this study, we explored the relationship between genetic variants in the sensory partition (organ of Corti and synapse) and the neural partition (spiral ganglion) with CI performance in a large cohort of adult CI recipients. All patients underwent comprehensive genetic testing by massively parallel sequencing all genes implicated in non-syndromic hearing loss. We hypothesized that performance on measures of speech perception would be poorer for CI recipients who carry deleterious genetic variants in the neural partition as compared to CI recipients who carry deleterious genetic variants in the sensory partition.
Materials and Methods
Study Population
240 subjects with post-lingual sensorineural hearing loss who received a CI after the age of 18 were eligible for this study. We excluded patients with inner ear malformations, those who suffered intraoperative or postoperative complications requiring explantation/reimplantation, and those who did not have a modern device implanted (Table 1 shows included devices). Devices were restricted in order to reduce heterogeneity in our subject population that can arise from changes in technology, changes in medical and audiological management, as well as changes in candidacy criteria for cochlear implantation.
Table 1.
Patient Characteristics
| n | % | ||
|---|---|---|---|
| Total Subjects Studied | 240 | ||
| Subjects Included | 155 | ||
| Clinical Characteristics | |||
| Male | 80 | 52% | |
| Female | 75 | 48% | |
| Age at implant 18–29 | 7 | 4% | |
| Age at implant 30–64 | 75 | 48% | |
| Age at implant >64 | 75 | 48% | |
| Device Implanted | |||
| Clarion HiRes 90K | 23 | 15% | |
| Clarion HiRes 90K Mid-Scala | 12 | 8% | |
| Clarion HiRes 90K with Helix | 3 | 2% | |
| Med-El Concert Flex 24 | 2 | 1% | |
| Med-El Concert Flex 28 | 1 | 1% | |
| Med-El Synchrony Flex 28 | 1 | 1% | |
| Med-El Synchrony Standard | 1 | 1% | |
| Nucleus CI 24RE(CA) | 17 | 11% | |
| Nucleus CI422 | 45 | 29% | |
| Nucleus CI512(CA) | 15 | 10% | |
| Nucleus CI512 (profile) | 3 | 2% | |
| Nucleus Hybrid S8 (24M) | 3 | 2% | |
| Nucleus Hybrid S8 (24RE) | 3 | 2% | |
| Nucleus Hybrid L24 | 20 | 13% | |
| Nucleus Hybrid S12 | 6 | 4% | |
| Analysis Group | |||
| Neural - Genetic | 24 | 16% | |
| Sensory - Genetic | 12 | 8% | |
| Bilateral SSNHL | 13 | 8% | |
| Ménière’s Disease | 6 | 4% | |
| Otosclerosis | 5 | 3% | |
| Single sided deafness | 26 | 17% | |
| Other | 10 | 6% | |
| Unknown | 60 | 38% |
155 subjects met inclusion criteria. These subjects were implanted between the years 2003 and 2016. The Institutional Review Board of the University of Iowa approved this study.
Audiometric Testing
Three standard measures of speech understanding were used to quantify speech recognition outcomes: the AzBio sentence test (Spahr et al., 2012), the CNC test (Consonant-vowel Nucleus-Consonant, Peterson and Lehiste, 1962), and the HINT (Hearing in Noise Test, Nilsson et al., 1998). All three speech tests were administered in quiet. Most subjects were tested at multiple post-operative visits but not all subjects were tested using each of the three speech perception tests. To ascertain optimal performance levels, we examined only scores collected after at least 6 months of device use; we used the single most recent postoperative score available for each test for analysis. In total, scores were available for 119 patients on the AzBio test, 111 completed CNC testing, and 40 had been tested using HINT sentences. These three audiometric measures were highly correlated (correlation of R = 0.873, 0.753, and 0.887 for Azbio to CNC and HINT and CNC to HINT, respectively for the 40 patients who completed all three tests). We computed z-scores for each audiometric measure to account for within-test scaling differences and unequal variances. The mean z-score of all three audiometric tests for each patient was then computed and used for analysis (“combined Z score”).
