Abstract
Objective
Laminar flow activates MEF2 transcription factors in vitro to induce expression of atheroprotective genes in the endothelium. Here we sought to establish the role of Mef2c in the vascular endothelium in vivo.
Approach and Results
To study endothelial Mef2c, we generated endothelial-specific deletion of Mef2c using Tie2-Cre or Cdh5-Cre-ERT2 and examined aortas and carotid arteries by en face immunofluorescence. We observed enhanced actin stress fiber formation in the Mef2c-deleted thoracic aortic endothelium (laminar flow region), similar to those observed in normal aortic inner curvature (disturbed flow region). Furthermore, Mef2c deletion resulted in the de novo formation of subendothelial intimal cells expressing markers of differentiated smooth muscle in the thoracic aortas and carotids. Lineage-tracing showed that these cells were not of endothelial origin. To define early events in intimal development, we induced endothelial deletion of Mef2c and examined aortas at 4 and 12 weeks post induction. The number of intimal cell clusters increased from 4 to 12 weeks, but the number of cells within a cluster peaked at 2 cells in both cases, suggesting ongoing migration but minimal proliferation. Moreover, we identified cells extending from the media through fenestrations in the internal elastic lamina into the intima, indicating trans-fenestral smooth muscle migration. Similar trans-fenestral migration was observed in wild-type carotid arteries ligated to induce neointimal formation.
Conclusions
These results indicate that endothelial Mef2c regulates the endothelial actin cytoskeleton and inhibits smooth muscle cell migration into the intima.
Keywords: Mef2, endothelial cell, intimal smooth muscle, migration, fenestration
Subject codes: Gene Expression and Regulation, Genetically Altered and Transgenic Models, Vascular Disease, Smooth Muscle Proliferation and Differentiation, Endothelium/Vascular Type/Nitric Oxide
INTRODUCTION
Migration of smooth muscle (SM) into the sub-endothelial intima is an essential process in vascular development and disease1–4. Although the endothelium is an important regulator of SM migration with many identified components5, our understanding of how the endothelium regulates migration is incomplete. Humans have extensive preexisting intimal smooth muscle (ISM) in which atherosclerotic lesions develop2–4. This ISM arises during fetal development at branch points and progresses to the extent that the intima is thicker than the media in adult coronary arteries4. Locations where ISM formation initiates are subjected to disturbed flow (DF), suggesting a link between DF and ISM that is supported by experimental models of neointima formation6–8. These DF regions are also where lesions develop in human atherosclerosis and animal models3,9. Although the role of hemodynamics in atherosclerosis is well established, how flow dynamics are sensed and transduced to induce ISM formation is not fully understood. Moreover, it is not clear to what extent DF actively induces ISM formation and/or laminar flow (LF) actively represses formation.
DF and LF activate many distinct pathways in endothelial cells (ECs) to modulate transcription factor activity resulting in either an atheroprone or atheroprotective phenotype respectively10,11. One such transcription factor family regulated by hemodynamics is the Myocyte Enhancer Factor 2 (Mef2) family. The four Mef2s (Mef2a - d) are important transcriptional regulators in a number of cell types that can activate or repress transcription depending on modifications and interacting partners12–14. The most studied in vitro targets for Mef2 in ECs are Klf2 and Klf4, which regulate an anti-thrombotic and anti-inflammatory transcriptional program15–21. Two stimuli that protect against atherosclerosis increase the ability of Mef2 to activate transcription in vitro: LF and statins. LF activates through Erk5 which phosphorylates Mef2s to increase transcriptional activity and that physically interacts to function as a transcriptional coactivator16,22. LF also activates calcium-dependent phosphorylation of class II histone deacetylases (HDACs) causing their nuclear export and reducing Mef2 interaction, resulting in transcriptional de-repression23,24. Statins activate Mef2 in ECs through ERK5 activation25 and RhoA inhibition19. Pitavastatin induction of Klf4 requires Mef2a, -c, and -d, suggesting functional redundancy of these factors26. In contrast to LF, DF represses Mef2 activity. One mechanism is by DF increasing the amount and nuclear localization of class II HDACs23,24. Another is by inducing CpG methylation, which reduces Mef2 binding to the Klf4 promoter27. These in vitro studies point towards a central role for Mef2-dependent transcription in the transduction of flow dynamics in vascular endothelium; however, in vivo studies have been limited.
In vivo experiments supporting a role for Mef2 in the endothelium have been restricted to the retinal vasculature and developing embryo15,28,29. These experiments show that Mef2c is important for protection to hyperoxia and pathological angiogenesis, partly through Hmox1 regulation28. They further revealed a protective role for Mef2c in inflammation in that LPS-induced leukocyte adhesion in retinal vessels is increased in Mef2c deleted endothelium15. While deletion of Mef2c alone did not affect angiogenesis, combined endothelial-specific deletion of Mef2a and -c revealed that they regulate angiogenic sprouting and are direct transcriptional regulators of Dll4 in angiogenic tip cells29.
