Abstract
In order to generate new hypotheses, sometimes a “systems” approach is needed. In this review I focus on the mitogen activated kinase p38 because it has been recently shown to play an important role in the developmental programing and senescence of normal and stressed reproductive tissues. What follows is an overview of 1) pathways of p38 activation and their involvement in basic biological processes 2) evidence that p38 is involved in the homeostasis of reproductive tissues 3) how focus on p38 can be incorporated into investigation of normal and stressed pregnancies. Existence of excellent reviews will be mentioned as well as relevant animal models.
Keywords: MAPK14 p38 pregnancy, reproduction, immunology
1. Why p38?
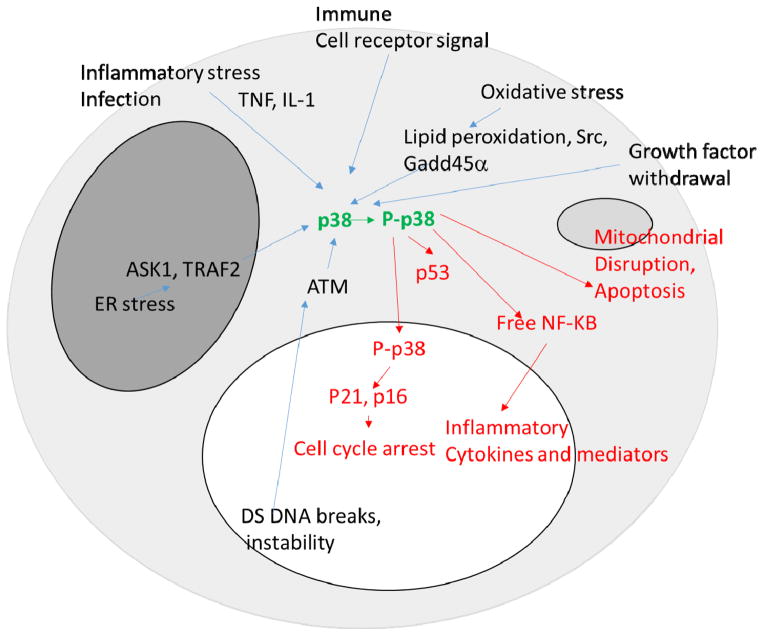
Efforts to delineate the molecular pathways critical to normal parturition and preterm birth have highlighted the importance of inflammation1–6. Further, analysis of the molecular events down-stream of senescence, oxidative stress, infection, and metabolic dysregulation in reproductive and other tissues have suggested that these events all involve the professional mediator p38 in the generation of inflammatory responses7–11. This and other data gives support to the development of a “p38-centric” (Fig. 1) view of cellular homeostasis that is relevant to reproduction, including normal and premature parturition.
Fig. 1. A p38-centric view of cellular homeostasis.
A stylized cell with dark grey endoplasmic reticulum, light grey mitochondria and a while nucleus is shown. A sampling of factors leading to increased presence of activated (phosphorylated, P-p38) p38 are shown with blue arrows/black writing, while events downstream of p38 activation (green) are shown with red arrows and writing. ASK-1 (Apoptosis signaling kinase 1), TRAF2 (TNF receptor-associated factor 2), GADD45α (Growth arrest and DNA-damage-inducible protein GADD45 alpha), TNF (Tumor necrosis factor), IL-1 (Interleukin 1), NF-κb (nuclear factor kappa B) are all shown as representative molecules in the p38 ‘universe’.
2. Introduction to p38
Extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK) and p38, are members of a serine/threonine kinases family with related molecules present in unicellular organisms, and thus have a long evolutionary history12, 13. These molecules are key in mediating cellular responses and are activated through the phosphorylation of a Thr-X-Tyr motif by upstream mitogen activated protein kinases (MAPK). While ERK pathways are typically associated with growth or proliferation signals, JNK and p38 are associated with the response to environmental and infectious/inflammatory stress. There are different pathways for activation of ERK, JNK, and p38, but it is likely that there is cross regulation between the pathways14, and frequently stimuli will activate more than one pathway. The p38s comprise a group of 4 proteins p38α, p38β, p38γ, and p38δ that represent different branches on a phylogenetic tree and are each conserved among species 15. The MAPK 14 family includes p38a in mice, rats, humans Xenopus laevis and zebra fish. The MAPK11 family similarly comprises p38β, while the MAPK 13 family corresponds to more loosely related p38δ. p38γ, also called ERK6 is part of the MAPK 12 family15. Of the p38 MAPK, p38α is most widely expressed and studied. While p38 β and δ16, are expressed in immune cells, p38γ is not and seems to have a highly restricted expression that as yet is not completely elucidated. From here on discussion will focus on p38α and for convenience it will be referred to as p38. This molecule is activated by three upstream kinases MKK3, MKK4, and MKK6. While MKK3 and MKK6 are p38’s main MAPKKs17 specificity of the outcome of external stimuli as noted above, is determined by cell and tissue context and other associated molecules. For example, in mice, all three are involved in activation after ultra violet radiation, while only MKK3 and MKK6 are critical for TNF-a mediated activation.18 Further upstream to the MKKs are the MEKKs 1 to 4 (also called the MAP3Ks for MAP kinase kinase kinases), MLK2/3, and ASK1 (Apoptosis signaling kinase 1), and TAK-1 (TGF-β activated protein kinase 1). Other upstream molecules will likely be delineated with further study. Another point of specificity in p38 regulation comes from the site of phosphorylation. Sites for phosphorylation pf p38 include Thr180 and Tyr182 and another level of control is exemplified by cyclophilin-dependent isomerization of p38 that modifies the chance that upstream kinases can phosphorylate p3819.