Comprehensive Genetic Testing
DNA was extracted from peripheral blood according to standard practices. Genetic testing was completed using the OtoSCOPE® platform, which sequences all genes known to cause non-syndromic hearing loss and non-syndromic hearing loss mimic genes (see https://morl.lab.uiowa.edu/genes-included-otoscope for complete gene list). Full details of testing are described elsewhere (Shearer et al., 2013). Genetic testing results were discussed at a multidisciplinary meeting with geneticists, bioinformaticians, and otolaryngologists to determine the likely genetic cause of deafness, if any, for each individual. OtoSCOPE v6 or v7 was used with the primary difference being the addition of newly discovered deafness genes. OPA1, a gene included in the neural gene analysis was not included on OtoSCOPE v6 and so patients run on the two platforms underwent genetic variant analysis (detailed below) separately.
Neural Pathogenic Variant Analysis
Patient variant call format (VCF) files were analyzed using a local installation of ANNOVAR (Wang et al 2014). Variants were annotated with gene, variant effect, and Combined Annotation Dependent Depletion (CADD) score (Kircher et al., 2014). Damaging variants (those with phred-like CADD score > 10, predicted to be among the top 10% most likely damaging variants possible in the human genome) were examined. CADD scores were extracted for genes implicated in auditory neuropathy (not synaptopathy) or with an expression pattern and previously reported function in the spiral ganglion (Moser and Starr 2016; Nishio et al., 2015). The complete list of these neural genes is as follows: OPA1, DIAPH3, DFNB59, AIFM1, TBC1D24, MT-RNR1, and TMPRSS3 (Figure 1). No filtering was performed based on minor allele frequency. For each patient, the total raw CADD score was summed to generate a neural CADD score. A higher neural CADD score would indicate more deleterious variants in neural genes. Patients with neural CADD scores >1 SD above the mean were selected for further analysis as part of the Neural-Genetic Group, regardless of their previous categorization based on etiology.
Subject Group Definitions
We grouped CI recipients by etiology of hearing loss and incorporating genetic testing results (Table 2). Etiology of deafness included bilateral sudden sensorineural hearing loss (Bilateral-SSNHL), Ménière's disease, otosclerosis, other causes of deafness (infection, trauma, vestibular schwannoma), and patients with single sided deafness (SSD, significant asymmetry of hearing loss with near-normal opposite-ear hearing). The Neural-Genetic Group included subjects with either: 1) Genetic hearing loss due to causative mutations in OPA1, DIAPH3, DFNB59, AIFM1, TBC1D24, MT-RNR1, or TMPRSS3; or 2) Genetic variants in the same 7 genes predicted to be deleterious by CADD score analysis. The Sensory-Genetic Group included subjects with genetic hearing loss due to causative mutations in genes affecting the organ of Corti or synapse (Figure 1).
Table 2.
Speech Perception Test Results by Groups
| Azbio | CNC | HINT | Combined Z Score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total n | n | Avg Score | n | Avg Score | n | Avg Score | n | Avg Score | |
| All subjects | 155 | 119 | 64.2% | 111 | 47.0% | 40 | 59.8% | 155 | −0.13 |
| Neural-Genetic | 24 | 19 | 52.8% | 17 | 36.1% | 10 | 55.2% | 24 | −.42 |
| Sensory-Genetic | 12 | 9 | 85.0% | 8 | 70.1% | 4 | 59.3% | 12 | 0.52 |
| Bilateral SSNHL | 13 | 11 | 80.2% | 7 | 73.9% | 3 | 74.7% | 13 | 0.58 |
| Ménière's Disease | 6 | 3 | 84.3% | 2 | 63.0% | 3 | 63.7% | 6 | 0.17 |
| Otosclerosis | 5 | 5 | 84.5% | 4 | 70.0% | 0 | - | 5 | 0.66 |
| Single-Sided Deafness* | 26 | 25 | 53.1% | 24 | 33.3% | 1 | 0.0% | 26 | −0.59 |
| Other Causes | 10 | 6 | 41.7% | 7 | 35.4% | 5 | 64.4% | 10 | −0.76 |
| Unknown | 60 | 42 | 65.5% | 43 | 49.7% | 14 | 61.8% | 60 | −0.11 |
Includes subjects with single sided deafness due to Ménière's disease, otosclerosis and sudden sensorineural hearing loss
Statistical Analysis
Statistical analysis was performed using SPSS version 26. Non-parametric testing was performed using analysis of variance (ANOVA) with post-hoc testing using Fisher’s least significant difference (LSD). P < 0.05 was considered significant.