Here we investigated the endothelial functions of Mef2c in LF regions. Endothelial-specific Mef2c deletion resulted in marked changes in the endothelial actin cytoskeleton and SM migration. This ISM migration was through pores in the internal elastic lamina that are of the same size and shape as pre-existing fenestrations. Trans-fenestral migration of SM to populate the intima was also induced by artery flow cessation following complete carotid ligation. Our results suggest that endogenous Mef2c activity regulates the endothelial cell actin cytoskeleton and provide new in vivo evidence that endothelial MEF2 is a key transducer of flow dynamics regulating underlying medial smooth muscle migration and accumulation in the neointima.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Mef2c regulates in vitro actin stress fiber formation through a ROCK-dependent pathway
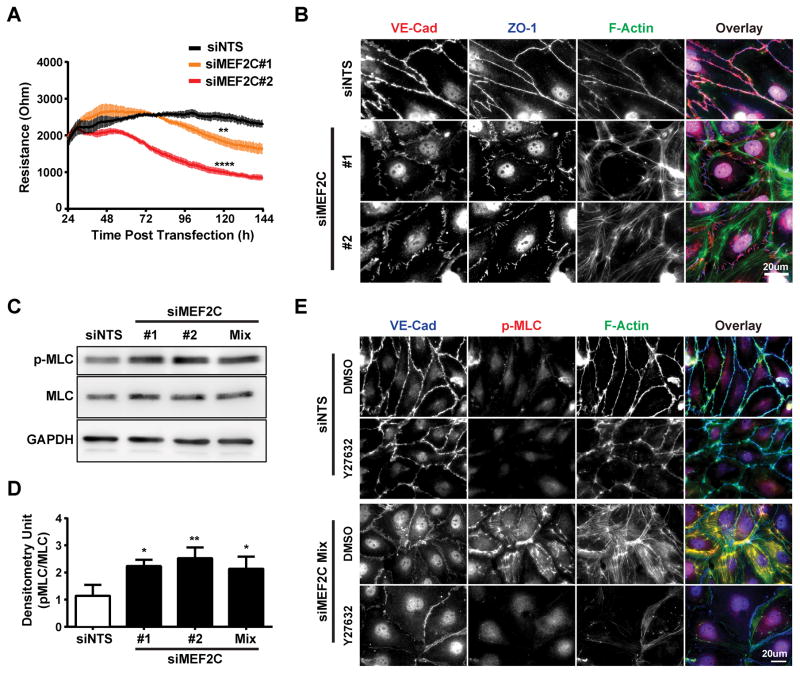
We used siRNA-directed knockdown of Mef2a, c, or -d (the major forms in ECs) to examine their individual roles in the integrity of a mature human dermal microvascular endothelial cell (HDMEC) monolayer by Electric Cell-substrate Impedance Sensing30. This showed that Mef2c knockdown reduced barrier function (Figure 1A) but knockdown of Mef2a or -d did not (Figure IC and ID in the online-only Data Supplement). Examination of these cells by immunofluorescence (IF) revealed that Mef2c knockdown caused an increase in actin stress fibers (ASF) and disruption of endothelial junctions as indicated by discontinuous ZO-1 staining (Figure 1B). Mef2a or -d knockdown had no effect on cytoskeletal organization or junctional integrity (Figure IE in the online-only Data Supplement). Because of the well-established role of regulatory Myosin Light Chain (MLC) phosphorylation in stress fiber formation, we examined its phosphorylation state by western blotting and found that Mef2c knockdown increased MLC phosphorylation (Figure 1C and 1D). Since Rho-associated protein kinase (ROCK) regulates MLC phosphorylation, we tested the ROCK inhibitor, Y-27632, and found that it blocked MLC phosphorylation and ASF formation; however, the endothelial junctions were still disrupted (Figure 1E). These data indicate that Mef2c regulates the endothelial cytoskeleton by a ROCK-dependent pathway but junctional integrity through a ROCK-independent pathway.
Figure 1. siRNA mediated Mef2c knockdown in HDMECs lead to junction disassembly and ROCK-dependent stress-fiber formation.
A, HDMECs were treated with siRNA to MEF2C (#1, #2) or non-targeting sequence (siNTS) at the time of seeding. Monolayers were allowed to mature for 24 hours before the electric resistance was measured by ECIS at 4000 Hz (n=3 independent experiments with duplicates). Results are presented as mean ± SEM, and the value recorded at 120 hours were used to perform one-way ANOVA analysis with Dunnett test to correction for multiple comparison (**, p<=0.01; ****, p<=0.0001 when compared to siNTS). B, siRNA transfected HDMECs were fixed 72 hrs post transfection and subsequently stained for VE-cadherin, ZO-1, F-actin (phalloidin) and nuclei (DAPI). Representative of two independent siRNA sequences to MEF2C (n=3 independent experiments). C, Representative western blot of HDMEC lysates 72 hrs after siRNA transfection for myosin light chain (MLC) and its di-phosphorylation (p-MLC). D, Densitometric quantification for MLC and p-MLC from the western blots in (C); the relative p-MLC levels were normalized to that of MLC. (n=3 independent experiments; *P < 0.05; ** P < 0.01 when compared with siNTS.) E, HDMECs were treated with siRNA to Mef2c as described and Y27632 or DMSO were added at 48 hours. Samples were subsequently fixed at 72 hours post transfection and stained for VE-Cadherin, p-MLC and F-actin (phalloidin). Representative of the siRNA mixture to Mef2c (n=3 independent experiments).