3. UPSTREAM: how does p38 get activated?
There are several “stresses” that result in p38 activation. 20
ER stress
Response to a stimulus includes generation of new proteins. The proper folding and transport of these proteins is monitored by the cell. The presence of excess unfolded protein generates the unfolded protein response that includes upregulation of PERK (Protein kinase (RNA)-like endoplasmic reticulum kinase), IRE1 (Inositol requiring protein 1) and ATF6 (Activating transcription factor 6), among others. This leads to an increase in XBP-1 (X-box binding protein 1) and free BiP (binding Ig protein) that can assist in chaperoning and folding the proteins. If however, such ER stress is not relived, this can lead to the interaction between ASK1 and TRAF2 and auto-phosphorylation of ASK1 which can feed into the pathway that causes MKK activation and leads to activation of p3821. Advanced glycation end products (AGEs), produced by the non-enzymatic glycation of macromolecules can accumulate in different tissues and mediate p38 activation through the generation of ER stress.22 ER stress induced by infection can also elevate p38 activation.23
Oxidative stress
Oxidative stress leads to the activation of p38 in many tissues. For examples, direct application of cigarette smoke extract to fetal membranes leads to activation of p38.24 In an endothelial cell line, lipid peroxidation can lead to activation of Src which in turn leads to the activation of p38. A first trimester human trophoblast cell line responds to hypoxia followed by reoxygenation by increasing growth arrest and DNA damage-inducible 45 alpha (Gadd45alpha) expression which in turn activates p38. Data such as this supports the growing hypothesis of the close link between inflammation and oxidative stress and further places p38 as playing a role in the crosstalk between the two processes.
Inflammatory stress
Signaling through receptors for pathogen or “Danger” associated molecular patterns, such as the Toll like receptors leads to activation of the MAP3 Kinases, including TAK-1 (TGF-b activated protein kinase 1) which then activates one of the downstream MAP2 Kinases such as MKK3/6 or MKK4. This in turn activates p38. Cytokines involved in inflammatory processes, such as IL-1 and Tumor necrosis factor-α bind to their cognate receptors and via the small GTPases (e.g. RAC1 and CDC42) also lead to p38 activation (reviewed in25). Specific receptors on different types of inflammatory cells mediate their functions via activation of p38, and this includes the T cell receptor. Binding of this receptor includes activation of ZAP70 and LCK, and ultimately activation of p38.26 Viral infection can also lead to p38 activation.27 In neutrophils, TGF-β, which with IL-6 helps to generate the TH-17 response28, activates p38 and MKK2 and promotes chemotaxis29.
Metabolic stress
This is a likely more broadly inclusive family of cellular stress that encompasses oxidative stress as well as other processes. Insulin, for example exerts an anti-proliferative and hypertrophic effect in an extra villous cell line, and this may be partially reversed by an inhibitor of p38,30 suggesting a role for this molecule. Exposure of kidney cells to high salt conditions leads to p38 activation.31 Embryonic stem cells starved of leukemia inhibitory factor die by apoptosis, and this is mediated by activated p38 and increased cleavage of caspase 3.32
Earlier studies have also delineated the potential role of p 38 in term trophoblast cell response to placenta derived growth factor and protection from serum withdrawal-induced apoptosis.20 Other growth factors such as VEGF and erythropoietin all trigger p38 activation in tissue specific manner33.
DNA damage
Ionizing radiation and other inducers of DNA double strand breaks lead immediately to histone modifications and sensors of DNA damage, including the PCNA-like complex facilitate the activation of upstream kinases such as Ataxia telangiectasia mutated (ATM), and Ataxia-telangiectasia and rad3 (ATR) 34, 35. This in turn can trigger phosphorylation p38 and cause its nuclear translocation.36 ATM in addition can work with NEMO (NF-κB essential modifier) to release NF-κB from IκB kinase α and β, Unprotected chromosomes induce DNA damage checkpoint cascades and has been associated with increased activation of p3837 and may explain the association between telomere shortening and the presence of phosphorylated p38.38, 39
4. DOWNSTREAM: what happens when p38 gets activated?
p38 and inflammation
Several effector pathways are induced by the activation of p38. In the so-called ‘classical’ p38 pathway, phosphorylated p38 in the cytosol can mediate the activation and movement of several transcription factors including AP-1 and SP-1 to the nucleus which could be followed by transcription of several inflammatory cytokines and mediators33. A particular example is that p38 activates mitogen- and stress-activated protein kinases, MSK1 and MSK2, which can phosphorylate the trans-activating p65 subunit of the NF-κB complex at Ser276 and thus potentiate NF-κB signaling.40 While in the cytoplasm, activated p38 can also mediate its effects through the activation of other specific proteins including MAP-kinase activated protein kinase 2 (MAPKAPK2, MK2) and MAPKAPK5 which in turn can phosphorylate heat shock (e.g. 25/27) and other proteins in and out of the nucleus 41 Down-stream molecules of p38 activation can also stimulate the transcription factor STAT3 which can potentiate the actions of NK cells and IL-642.