Results
Our genetic diagnostic rate (i.e. subjects for whom we were able to identify a causative genetic mutation) was 17% (27 of 155 patients, Supplemental Table 1). Causative mutations in TMPRSS3 were most common (5 patients, 19% of diagnoses), followed by MT-RNR1 (3 patients, 11% of diagnoses). There were two patients (7%) each with causative mutations in TECTA, MYO15A, LOXHD1, and COCH. There was one patient (4%) with causative mutations in each of TMC1, SLC17A8, SCN7A, POU4F3, OTOG, MYO7A, MYH9, MYH14, KCNQ4, GJB2 and CDH23.
Statistical analysis was performed on 8 subject groups (Table 1 and Supplemental Table 1). There were 25 subjects (16%) in the Neural-Genetic Group and 12 subjects (8%) in the Sensory-Genetic Group. In addition, 12 subjects (8%) were included in the Bilateral SSNHL group, 6 subjects (4%) had Ménière’s disease, 5 subjects (3%) had otosclerosis, and 26 patients (17%) had significant asymmetry of hearing loss with near-normal opposite-ear hearing (Single-Sided Deafness Group). Nine patients (6%) had other causes of deafness, including infection (5), ototoxicity (2), osteogenesis imperfecta (1), and noise trauma (1).
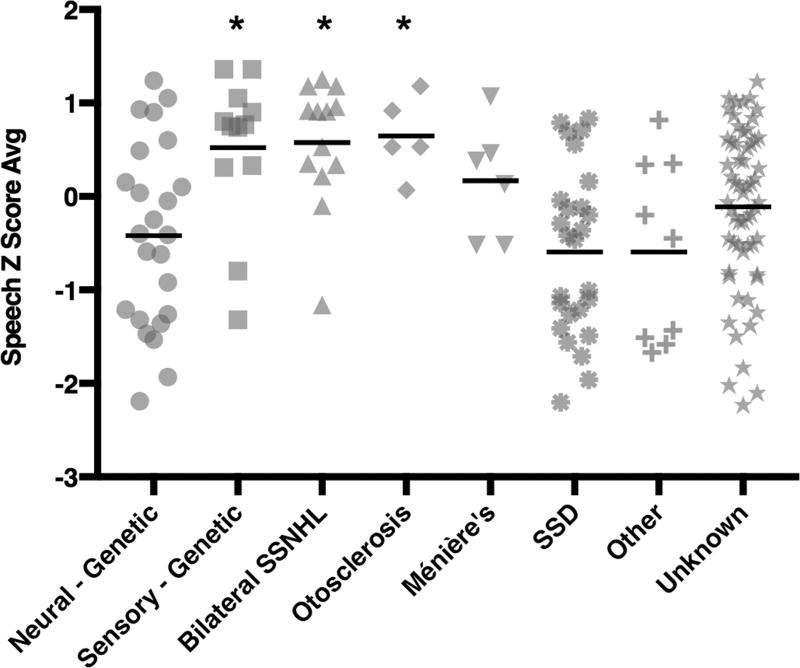
Post-operative speech testing with the AzBio, CNC, and HINT tests was used to evaluate CI outcomes and a Z-scored average was used to normalize and summarize the results (Table 2, Figure 2). ANOVA testing revealed a statistically significant difference in outcome based on the groups described above (p < 0.001). The eta squared for the effect of the statistical groups on the Z score average was 0.183, indicating that this single factor accounts for 18.3% of the variance in post-operative speech perception testing outcomes.