Endothelial-specific deletion of Mef2c induces ASFs in aortic endothelium but no change in basal barrier function
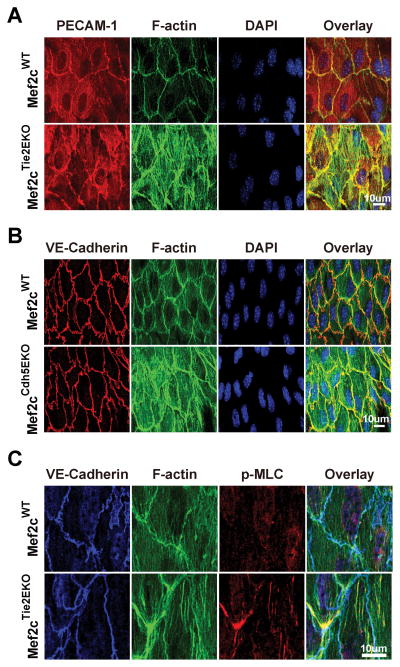
Two endothelial-specific Cre lines were used to delete Mef2c to test its function in vivo: Tie2-Cre for deletion early in vascular development, and Cdh5-Cre-ERT2 for tamoxifen-induced endothelial-specific deletion31. Deletions with Tie2-Cre are denoted as Mef2cTie2EKO, and deletions with Cdh5-Cre-ERT2 are denoted as Mef2cCdh5EKO. We have reported use of both these lines previously28,29. To assay the extent of deletion, we isolated RNA directly from aortic ECs by passing TRIzol through the lumen of the thoracic aorta and performed reverse transcriptase - quantitative PCR to the targeted exon32. Mef2c was substantially deleted in Mef2cTie2EKO and Mef2cCdh5EKO without affecting expression of Mef2a or Mef2d. Mef2b expression was too low to accurately quantify. We then performed en face IF on the aortas of Mef2c-deleted mice to investigate the consequences of Mef2c deletion on endothelial junctions and cytoskeleton. This showed normal organization of the junctions but enhanced ASFs with deletion of Mef2c either developmentally (Mef2cTie2EKO) (Figure 2A) or 2 weeks prior to analysis (Mef2cCdh5EKO) (Figure 2B). To examine ASFs further, en face IF was performed with antibodies to phospho-MLC (Figure 2C) and activated Focal Adhesion Kinase (pY397-FAK) (Figure III in the online-only Data Supplement). These showed increased phospho-MLC and pY397-FAK with deletion of Mef2c. By comparison, the aortic inner curvature, which experiences DF, has a similar increase in pY397-FAK but even more enhanced ASF and partial junctional disruption (Figure III in the online-only Data Supplement). Because in vitro knockdown and in vivo deletion of Mef2c caused similar ASF formation and enhanced MLC phosphorylation, we compared permeability in Mef2cTie2EKO and control aortas using Evan’s Blue autofluorescence. While permeability was higher in the inner-curvature than the thoracic aortas of both, it was unchanged by deletion of Mef2c (Figure IV in the online-only Data Supplement). Taken together, these data indicate that Mef2 regulates the actin cytoskeleton in the endothelium, possibly through phosphorylation and activation of FAK and MLC.
Figure 2. Endothelial Mef2c deletion leads to MLC phosphorylation and actin stress-fiber formation in the thoracic aorta.
En face preparation and immunostaining were performed on the thoracic aorta as described in methods. A, B, thoracic aortas from Mef2cTie2EKO and control Mef2cWT mice were immunostained for PECAM-1, F-actin (phalloidin), and nuclei (DAPI). B, Mef2cCdh5EKO (14-days post tamoxifen) and vehicle control were immunostained for VE-Cadherin, F-actin (phalloidin) and nuclei (DAPI); C, thoracic aortas from Mef2cTie2EKO and Mef2cWT control were immunostained for VE-Cadherin, p-MLC, and F-actin (phalloidin). Representative confocal images are shown (n=4).
Endothelial-specific deletion of Mef2c produced F-actin rich cells between the endothelium and internal elastic lamina
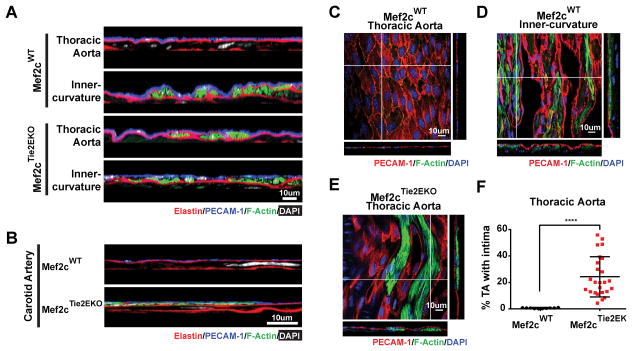
We anticipated other possible roles for Mef2c in endothelial functions that differ between LF and DF regions of the vasculature such as regulation of SM migration and proliferation. Our initial indication of a role was the observation of F-actin-rich clusters of cells located between the endothelium and the internal elastic lamina in the LF region of the Mef2cTie2EKO thoracic aortas (Figure 3A and 3E) and carotid arteries (Figure 3B) that were not found in control arteries (Figure 3A – C). These cells were aligned longitudinally in the aorta in the same direction as flow and not circumferentially as the innermost medial SM cells (Figure 3E). A similar layer of F-actin-rich cells was observed in the inner curvature of the aorta in both Mef2cTie2EKO and control mice (Figure 3A), as well as at branches into the intercostal arteries (Figure 5).