Autophagy43
This is an important mechanism for homeostasis in several tissues in response to stresses such as inflammation, and evidence of dysregulation of this process has been associated with preterm birth in humans44. p38 can up or down-regulate autophagy45, 46. p38 can block autophagy through the mechanistic target of rapamycin (mTOR) pathway and pathways independent of this molecule45
Senescence
Senescence occurs as the result of irreversible cell cycle arrest, and is associated with DNA fragments, and stereotypic histologic changes and inflammation40. Activated p38 can arrest cell division by multiple pathways, including activation of p5347 and in the so-called ‘non classical’ pathway p38 can move to the nucleus itself and enhance the activity of molecules such as p21 and p16 to block cell cycling10 at both the G2M48 and G1/S checkpoints.47 Genomic instability evoked by senescence triggers epigenetic changes, e.g. release of HMGB1 proteins which are also potent enhancers of inflammatory responses (see feedback loops, below). Another molecule upregulated by senescence is p19ARF that, along with are thought to also behave as tumor suppressors40. It is possible that senescent cells sit on a precipice between cancer and death and that p38 is critically involved in the balance.
Cell death
The death of a cell can proceed along several specific molecular pathways (reviewed in49, 50), and p38 may be directly or indirectly involved.51 The two major apoptotic pathways that are well documented in mammalian cells are the death receptor and the mitochondrial pathway in the death receptor pathway. The death receptor pathway involves the cysteine proteases Caspase 8, 10, and 3, and is initiated by expression of molecules like TNF. The mitochondrial pathway is an intrinsic pathway is activated in response to ionizing radiation, growth factor withdrawal, chemotherapeutics drugs, reactive oxygen and nitrogen species, and DNA damage. Such insults lead to expression of BCL-2 family members, mitochondrial membrane permeability, release of cytochrome c and activation of Caspases 9 and 3. Some of the pathways can be quite complex. For example, Glycogen synthase kinase 3β (GSK3β) is a constitutively active kinase that supports apoptosis through inhibition of the pro-survival transcription factors, and facilitating pro-apoptotic transcription factors such as p5352. In developing T cells, GSK3β phosphorylates beta-catenin and causes its degradation53 which prevents it from supporting the transcription of pro-survival genes. However, activated p38 can phosphorylate GSK3β on Ser 389 and inactivate it, thus preserving survival.54 Recently, inability to inactivate GSK3β has been linked to promotion of cell death via a process now called necroptosis.49
Feedback loops and “rogue” pathways
The “upstream/downstream” construct for p38 may be a gross oversimplification. For example, molecules such as tumor progression locus 2 (TPL-2) TPL2 participate in a circuit that is dependent on p38 activation and itself activates the MKK3/6 which in turn activates p3855. For another example, stressors that generate p38 activation can also result in expression of High Mobility Group B1 protein (HMGB1), but this in turn can act via internal pathways to activate p38.56 For yet a thrid example, senescent T cells emply neither the canonical nor alternative pathways and instead cause auto phosphorylation of p38 by engaging AMPK to cause p38 recruitment to the scaffold TAB1. This process leads to loss of telomerase57 which may lead to shortened telomeres and may itself be associated with p38 activation.
5. Tissue specific regulation of p38 and implications for normal and abnormal function
The ovary
Activation of p38 might regulate proliferation. For example, in mouse granulosa cells, exposure to FSH leads to p38-mediated de-phosphorylation of the transcription factor STAT1. This leads to decreased transcription of the cytochrome P450 1 subfamily member Cyp1b1 and altered estrogen metabolism. The result is decreased proliferation, and it is hypothesized that this assists in the maintenance of estradiol levels in the dominant follicle in vivo.58 In addition, p38 regulates the response to prolactin in a model premature ovarian failure59
The cervix
Cervical mucosal epithelium responds to infection with production of TNF-α and this is in part dependent on activation of p3860. In human cervix, p38 activation has been associated with parturition but this is likely due to inflammatory cell traffic macrophages61–63 and or association with tissues such as the fetal membranes overlying the cervix at term64, 65 as this tissue expresses an increased inflammatory signature.66
The myometrium
This kinase is expressed in myometrial cells where it has been found to mediate may mediate oxytocin receptor signaling and downstream inflammatory responses67. Histamine also induces p38 activation in myometrial cells and this may in part explain the cross-talk between histamine and Toll like receptor triggered inflammation and further, that there is an increase in premature labor in patients with severe allergy68 or asthma69. Stretch also activates the p38 pathway within myometrial cells2, 70, and this may contribute to the inflammatory signature occurring with multiple gestation, or with the end of a singleton pregnancy.
The decidua
Overall, activation of p38 increases with gestational age in mouse decidua 38 and may be increased by labor in humans.71 The decidua is a complex tissue72, 73 comprised of stromal, leukocyte, glandular, and vascular elements. Each is likely to have its own regulation with regard to the mitogen activated kinases. For examples, preimplantation factor, PIF supports implantation in a process that involves decreasing p38 activation and endo-cannabinoids can induce apoptosis in decidual cells in a process that includes activation of p38, mitochondrial stress, reactive oxygen species elaboration and apoptosis.74 Decidual stromal cell dysfunction has been implicated in early and late pregnancy loss, related to a mouse model of progesterone receptor targeted insufficiency in p53, but the role of p38 in that model is unclear75. In humans, p38 may mediate the inflammation related to functional progesterone withdrawal76,72.