Figure 2.
Post-implantation speech understanding varies significantly based on etiology of deafness and genetic testing results. Comprehensive genetic analysis is included with subject groups as defined in the text. Speech values are average Z scores derived from the mean of CNC, HINT and Azbio audiometric testing. SSNHL, sudden sensorineural hearing loss; SSD, single-sided deafness).
Post-hoc testing showed significantly lower combined Z score average speech scores for the Neural-Genetic Group when compared with the Sensory-Genetic Group, and also when compared to the Bilateral sudden sensorineural hearing loss (Bilateral-SSNHL) and Otosclerosis Groups (p = 0.003, 0.001, and 0.014, respectively). The differences between the Neural-Genetic Group and the Ménière’s Disease, Single-Sided Deafness, Other Causes, and Unknown Groups were not significant (p = 0.143, 0.473, 0.601, and 0.148 respectively).
Discussion
Our results suggest that genetic testing can be used as a non-invasive measure of spiral ganglion health. We restricted our analysis to genes included on the OtoSCOPE panel because the pathogenic potential of variation in these genes as a cause of deafness is incontrovertible. We chose not to group all genes implicated in auditory neuropathy spectrum disorder (ANSD) together as ANSD is a spectrum of phenotypes resulting from genetic alterations to different portions of the peripheral neural pathway (Moser and Starr, 2016). Mutations in four ANSD genes (OPA1, DIAPH3, AIFM1 and DFNB59) cause damage directly to spiral ganglion cells while mutations in four other genes cause damage to pre- or post-synaptic sites (OTOF, SLC17A8, CACNA1D, CABP2). Thus, from a molecular physiology standpoint, a genetic variant negatively affecting the spiral ganglion itself could negatively affect the CI outcome whereas a variant that affects the synapse (e.g. OTOF) may not.
We found that CI recipients with deleterious variants in spiral ganglion cells perform significantly worse on post-implant measures of speech perception than CI recipients with genetic lesions confined to the cochlea or other forms of cochlea-specific damage such as Ménière’s disease, otosclerosis and bilateral SSNHL. Our data show that assigning patients in to groups that include quantitation of the deleterious genetic load in spiral ganglion cells accounts for 18.3% of total variance in post-operative CI outcome, making this metric the single most predictive factor identified to date. In aggregate, these data suggest that the physiological status of the spiral ganglion itself is an important predictor of CI outcomes. Outcomes for the Neural – Genetic Group were more variable than the Sensory – Genetic Group (Figure 1). This may reflect the relative damaging mutational burden on the spiral ganglion cells, or another factor not accounted for here, but will require further study.
Intraoperative electrocochleography is predictive of post-operative CI outcomes, likely because it measures overall health of the spiral ganglion. However, this method requires surgical exposure of the round window thus precluding pre-operative applications (McClellan et al., 2014). Ideally, a potential CI recipient would receive pre-operative clinical counseling of expected CI outcomes, using this information as part of a shared decision model for choosing to undergo cochlear implantation. This information could also temper expectations and underscore the importance of post-activation training and practice, which are known to improve outcomes (Fu and Galvin, 2007).
The other group of subjects who experienced relatively poor outcomes with a CI were individuals who were diagnosed with single sided deafness (SSD). Speech outcomes based on CI only scores in patients with SSD typically are lower than other groups of patients (Sladen et al., 2016; Tokita et al., 2014). Presumably the lower performance is because SSD patients hear normally or near normally in the contralateral, non-implanted ear and are less dependent on the CI for overall hearing performance.
The primary weakness of this study is the limited genetic analysis that was completed. Future studies must address the genetic breadth and complexity of the spiral ganglion in large cohorts of clinically well-characterized CI recipients. Spiral ganglion-enriched genes are known to govern auditory-specific features essential to the complex process of circuit assembly. By interrogating genes essential for auditory wiring and maintenance, not only we will be able to help CI recipients by improving on the predictive power of spiral ganglion genetic analyses, but we will also identify a new source of candidate genes for human deafness and auditory processing disorders (Lu et al., 2011). The latter will have important clinical ramifications beyond the CI population as an important source of genes likely to be important in age- and noise-related forms of hearing loss.