Figure 3. F-actin rich cell clusters develop in Mef2cTie2EKO between the endothelium and the internal elastic lamina.
The thoracic and inner curvature of aortas (A) or carotids (B) from Mef2cWT and Mef2cTie2EKO mice were subjected to en face preparation and labeled for elastin (hydrazide), F-actin (phalloidin), nuclei (DAPI) and PECAM-1. Y-plane confocal images revealed F-actin rich cells between the endothelium and the internal elastic lamina (n=3). C–E, The extent of F-actin rich cell coverage was investigated in en face images with PECAM-1, F-actin (phalloidin) and nuclei (DAPI) labeling of Mef2cWT thoracic aorta (C), inner curvature of Mef2cWT aorta (D), and Mef2cTie2EKO thoracic aorta (E). Representative slice of a z-stack confocal image is shown encompassing the endothelium and the actin-rich intimal cell clusters, with the corresponding x-plane and y-plane. F, The relative area covered by these F-actin labeled intimal cells was quantified on 12–15 independent fields per thoracic aorta on mice from 10 to 69 weeks of age. Results are displayed as the percentage of thoracic aorta with intimal cells (Mef2cWT, n=10; Mef2cTie2EKO, n=25; ****, p<=0.0001 by student t-test).
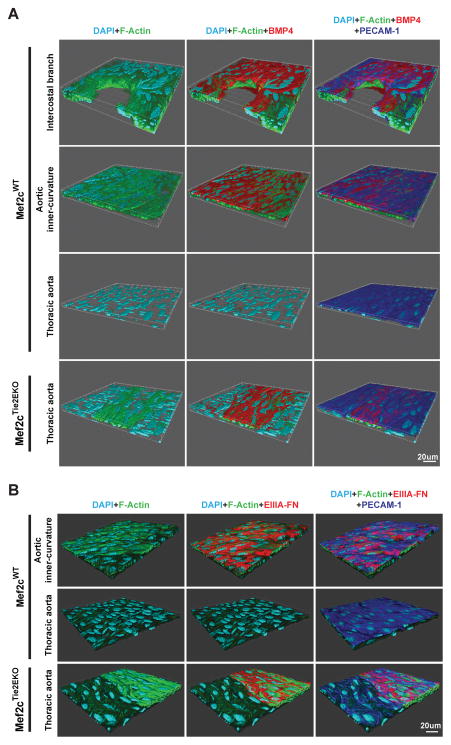
Figure 5. BMP 4 and EIIIA FN are induced in disturbed flow regions and ISM lesions of Mef2cTie2EKO mice.
En face immunofluorescence labeling of the endothelium (PECAM-1), ISM (F-actin) and BMP4 (A) or EIIIA FN (B) showed expression of BMP4 and EIIIA FN at regions of disturbed flow (intercostal branch point and aortic inner curvature) but not in regions of laminar flow (thoracic aorta) in wildtype. By contrast, BMP4 and EIIIA FN were expressed in the Mef2cTie2EKO thoracic aorta but only in regions with ISM. Representative z-stack images (n=4) are shown as 3D renders to highlight the spatial localization.
Because these intimal cells were in isolated groups, we speculated that they were clones of cells that would increase in size with age. To examine this, we isolated thoracic aortas from 10 to 69 weeks old Mef2cTie2EKO mice and measured intimal coverage by en face IF. The total area covered by F-actin-rich cells ranged up to 56% but averaged about 24% for all ages combined (Figure 3F). Contrary to expectations, the average was not significantly different between age groups (Figure V in the online-only Data Supplement), suggesting that stasis is reached by 10 weeks of age. In this restricted growth they are similar to the intimal cells of DF regions that also do not expand to form an extensive smooth muscle intima.
Intimal F-actin rich cells are not of endothelial or hematopoietic origin, express SM markers and resemble intimal SM cells located in DF regions
To determine if these intimal cells are SM, we performed IF on en face and frozen sections with the SM markers, Acta2 and Myh11; and S100a4, which is expressed in neointimal SM cells and ECs undergoing endothelial to mesenchymal transition33,34. In both the aortic inner curvature of control mice and thoracic aortas of Mef2cTie2EKO mice, F-actin rich cells co-expressed all three SM markers (Figure 4A – C). Interestingly, the intensity of IF with Acta2 and Myh11 was similar in intimal and medial cells but S100a4 was considerably higher in intimal cells, as previously reported in human and porcine intimal cells (Figure VI in the online-only Data Supplement)33. Thus, we conclude that the intimal cells located in regions of DF and in regions of LF in the Mef2cTie2EKO are SM cells, the source of which could be endothelial (by endothelial to mesenchymal transition), hematopoietic, medial vascular smooth muscle, or even adventitial progenitors.
Figure 4. Actin-rich intimal cells express smooth muscle markers and are not of endothelial or hematopoietic origin.