The activation of leukocyte populations by Toll-like signalling largely involves activation of p38. For example, NK T cells are activated by Toll-like receptor, CD1, and inflammatory cytokine signaling that proceeds with activation of p38 and ERK pathways77. Decidual artery remodeling is an important aspect of normal pregnancy in the mouse78,79, 80 and deficient invasion of spiral arteries is thought to be an important initiator of preeclampsia. While many studies have reported the potential role of p38 activation or inhibition in systemic vascular remodelling81, few have examined decidual vessels. Human umbilical vein endothelial cells exposed to hypoxia/reperfusion upregulate growth arrest and DNA damage-inducible 45 alpha (Gadd45α) leading to p38 activation and ultimately an increase in fms-related tyrosine kinase-1 (sFlt-1) and soluble endoglin secretion, and both Gadd45α and activated p38 are found in placentas from preeclampsia pregnancies.82
Preimplantation embryo and trophoblast
Several studies have delineated the expression41 and importance83 of p38 signaling in the development and function of the preimplantation embryo. The p38 pathway is critical for glucose metabolism83 and differentiation41, as well as in the generation of molecules important in implantation. Early in gestation, VEGF activates p38 and contributes to VEGF-induced EVT migration and capillary-like tube formation in human chorio-decidual trophoblast84.
Fetal membranes
The fetal membranes represent a unique tissue that balances proliferation, renewal, senescence and death. The fetal membranes are a multilayered and multicellular tissue that includes the amnion and the chorion.85 Over time in normal pregnancy the fetal membranes undergo senescence associated with increased expression of p3834,6, 38, and express increased p38 with oxidative stress,24 and in the setting of premature rupture of membranes6, 71, 86, 87,72. Membranes express both classical and alternative p38 pathway molecules, but cell type specificity, and the exact up and downstream activators and effectors are not known.
6. The future of p38 in reproductive science
Animal models of reproductive outcome
There exist several models of reproductive outcome.88–90 With regard to the role of p38, animal models, particularly mice offer the opportunity to examine both the kinetics and the specific molecular pathways by which activation of this molecule occurs and causes downstream effect. Studies in normal mice mice38 suggest that progressive increase in activated p38 occurs in the decidua, placenta and fetal membranes. However, the relevant pathways and cells are unclear and will have to be examined in future studies and correlated with findings in humans. 71, 91, 92,49 Although some of the stressors leading to poor pregnancy outcome humans can be modeled in mice, the specific p38-involved pathways have yet to be fully explored.
Biomarkers of p38 activation
Peripheral blood cytokines and molecules such as sFlt have been used to predict abnormal pregnancy and may be down stream of p38 activation93. As activation of p38 is a pivotal event in many processes indicative of pregnancy stress or disregulation, it would be useful to have early indicators of p38 activation. In vitro, exosomes from aminon epithelial cells contain activated p38 and uptake into the cytoplasm of other cells activates inflammatory pathways.94 Vesicles, including exosomes95 eminate from several tissues of the reproductive tract and can be found in maternal blood. This raises the interesting idea that exosomes be used to diagnose early placental p38 activation.
Inhibitors and activators of p38 at the bedside
The clinical importance of modifiers of p38 activity and an approach to development of these drugs has been recently reviewed.96 Focus has been on inhibition of the catalytic activity of p38 is through competitive or non-competitive binding of ATP97. Many inhibitors of p38 are being considered, including SB203580 (a competitive binder in the ATP pocket or p38), and BIRB 79697 in diseases related to premature ageing (e.g. Werner Syndrome97) and autoimmunity. Focus on how to target drug activity and limit side effects continues. Further, there exists a pipeline to develop ways to modulate molecules down stream of p38 activation81, 96 in specific cell types98 order to increase specificity. Relevant clinical trials focusing on reproductive outcome need to be developed. Agents, such as statins99 are being considered for improvement of reproductive outcome, and have an indirect action on the p38 pathway through decreasing upstream stressors such as oxidative stress100, but further understanding of these agents is also needed.
7. Summary
p38 is part of an evolutionarily conserved family of molecules that plays a pivotal role in homeostasis in the reproductive tract. Certain cell types, including immune cells, employ canonical, alternative or “rogue” p38 pathways. Consideration p38’s roll in cell-cell interaction in the reproductive tract and beyond is crucial. Focus on the molecular stresses leading to increased expression of activated p38 as well as down-stream effector pathways will lead to greater understanding of normal and abnormal reproduction.
Acknowledgments
I am supported in part by the Vermont Center for Immunology and Infectious Diseases (NIH P30 GM118228) and the Department of Obstetrics, Gynecology and Reproductive Sciences. I am grateful for discussions with my colleagues in PREBIC, the Preterm Birth International Collaborative and apologize to my colleagues for work not mentioned due to space or time considerations.