Another weakness of this study was that we did not have accurate records of time from onset of profound deafness to cochlear implantation, a factor that has been shown to be important in post-operative outcomes (Gantz et al., 1993, Blamey et al., 2013; Rance and Starr, 2015). In addition, we did not directly address spiral ganglion degeneration that can result from damage to the cochlear sensory organ (Nadol et al., 1989). Our data would indicate that spiral ganglion degeneration due to inadequate stimulation from a cochlear defect may be less consequential to CI performance than lesions to the spiral ganglion itself. In vivo electrophysiological testing may be the key to understanding these differences and will be the topic of further study.
Another important question yet to be addressed is whether patients with deleterious mutations in spiral ganglion neurons would benefit from different CI mapping strategies compared to other CI recipients. If so, knowledge of a patient’s genetic profile may inform post-implantation mapping and rehabilitation strategies. This could be particular useful for patient who perform relatively poorly with their implants. Finally, it will be important to determine how patients with deleterious mutations in spiral ganglion neurons perform on more complex tasks such as understanding in noise and music appreciation compared to other CI cohorts.
Supplementary Material
Acknowledgments
This work was supported by NIDCD RO1s DC003544, DC002842 and DC012049 and P50 DC00242. We would like to thank George Johanson, PhD, Emeritus Professor of Educational Research and Evaluation, Ohio University for guidance on statistical analysis.
Footnotes
Author Contributions
RJHS conceived study; AES and RJHS designed study; AES, RWE, KF, CMSH, CD, VT, CB, PA, MRH and BJG completed patient collection, data collection and assembly; AES completed data analysis; AES and RJHS wrote the manuscript; all authors contributed to, edited and reviewed the final manuscript; RJHS takes final responsibility for the contents.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Blamey P, Artieres F, Baskent D, Bergeron F, Beynon A, Burke E, Dillier N, Dowell R, Fraysse B, Gallégo S, Govaerts PJ, Green K, Huber AM, Kleine-Punte A, Maat B, Marx M, Mawman D, Mosnier I, O'Connor AF, O'Leary S, Rousset A, Schauwers K, Skarzynski H, Skarzynski PH, Sterkers O, Terranti A, Truy E, Van de Heyning P, Venail F, Vincent C, Lazard DS. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol. Neurootol. 2013;18:36–47. doi: 10.1159/000343189. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ. Perceptual Learning and Auditory Training in Cochlear Implant Recipients. Trends in Amplification. 2007;11:193–205. doi: 10.1177/1084713807301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, woodworth G, Knutson J, Abbas P, Tyler R. Multivariate Predictors of Audiological Success with Multichannel Cochlear Implants. Annals of Otology, Rhinology & Laryngology. 1993;102:909–914. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. A General Framework for Estimating the Relative Pathogenicity of Human Genetic Variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazard DS, Vincent C, Venail F, Van de Heyning P, Truy E, Sterkers O, Skarzynski PH, Skarzynski H, Schauwers K, O'Leary S, Mawman D, Maat B, Kleine-Punte A, Huber AM, Green K, Govaerts PJ, Fraysse B, Dowell R, Dillier N, Burke E, Beynon A, Bergeron F, Baskent D, Artieres F, Blamey PJ. Pre-, Per- and Postoperative Factors Affecting Performance of Postlinguistically Deaf Adults Using Cochlear Implants: A New Conceptual Model over Time. PLoS ONE. 2012;7:e48739–11. doi: 10.1371/journal.pone.0048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CC, Appler JM, Houseman EA, Goodrich LV. Developmental Profiling of Spiral Ganglion Neurons Reveals Insights into Auditory Circuit Assembly. Journal of Neuroscience. 