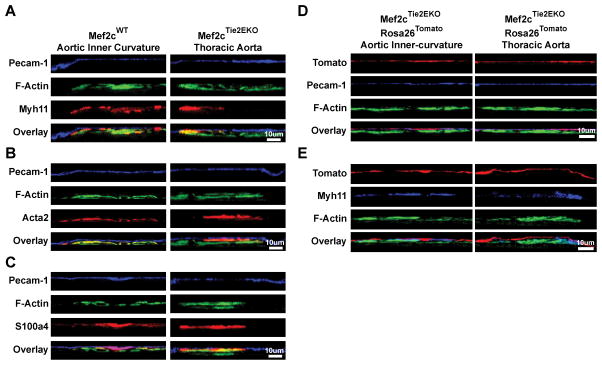
Mef2cWT aortic inner-curvature (left panel) and Mef2cTie2EKO thoracic aorta (right panel) were subjected to en face preparation, immunostained with Myh11 (A), Acta2 (B) and S100a4 (C) with co-labeling of endothelium (PECAM-1) and F-actin (phalloidin). D, Aortic inner-curvature and thoracic aorta from Mef2cTie2EKO Rosa26Tomato where endothelial and hematopoietic derived cells were labeled with tdTomato were subjected to en face preparation, and co-labeling of endothelium (PECAM-1) (D) or smooth muscle (Myh11) (E) and F-actin (phallodin). Confocal z-series were acquired and representative y-plane images were shown (n≥4).
Because Mef2c was deleted in the endothelium and it is in the pathway involved in Cerebral Cavernous Malformations35, which has aspects of endothelial to mesenchymal transition, we investigated this possibility through lineage tracing. For this we bred Tie2Cre; Mef2cF/F mice to a cre reporter, Gt(ROSA)26Sortm9(CAG-tdTomato)Hze, to mark all progeny of cells that expressed Tie2Cre with tdTomato. This marks all cells derived from Tie2Cre expressing cells in the endothelium and a large percentage of hematopoietic cells36. As expected, PECAM-1 positive ECs of the inner curvature of the aortic arch and descending thoracic aorta were tdTomato positive (Figure 4D), indicating the expected deletion in ECs. While extremely rare tdTomato positive cells were observed in medial and ISM cells, possibly from low-level leakiness of the cre, most of the intimal cells in the thoracic aorta, inner curvature, and branch points were tdTomato negative (Figure 4E). From these data, we conclude that ISM cells produced by endothelial-specific deletion of Mef2c and during normal intimal formation in DF regions are not of endothelial or hematopoietic origin.
BMP4 and EIIIA-FN are induced in regions of disturbed flow and overlying ISM in Mef2cTie2Cre mice
To explore further the correlation between the properties of the endothelium deleted of Mef2c with the endothelium in DF regions of control mice, we examined expression by en face IF of two proteins that are expressed in DF regions, BMP4 and the EIIIA splice variant of fibronectin (EIIIA-FN). BMP4 is expressed over atherosclerotic lesions in human coronaries and is highly expressed in the endothelium of DF but not LF regions of the vasculature37,38. As expected from previous reports37,38, we observed expression of BMP4 in the control endothelium of the aortic inner curvature and at branch points of the intercostal vessels in the thoracic aorta (Figure 5A). Remarkably, while BMP4 was not expressed in Mef2cTie2Cre endothelium of thoracic aortas normally, it was induced over sites with ISM (Figure 5A). The EIIIA splice form of fibronectin is induced in the endothelium by DF caused by partial carotid ligation and protects against subendothelial hemorrhage39. We found that EIIIA-FN was expressed in the inner curvature of control aortas, as predicted by induction though DF, and in Mef2cTie2Cre endothelium, but only in ECs with subjacent ISM (Figure 5B). These data indicate that endothelial deletion of Mef2c alone does not induce BMP4 or EIIIA-FN but that interaction between the endothelium and ISM recapitulates the induction that normally arises in DF regions of the vasculature.