References
- 1.Murtha AP, Menon R. Regulation of fetal membrane inflammation: a critical step in reducing adverse pregnancy outcome. Am Journal Obstet Gynrcol. 2015;213:447–448. doi: 10.1016/j.ajog.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Adams Waldorf KM, Singh N, Mohan AR, Young RC, Ngo L, Das A, Tsai J, Bansal A, Paolella L, Herbert BR, Sooranna SR, Gough GM, Astley C, Vogel K, Baldessari AE, Bammler TK, MacDonald J, Gravett MG, Rajagopal L, Johnson MR. Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. Am Journal Obstet Gynrcol. 2015;213:830e831–830.e819. doi: 10.1016/j.ajog.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendal-Wright CE. Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci. 2007;14:35–41. doi: 10.1177/1933719107310763. [DOI] [PubMed] [Google Scholar]
- 4.Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Molec Endocrinol. 2009;23:947–954. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovsky M, Menon R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Molec Hum Reprod. 2016;22:143–157. doi: 10.1093/molehr/gav074. [DOI] [PubMed] [Google Scholar]
- 7.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. American J Reprod Immunol 1989. 2016;75:3–7. doi: 10.1111/aji.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krementsov DN, Thornton TM, Teuscher C, Rincon M. The emerging role of p38 mitogen-activated protein kinase in multiple sclerosis and its models. Molec Cell Biol. 2013;33:3728–3734. doi: 10.1128/MCB.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segovia M, Haramaty L, Berges JA, Falkowski PG. Cell death in the unicellular chlorophyte Dunaliella tertiolecta. A hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant physiol. 2003;132:99–105. doi: 10.1104/pp.102.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Gomez C, Parages ML, Jimenez C, Palma A, Mata MT, Segovia M. Cell survival after UV radiation stress in the unicellular chlorophyte Dunaliella tertiolecta is mediated by DNA repair and MAPK phosphorylation. J Exp botany. 2012;63:5259–5274. doi: 10.1093/jxb/ers185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busca R, Pouyssegur J, Lenormand P. ERK1 and ERK2 Map Kinases: Specific Roles or Functional Redundancy? Frontiers in Cell and Developmental Biol. 2016;4:53. doi: 10.3389/fcell.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krens SF, Spaink HP, Snaar-Jagalska BE. Functions of the MAPK family in vertebrate-development. FEBS letters. 2006;580:4984–4990. doi: 10.1016/j.febslet.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol (Baltimore, Md : 1950) 1999;162:4246–4252. [PubMed] [Google Scholar]
- 17.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 18.Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brichkina A, Nguyen NT, Baskar R, Wee S, Gunaratne J, Robinson RC, Bulavin DV. Proline isomerisation as a novel regulatory mechanism for p38MAPK activation and functions. Cell Death Differ. 2016;23:1592–1601. doi: 10.1038/cdd.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai J, Holt-Shore V, Torry RJ, Caudle MR, Torry DS. Signal transduction and biological function of placenta growth factor in primary human trophoblast. Biol Reprod. 1999;60:887–892. doi: 10.1095/biolreprod60.4.887. [DOI] [PubMed] [Google Scholar]
- 21.Yan BC, Adachi T, Tsubata T. ER stress is involved in B cell antigen receptor ligation-induced apoptosis. Biochem Bioiophys Res Com. 2008;365:143–148. doi: 10.1016/j.bbrc.2007.10.137. [DOI] [PubMed] [Google Scholar]
- 22.Rasheed Z, Haqqi TM. Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2α, p38-MAPK and NF-κB in advanced glycation end products stimulated human chondrocytes. Biochimica Biophysica Acta. 2012;1823:2179–2189. doi: 10.1016/j.bbamcr.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YX, Ren YL, Fu HJ, Zou L, Yang Y, Chen Z. Hepatitis B Virus Middle Protein Enhances IL-6 Production via p38 MAPK/NF-kappaB Pathways in an ER Stress-Dependent Manner. PloS one. 2016;11:e0159089. doi: 10.1371/journal.pone.0159089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon R, Fortunato SJ, Yu J, Milne GL, Sanchez S, Drobek CO, Lappas M, Taylor RN. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta. 2011;32:317–322. doi: 10.1016/j.placenta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nature Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- 26.Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJ, Jr, Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nature immunol. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- 27.Sreekanth GP, Chuncharunee A, Sirimontaporn A, Panaampon J, Noisakran S, Yenchitsomanus PT, Limjindaporn T. SB203580 Modulates p38 MAPK Signaling and Dengue Virus-Induced Liver Injury by Reducing MAPKAPK2, HSP27, and ATF2 Phosphorylation. PLoS One. 2016;11:e0149486. doi: 10.1371/journal.pone.0149486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 29.Hannigan M, Zhan L, Ai Y, Huang CK. The role of p38 MAP kinase in TGF-beta1-induced signal transduction in human neutrophils. Biochem Bioiophys Res Com communications. 1998;246:55–58. doi: 10.1006/bbrc.1998.8570. [DOI] [PubMed] [Google Scholar]