2011;31:10903–10918. doi: 10.1523/JNEUROSCI.2358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa M, Nishio SY, Usami SI. A Comprehensive Study on the Etiology of Patients Receiving Cochlear Implantation With Special Emphasis on Genetic Epidemiology. Otology & Neurotology. 2016;37:e126–e134. doi: 10.1097/MAO.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan JH, Formeister EJ, Merwin WH, Dillon MT, Calloway N, Iseli C, Buchman CA, Fitzpatrick DC, Adunka OF. Otology & Neurotology. 2014;35:e245–e252. doi: 10.1097/MAO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- Mo B, Lindbaek M, Harris S. Cochlear Implants and Quality of Life: A Prospective Study. Ear and Hearing. 2005;26:186–194. doi: 10.1097/00003446-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Moberly AC, Bates C, Harris MS, Pisoni DB. The Enigma of Poor Performance by Adults With Cochlear Implants. Otology & Neurotology. 2016;37:1522–1528. doi: 10.1097/MAO.0000000000001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Starr A. Auditory neuropathy — Neural and Synaptic mechanisms. Nature Reviews Neurology. 2016;12:135–149. doi: 10.1038/nrneurol.2016.10. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Young Y-S, Glynn RJ. Survival of Spiral Ganglion Cells in Profound Sensorineural Hearing Loss: Implications for Cochlear Implantation. Ann. Otol. Rhinol. Laryngol. 98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Soli SD, Sullivan JA. Development of the Hearing In Noise Test for the measurement of speech reception thresholds in quiet and in noise. The Journal of the Acoustical Society of America. 1998;95:1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Nishio S-Y, Hattori M, Moteki H, Tsukada K, Miyagawa M, Naito T, Yoshimura H, Iwasa Y-I, Mori K, Shima Y, Sakuma N, Usami S-I. Ann. Otol. Rhinol. Laryngol. 2015;124:6S–48S. doi: 10.1177/0003489415575549. [DOI] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC Lists for Auditory Tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Raine CH, Summerfield Q, Strachan DR. The cost and analysis of nonuse of cochlear implants. Otology & Neurotology. 2008;29:221–224. doi: 10.1097/mao.0b013e31815c25a1. [DOI] [PubMed] [Google Scholar]
- Rance G, Starr A. Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. Brain. 2015;138:3141–3158. doi: 10.1093/brain/awv270. [DOI] [PubMed] [Google Scholar]
- Shearer AE, Black-Ziegelbein EA, Hildebrand MS, Eppsteiner RW, Ravi H, Joshi S, Guiffre AC, Sloan CM, Happe S, Howard SD, Novak B, DeLuca AP, Taylor KR, Scheetz TE, Braun TA, Casavant TL, Kimberling WJ, LeProust EM, Smith RJH. Advancing genetic testing for deafness with genomic technology. J Med Genet. 2013;50:627–634. doi: 10.1136/jmedgenet-2013-101749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladen DP, Frisch CD, Carlson ML, Driscoll CLW, Torres JH, Zeitler DM. Cochlear Implantation For Single-Sided Deafness: A Multicenter Study. Laryngoscope. 2016;127:223–228. doi: 10.1002/lary.26102. [DOI] [PubMed] [Google Scholar]
- Spahr AJ, Dorman MF, Litvak LM, Van Wie S, Gifford RH, Loizou PC, Loiselle LM, Oakes T, Cook S. Development And Validation of the Azbio Sentence Lists. Ear and Hearing. 2012;33:112–117. doi: 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita J, Dunn C, Hansen MR. Cochlear Implantation and Single Sided Deafness. Curr Opin Otolaryngol Head Neck Surg. 2014;22:353–358. doi: 10.1097/MOO.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Dorman M. Cochlear implants: A remarkable past and a brilliant future. Hear Res. 2008;242:3–21. doi: 10.1016/j.heares.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-C, Lin Y-H, Liu T-C, Lin K-N, Yang W-S, Hsu C-J, Chen P-L, Wu C-M. Identifying Children With Poor Cochlear Implantation Outcomes Using Massively Parallel Sequencing. Medicine. 2015;94:e1073. doi: 10.1097/MD.0000000000001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.