Endothelial Mef2c regulates trans-fenestral migration of SM cells
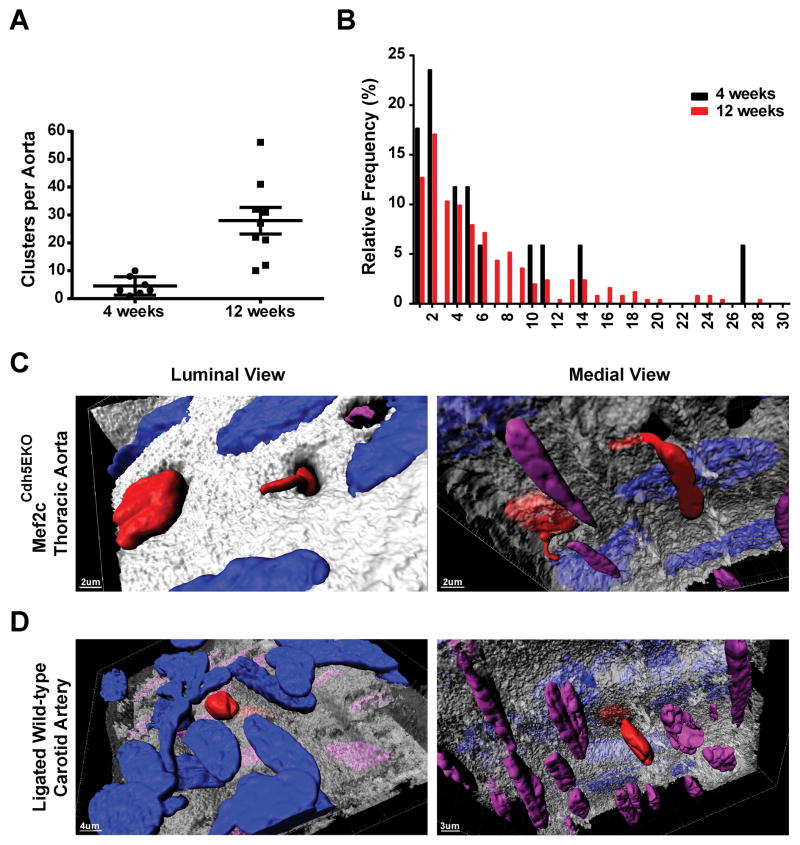
ISM in Mef2cTie2EKO aortas was usually composed of isolated clusters of cells that varied in number of cells per cluster, suggestive of an origin in either a single or small number of cells that migrated into the intima and subsequently expanded. This would be consistent with the reported clonal expansion of ISM following vascular injury40. To examine migration and expansion more carefully, we induced Mef2c deletion with tamoxifen in 3 week old Mef2cCdh5EKO mice, examined the aortas 4 and 12 weeks later by en face IF and counted the number of ISM clusters per aorta (Figure 6A) and the number of cells per cluster (Figure 6B). The number of ISM clusters observed per aorta increased from 4.6 +/− 1.3 clusters at 4 weeks to 28.0 +/− 4.8 clusters at 12 weeks, as would be expected from ongoing migration (Figure 6A). However, the distribution of cells per cluster was the same for 4 and 12 weeks with a peak frequency of only 2 cells per cluster and half of the clusters at 12 weeks containing 4 or less cells (Figure 6B). These data suggests that SM cells migrate into the intima for a substantial time following Mef2c deletion but that proliferation is very low with half of the cells undergoing 2 or fewer cell divisions.
Figure 6. Intimal smooth muscle cells from thoracic aorta of Mef2cCdh5EKO and ligated carotid artery of wild-type mice migrate through fenestrations in the internal-elastic lamina.
Mef2c deletion in Mef2cCdh5EKO Rosa26Tomato were generated by 5 daily tamoxifen injections starting at postnatal day 21. The thoracic aortas were harvested at 4 (A) or 12 (B) weeks after the start of injections. A, number of intimal smooth muscle clusters per aorta. B, Number of smooth muscle cells per ISM cluster. The frequency distribution shows that the median was 4.5 cells/cluster and the peak was 2 cells per cluster for both ages (4 weeks, n=4; 12 weeks, n=9). C, D, Thoracic aortas of Mef2cCdh5EKO Rosa26Tomato mice (C) and left carotid arteries of wild-type mice 10 days post-ligation (D) were processed for en face preparation and labeled for elastin (hydrazine), F-actin (phalloidin) and nuclei (DAPI). Confocal z-series were acquired and representative z-series were 3D rendered (Imaris, Bitplane) (n=7 for Mef2cCdh5EKO thoracic aorta, and n=5 for wild-type ligated carotid). The identity of the nuclei was determined by co-localization with tdTomato signal (endothelium nuclei, blue), co-localization with F-actin (not shown in rendering) but above the internal elastin lamina (intimal smooth muscle cell nuclei, red), or underneath the internal elastin lamina (media smooth muscle nuclei, purple).
Visualizing the internal elastic lamina with Alexa Fluor 633 hydrazine41,42 and nuclei with DAPI facilitated identifying migrating SM through visualizing nuclei passing through the internal elastic lamina of Mef2cCdh5EKO mice. This showed that SM cells migrated through pores in the internal elastic lamina (Figure 6C) that were of similar size and structure as the fenestrations through which myoendothelial junctions form41,43,44. This prompted us to investigate if SM similarly migrates through fenestrations in a mouse model of vascular injury7,45. To test this, we ligated the left common carotid artery of mice and examined migration by en face 10 days after ligation. As with Mef2c deletion, ligation of the carotid artery caused migration of SM through fenestrations in the internal elastic lamina (Figure 6D). Thus, endothelial deletion of Mef2c or disrupting blood flow both caused trans-fenestral SM migration.
Transcriptome analysis to identify Mef2c-dependent genes
To identify Mef2c-dependent genes in the endothelium that regulate smooth muscle migration, we performed transcriptome analysis with the GeneChip Mouse Transcriptome Assay 1.0 (Affymetrix). We identified only 37 differentially expressed genes using a cutoff of 1.8 fold change and P<0.05. A mechanistically promising differentially expressed gene is the neuronal guidance molecule, Sema3d. Decreased expression of Sema3d with Mef2c deletion was validated by RT-QPCR on RNA from Mef2cCdh5EKO and Mef2cTie2EKO aortas (GEO accession number GSE97089 and Figure VII in the online-only Data Supplement). Of note, we did not detect expression changes in the reported MEF2 target genes Klf2 and Klf4, or of reported regulators of smooth muscle migration with the exception of Nos3 reduction in Mef2cTie2EKO aortas only, which may be the result of long-term Mef2c deficiency (Figure VII in the online-only Data Supplement)16,26,27,46–48.