- 30.Silva C, Nunes C, Correia-Branco A, Araújo JR, Martel F. Insulin Exhibits an Antiproliferative and Hypertrophic Effect in First Trimester Human Extravillous Trophoblasts. Reprod Sci. 2016 Sep 22;2016 doi: 10.1177/1933719116667220. pii: 1933719116667220 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Dmitrieva NI, Bulavin DV, Fornace AJ, Burg MB. Rapid activation of G(2)/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. PNAS. 2002;99:184–189. doi: 10.1073/pnas.231623498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duval D, Trouillas M, Thibault C, Dembele D, Diemunsch F, Reinhardt B, Mertz AL, Dierich A, Boeuf H. Apoptosis and differentiation commitment: novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ. 2005;13:564–575. doi: 10.1038/sj.cdd.4401789. [DOI] [PubMed] [Google Scholar]
- 33.Ono K, Han J. The p38 signal transduction pathway Activation and function. Cellular signalling. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 34.Tivey HS, Rokicki MJ, Barnacle JR, Rogers MJ, Bagley MC, Kipling D, Davis T. Small molecule inhibition of p38 MAP kinase extends the replicative life span of human ATR-Seckel syndrome fibroblasts. J Gerontol Series A. 2013;68:1001–1009. doi: 10.1093/gerona/gls336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misri S, Pandita S, Kumar R, Pandita TK. Telomeres, histone code, and DNA damage response. Cytogenetic Genome Res. 2008;122:297–307. doi: 10.1159/000167816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood CD, Thornton TM, Sabio G, Davis RA, Rincon M. Nuclear localization of p38 MAPK in response to DNA damage. IJBS. 2009;5:428–437. doi: 10.7150/ijbs.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derradji H, Bekaert S, De Meyer T, Jacquet P, Abou-El-Ardat K, Ghardi M, Arlette M, Baatout S. Ionizing radiation-induced gene modulations, cytokine content changes and telomere shortening in mouse fetuses exhibiting forelimb defects. Dev Biol. 2008;322:302–313. doi: 10.1016/j.ydbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 38.Bonney EA, Krebs K, Saade G, Kechichian T, Trivedi J, Huaizhi Y, Menon R. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta. 2016;43:26–34. doi: 10.1016/j.placenta.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillippe M. Cell-Free Fetal DNA, Telomeres, and the Spontaneous Onset of Parturition. Reprod Sci. 2015;22:1186–1201. doi: 10.1177/1933719115592714. [DOI] [PubMed] [Google Scholar]
- 40.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell Sig. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Natale DR, Paliga AJ, Beier F, D’Souza SJ, Watson AJ. p38 MAPK signaling during murine preimplantation development. Dev Biol. 2004;268:76–88. doi: 10.1016/j.ydbio.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Braunschweig A, Poehlmann TG, Busch S, Schleussner E, Markert UR. Signal transducer and activator of transcription 3 (STAT3) and Suppressor of Cytokine Signaling (SOCS3) balance controls cytotoxicity and IL-10 expression in decidual-like natural killer cell line NK-92. Am J Reprod Immunol. 2011;66:329–335. doi: 10.1111/j.1600-0897.2011.00989.x. [DOI] [PubMed] [Google Scholar]
- 43.Choi AMK, Ryter SW, Levine B. Autophagy in Human Health and Disease. New Eng J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 44.Avagliano L, Massa V, Zullino S, Doi P, Marconi AM, Ferrazzi E, Bulfamante GP. Inflammation modulates LC3 expression in human preterm delivery. J Maternal-fetal Neonat Med. 2016:1–7. doi: 10.1080/14767058.2016.1183630. [DOI] [PubMed] [Google Scholar]
- 45.Henson SM, Lanna A, Riddell NE, Franzese O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK, Tooze SA, Akbar AN. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J Clin Invest. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simone C. Signal-dependent control of autophagy and cell death in colorectal cancer cell: the role of the p38 pathway. Autophagy. 2007;3:468–471. doi: 10.4161/auto.4319. [DOI] [PubMed] [Google Scholar]
- 47.Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. IJBS. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature. 2001;411:102–107. doi: 10.1038/35075107. [DOI] [PubMed] [Google Scholar]
- 49.Tait SWG, Ichim G, Green DR. Die another way – non-apoptotic mechanisms of cell death. J Cell Science. 2014;127:2135–2144. doi: 10.1242/jcs.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/Threonine Protein Kinases and Apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- 52.Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GV, Jope RS. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. PNAS. 2002;99:7951–7955. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. GSK-3β: A Bifunctional Role in Cell Death Pathways. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thornton TM, Delgado P, Chen L, Salas B, Krementsov D, Fernandez M, Vernia S, Davis RJ, Heimann R, Teuscher C, Krangel MS, Ramiro AR, Rincon M. Inactivation of nuclear GSK3beta by Ser(389) phosphorylation promotes lymphocyte fitness during DNA double-strand break response. Nat Com. 2016;7:10553. doi: 10.1038/ncomms10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pattison MJ, Mitchell O, Flynn HR, Chen CS, Yang HT, Ben-Addi H, Boeing S, Snijders AP, Ley SC. TLR and TNF-R1 activation of the MKK3/MKK6-p38alpha axis in macrophages is mediated by TPL-2 kinase. Biochem J. 2016;473:2845–2861. doi: 10.1042/BCJ20160502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One. 2014;9:e113799. doi: 10.1371/journal.pone.0113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanna A, Henson SM, Escors D, Akbar AN. AMPK-TAB1 activated p38 drives human T cell senescence. Nature Immunol. 2014;15:965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du XH, Zhou XL, Cao R, Xiao P, Teng Y, Ning CB, Liu HL. FSH-induced p38-MAPK-mediated dephosphorylation at serine 727 of the signal transducer and activator of transcription 1 decreases Cyp1b1 expression in mouse granulosa cells. Cell Sig. 2015;27:6–14. doi: 10.1016/j.cellsig.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Devi YS, Seibold AM, Shehu A, Maizels E, Halperin J, Le J, Binart N, Bao L, Gibori G. Inhibition of MAPK by prolactin signaling through the short form of its receptor in the ovary and decidua: involvement of a novel phosphatase. J Biol Chem. 2011;286:7609–7618. doi: 10.1074/jbc.M110.166603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang JB, Quan JH, Kim YE, Rhee YE, Kang BH, Choi IW, Cha GH, Yuk JM, Lee YH. Involvement of PI3K/AKT and MAPK Pathways for TNF-alpha Production in SiHa Cervical Mucosal Epithelial Cells Infected with Trichomonas vaginalis. Korean J Parasitol. 2015;53:371–377. doi: 10.3347/kjp.2015.53.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubicke A, Ekman-Ordeberg G, Mazurek P, Miller L, Yellon SM. Density of Stromal Cells and Macrophages Associated With Collagen Remodeling in the Human Cervix in Preterm and Term Birth. Reprod Sci. 2015;23(5):595–603. doi: 10.1177/1933719115616497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage Trafficking in the Uterus and Cervix Precedes Parturition in the Mouse. Biol Reprod. 1999;61:879–883. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- 63.Dobyns AE, Goyal R, Carpenter LG, Freeman TC, Longo LD, Yellon SM. Macrophage gene expression associated with remodeling of the prepartum rat cervix: microarray and pathway analyses. PloS one. 2015;10:e0119782. doi: 10.1371/journal.pone.0119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsen B, Hwang J. Progesterone interactions with the cervix: translational implications for term and preterm birth. Infect Dis Obstet Gynecol. 2011;2011:353297. doi: 10.1155/2011/353297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lappas M, Riley C, Lim R, Barker G, Rice GE, Menon R, Permezel M. MAPK and AP-1 proteins are increased in term pre-labour fetal membranes overlying the cervix: regulation of enzymes involved in the degradation of fetal membranes. Placenta. 2011;32:1016–1025. doi: 10.1016/j.placenta.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 66.Lappas M, Odumetse TL, Riley C, Reti NG, Holdsworth-Carson SJ, Rice GE, Permezel M. Pre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signalling pathways. Placenta. 2008;29:995–1002. doi: 10.1016/j.placenta.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Kim SH, MacIntyre DA, Firmino Da Silva M, Blanks AM, Lee YS, Thornton S, Bennett PR, Terzidou V. Oxytocin activates NF-kappaB-mediated inflammatory pathways in human gestational tissues. Mol Cell Endocrinol. 2015;403:64–77. doi: 10.1016/j.mce.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Romero R, Kusanovic JP, Munoz H, Gomez R, Lamont RF, Yeo L. Allergy-induced preterm labor after the ingestion of shellfish. J Matern Fetal Neonatal Med. 2010;23:351–359. doi: 10.3109/14767050903177193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bakhireva LN, Schatz M, Jones KL, Chambers CD. Asthma control during pregnancy and the risk of preterm delivery or impaired fetal growth. Ann Allergy Asthma Immunol. 2008;101:137–143. doi: 10.1016/S1081-1206(10)60201-3. [DOI] [PubMed] [Google Scholar]
- 70.Sooranna SR, Engineer N, Loudon JA, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: the differential effect of stretch and interleukin-1{beta} J Clin Endocrinol Metab. 2005;90:3517–3527. doi: 10.1210/jc.2004-1390. [DOI] [PubMed] [Google Scholar]
- 71.Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging. 2016 doi: 10.18632/aging.100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22:535–560. doi: 10.1093/humupd/dmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norwitz ER, Bonney EA, Snegovskikh VV, Williams MA, Phillippe M, Park JS, Abrahams VM. Molecular Regulation of Parturition: The Role of the Decidual Clock. Cold Spring Harbor Perspect Med. 2015;5:11. doi: 10.1101/cshperspect.a023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fonseca BM, Correia-da-Silva G, Teixeira NA. The endocannabinoid anandamide induces apoptosis of rat decidual cells through a mechanism involving ceramide synthesis and p38 MAPK activation. Apoptosis. 2013;18:1526–1535. doi: 10.1007/s10495-013-0892-9. [DOI] [PubMed] [Google Scholar]
- 75.Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120:803–815. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzeloglu-Kayisli O, Kayisli UA, Semerci N, Basar M, Buchwalder LF, Buhimschi CS, Buhimschi IA, Arcuri F, Larsen K, Huang JS, Schatz F, Lockwood CJ. Mechanisms of chorioamnionitis-associated preterm birth: interleukin-1beta inhibits progesterone receptor expression in decidual cells. J Pathol. 2015;237:423–434. doi: 10.1002/path.4589. [DOI] [PubMed] [Google Scholar]
- 77.Li L, Yang J, Jiang Y, Tu J, Schust DJ. Activation of decidual invariant natural killer T cells promotes lipopolysaccharide-induced preterm birth. Molec Hum Reprod. 2015;21:369–381. doi: 10.1093/molehr/gav001. [DOI] [PubMed] [Google Scholar]