DISCUSSION
Endothelial-specific deletion of Mef2c revealed that it regulates the endothelial actin cytoskeleton and migration of SM into the intima. In vitro, knockdown of Mef2c caused MLC phosphorylation and ASF formation in a ROCK-dependent manner. Interestingly, knockdown also led to junctional disassembly resulting in increased permeability that was not ROCK-dependent, suggesting a separate pathway for junctional regulation (Figure 1). In vivo deletion also resulted in ASF formation, and MLC and FAK phosphorylation, but did not alter endothelial junctions or increase aortic permeability (Figures 2 and IV in the online-only Data Supplement). These differences with the in vitro results for the junctional role of Mef2c could reflect in vivo compensation by the other MEF2 proteins or the distinct environments of blood vessels and in vitro culture. Compensation may also explain the unchanged expression of presumptive Mef2 targets (Klf2, Klf4) by Mef2c deletion (Figure VII in the online-only Data Supplement). Although determining the mechanism by which Mef2c regulates the cytoskeleton will require further research, a cytoskeletal role of Mef2c may relate to the structure of the cytoskeleton in DF regions, which exhibited enhanced actin cytoskeleton, stress fibers, and permeability (Figures III and IV in the online-only Data Supplement). Because Mef2c is less active under DF23,24, we speculate that Mef2c-dependent genes regulating the cytoskeleton under LF are downregulated in DF regions to contribute to the cytoskeletal phenotype of these regions.
Another similarity between DF regions and Mef2c deletion is expression of BMP4 and EIIIA-FN37–39. These differ in that endothelial-specific deletion of Mef2c in itself does not cause induction of these genes, which were only induced where there is contact between the Mef2c-deleted endothelium and ISM (Figure 5). This indicates that interaction between ECs and the subjacent ISM may induce these genes in addition to DF. Whether these changes would promote atherosclerotic lesion development is an interesting question for further research.
A role for endothelial Mef2c in SM migration into the intima is a major result revealed by Mef2c deletion. Another finding is that Mef2c deletion causes SM cells migration through pores in the IEL that are of the same size and structure as pre-existing fenestrations (Figure 6C)43. We also observed trans-fenestral migration in the carotid artery following carotid ligation (Figure 6D), and it has been reported in macaque iliac artery denuded of endothelium49, suggesting a similar mode of migration whether the endothelium is dysfunctional or removed. We speculate that the difficulty of migrating through small fenestrations may at least partially explain the small number of neointimal progenitor cells in mouse models40. However, this cannot be the sole reason because we observed large numbers of fenestrations not associated with ISM. Interestingly, the size of fenestrations is such that migration through them would require nuclear envelope breakage to allow nuclear passage and possibly induce DNA damage50. It is interesting to speculate that nuclear envelope breakage during passage through fenestrations could in itself lead to changes in gene expression that affect the differentiation state of neointimal SM cells.
ISM produced by postnatal Mef2c deletion through Cdh5-Cre-ERT2 appear to replicate at a lower rate than ISM arising from injury such as carotid ligation in that the most frequent number of cells in an ISM cluster is only 2 even 12 weeks after inducing deletion (Figure 6B). However, deletion with Tie2-Cre produced much more extensive ISM coverage with an average of 24% coverage and very large ISM clusters (Figure 3F). This is probably a consequence of embryonic deletion when cells are highly proliferative. For both Cre lines, these cells retain SM markers such as Myh11 that are usually downregulated in neointimal SM, indicating that these cells are differentiated SM (Figure 4). Thus endothelial Mef2c deletion separates SM migration from differentiation and proliferation. As such, endothelial deletion of Mef2c provides a novel model system to explore events in SM differentiation and proliferation after migration into the intima.
At first consideration, the relatively small number of ISM cells produced by postnatal Mef2c deletion (Figure 6A and B) would not appear to be relevant to vascular disease where the neointima is composed of thousands of cells. However, a recent publication of an elegant clonal analysis of neointimal cells shows that only a small number of cells produce the neointima through extensive clonal replication in murine atherosclerotic plaques or following carotid ligation40. The numbers of individual ISM clusters produced by Mef2c deletion (Figure 6A) was of similar or greater magnitude to the number of progenitor cells in an atherosclerotic lesion or carotid neointima and would thus be sufficient to produce an extensive neointima if these ISM cells were highly proliferative as in the carotid neointima where the median clonal patch size is 435 cells 4 weeks after injury40. By contrast, Mef2c deletion produces intimal cell clusters with a median of 4.5 cells after 12 weeks (Figure 6B). We therefore speculate that endothelial Mef2c activity may be reduced in vascular disease to permit SM migration into the intima but that additional factors regulate proliferation of these ISM cells.