- 78.Dixon ME, Chien EK, Osol G, Callas PW, Bonney EA. Failure of decidual arteriolar remodeling in the CBA/J x DBA/2 murine model of recurrent pregnancy loss is linked to increased expression of tissue inhibitor of metalloproteinase 2 (TIMP-2) Am J Obstet Gynecol. 2006;194:113–119. doi: 10.1016/j.ajog.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 79.Felker AM, Chen Z, Foster WG, Croy BA. Receptors for non-MHC ligands contribute to uterine natural killer cell activation during pregnancy in mice. Placenta. 2013;34:757–764. doi: 10.1016/j.placenta.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal pregnancy. J Exp Med. 2000;192:259–269. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Potthoff SA, Stamer S, Grave K, Konigshausen E, Sivritas SH, Thieme M, Mori Y, Woznowski M, Rump LC, Stegbauer J. Chronic p38 mitogen-activated protein kinase inhibition improves vascular function and remodeling in angiotensin II-dependent hypertension. JRAAS. 2016;17(3) doi: 10.1177/1470320316653284. pii: 1470320316653284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo X, Yao Z-w, Qi H-b, Liu D-d, Chen G-q, Huang S, Li Q-s. Gadd45α as an upstream signaling molecule of p38 MAPK triggers oxidative stress-induced sFlt-1 and sEng upregulation in preeclampsia. Cell Tiss Res. 2011;344:551. doi: 10.1007/s00441-011-1164-z. [DOI] [PubMed] [Google Scholar]
- 83.Sozen B, Ozturk S, Yaba A, Demir N. The p38 MAPK signalling pathway is required for glucose metabolism, lineage specification and embryo survival during mouse preimplantation development. Mech Dev. 2015;138(Pt 3):375–398. doi: 10.1016/j.mod.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Lala N, Girish GV, Cloutier-Bosworth A, Lala PK. Mechanisms in decorin regulation of vascular endothelial growth factor-induced human trophoblast migration and acquisition of endothelial phenotype. Biol Reprod. 2012;87:59. doi: 10.1095/biolreprod.111.097881. [DOI] [PubMed] [Google Scholar]
- 85.Bourne G. The Fœtal Membranes: A Review of the Anatomy of Normal Amnion and Chorion and Some Aspects of Their Function. Postgrad Med J. 1962;38:193–201. doi: 10.1136/pgmj.38.438.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol. 2014;184:1740–1751. doi: 10.1016/j.ajpath.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 87.Menon R, Papaconstantinou J. p38 Mitogen activated protein kinase (MAPK): a new therapeutic target for reducing the risk of adverse pregnancy outcomes. Expert Opin Therapeut Targets. 2016:1–16. doi: 10.1080/14728222.2016.1216980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nielsen BW, Bonney EA, Pearce BD, Donahue LR, Sarkar IN. A Cross-Species Analysis of Animal Models for the Investigation of Preterm Birth Mechanisms. Reprod Sci. 2016;23:482–491. doi: 10.1177/1933719115604729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bonney EA. Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside. Am J Reprod Immunol. 2013;69:567–584. doi: 10.1111/aji.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonney EA, Brown SA. To drive or be driven: the path of a mouse model of recurrent pregnancy loss. Reproduction. 2014;147:R153–167. doi: 10.1530/REP-13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, Saade GR, Menon R. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015;213:359e351–316. doi: 10.1016/j.ajog.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 92.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere Fragment Induced Amnion Cell Senescence: A Contributor to Parturition? PLoS One. 2015;10:e0137188. doi: 10.1371/journal.pone.0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiong Y, Liebermann DA, Tront JS, Holtzman EJ, Huang Y, Hoffman B, Geifman-Holtzman O. Gadd45a stress signaling regulates sFlt-1 expression in preeclampsia. J Cell Physiol. 2009;220:632–639. doi: 10.1002/jcp.21800. [DOI] [PubMed] [Google Scholar]
- 94.Sheller S, Papaconstantinou J, Urrabaz-Garza R, Richardson L, Saade G, Salomon C, Menon R. Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS ONE. 2016;11:e0157614. doi: 10.1371/journal.pone.0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foster BP, Balassa T, Benen TD, Dominovic M, Elmadjian GK, Florova V, Fransolet MD, Kestlerova A, Kmiecik G, Kostadinova IA, Kyvelidou C, Meggyes M, Mincheva MN, Moro L, Pastuschek J, Spoldi V, Wandernoth P, Weber M, Toth B, Markert UR. Extracellular vesicles in blood, milk and body fluids of the female and male urogenital tract and with special regard to reproduction. Crit Reviews Clinical Lab Sci. 2016;53:379–395. doi: 10.1080/10408363.2016.1190682. [DOI] [PubMed] [Google Scholar]
- 96.Menon R, Papaconstantinou J. p38 Mitogen activated protein kinase (MAPK): a new therapeutic target for reducing the risk of adverse pregnancy outcomes. Expert Opin Therapeut Targets. 2016;20:1397–1412. doi: 10.1080/14728222.2016.1216980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bagley MC, Davis T, Murziani PGS, Widdowson CS, Kipling D. Use of p38 MAPK Inhibitors for the Treatment of Werner Syndrome. Pharmaceuticals. 2010;3:1842–1872. doi: 10.3390/ph3061842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kragholm K, Newby LK, Melloni C. Emerging treatment options to improve cardiovascular outcomes in patients with acute coronary syndrome: focus on losmapimod. Drug Design, Dev Therapy. 2015;9:4279–4286. doi: 10.2147/DDDT.S69546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramma W, Ahmed A. Therapeutic potential of statins and the induction of heme oxygenase-1 in preeclampsia. J Reprod Immunol. 2014;101–102:153–160. doi: 10.1016/j.jri.2013.12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bao X-m, Wu C-f, Lu G-p. Atorvastatin attenuates homocysteine-induced apoptosis in human umbilical vein endothelial cells via inhibiting NADPH oxidase-related oxidative stress-triggered p38MAPK signaling. Acta Pharmacologica Sinica. 2009;30:1392–1398. doi: 10.1038/aps.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]