Based on our results and published reports, we propose (Figure VIII in the online-only Data Supplement) that Mef2c functions as an endothelial transcriptional activator to suppress SM migration in LF regions whereas in DF regions Mef2c activity is repressed to permit SM migration. How might endothelial Mef2c regulate SM migration? A possible mechanism is through reduced expression of Sema3d, which is decreased in both Mef2cCdh5EKO and Mef2cTie2EKO (GEO accession number GSE97089 and Figure VII in online-only Data Supplement). It is a secreted, repulsive molecule for neurons, ECs, and other cell types that signals through two neuropilin co-receptors (Nrp1 and Nrp2) in combination with any of 4 Plexin A receptors51–53. Interestingly, the neuropilin receptors are in SM where they are co-receptors for Pdgfrβ and required for neointimal formation54–56. Therefore, Sema3d may prevent SM migration into the intima either directly by repulsion through Nrp/Plxna signaling or indirectly through inhibiting Pdgfb signaling by competition with neuropilin/Pdgfrβ similar to how Sema3f interferes with VEGF signaling57. We hypothesize that reduced Sema3d caused by Mef2c deletion reduces this inhibition to allow SM migration.
Supplementary Material
HIGHLIGHTS.
Endothelial-specific deletion of Mef2c induced actin stress fibers in endothelial cells.
Embryonic, endothelial-specific deletion of Mef2c resulted in extensive regions of intimal smooth muscle in the thoracic aorta and carotid arteries.
Inducible, endothelial-specific postnatal deletion of Mef2c revealed smooth muscle migration through fenestrations in the internal elastic lamina.
Acknowledgments
We gratefully acknowledge Dr. Livingston Van De Water for antibody to EIIIA-FN. Confocal imaging was performed in the Albany Medical College Imaging Core Facility.
SOURCES OF FUNDING
American Heart Association Scientist Development Grants (13SDG17100110 to A.P.A.); (12SDG12050083 to G.D.). National Heart, Lung, and Blood Institute (HL092510 and HL49426 to H.A.S); (HL118245 to G.D.).
Nonstandard Abbreviations and Acronyms
- ASF
actin stress fibers
- DF
disturbed flow
- EC
endothelial cell
- HDAC
Histone deacetylase
- ISM
intimal smooth muscle
- LF
laminar flow
- MEF2
myocyte enhancer factor 2
- MLC
myosin light chain
- ROCK
Rho-associated protein kinase
- SM
smooth muscle
Footnotes
DISCLOSURES
None.
References
- 1.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circulation research. 2007;100(5):607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(5):1159–1165. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovascular research. 2008;79(1):14–23. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima Y, Chen Y-X, Kinukawa N, Sueishi K. Distributions of diffuse intimal thickening in human arteries: preferential expression in atherosclerosis-prone arteries from an early age. Virchows Archiv: an international journal of pathology. 2002;441(3):279–288. doi: 10.1007/s00428-002-0605-1. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 6.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(12):2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arteriosclerosis, thrombosis, and vascular biology. 1997;17(10):2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 8.Hui DY. Intimal hyperplasia in murine models. Current drug targets. 2008;9(3):251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arteriosclerosis, thrombosis, and vascular biology. 1998;18(5):842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 10.Gimbrone MA, Jr, García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circulation research. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill PA, Redmond EM. Vascular endothelium - Gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canté-Barrett K, Pieters R, Meijerink JPP. Myocyte enhancer factor 2C in hematopoiesis and leukemia. Oncogene. 2014;33(4):403–410. doi: 10.1038/onc.2013.56. [DOI] [PubMed] [Google Scholar]
- 13.Clark RI, Tan SWS, Péan CB, Roostalu U, Vivancos V, Bronda K, Pilátová M, Fu J, Walker DW, Berdeaux R, Geissmann F, Dionne MS. MEF2 is an in vivo immune-metabolic switch. Cell. 2013;155(2):435–447. doi: 10.1016/j.cell.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134(23):4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Yoshida T, Wu L, Maiti D, Cebotaru L, Duh EJ. Transcription factor MEF2C suppresses endothelial cell inflammation via regulation of NF-κB and KLF2. Journal of cellular physiology. 2015;230(6):1310–1320. doi: 10.1002/jcp.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. The Journal of clinical investigation. 2006;116(1):49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, García-Cardeña G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. The Journal of biological chemistry. 2005;280(29):26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 18.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr, Balasubramanian V, García-Cardeña G, Jain MK. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circulation research. 2005;96(5):e48–57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 19.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112(5):720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 20.Atkins GB, Jain MK. Role of Krüppel-like transcription factors in endothelial biology. Circulation research. 2007;100(12):1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 21.Sangwung P, Zhou G, Nayak L, et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI insight. 2017;2(4):e91700. doi: 10.1172/jci.insight.91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villarreal G, Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, García-Cardeña G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochemical and biophysical research communications. 2010;391(1):984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin Z-G. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115(14):2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee D-Y, Lee C-I, Lin T-E, Lim SH, Zhou J, Tseng Y-C, Chien S, Chiu J-J. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(6):1967–1972. doi: 10.1073/pnas.1121214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le N-T, Takei Y, Izawa-Ishizawa Y, Heo K-S, Lee H, Smrcka AV, Miller BL, Ko KA, Ture S, Morrell C, Fujiwara K, Akaike M, Abe J-I. Identification of activators of ERK5 transcriptional activity by high-throughput screening and the role of endothelial ERK5 in vasoprotective effects induced by statins and antimalarial agents. Journal of immunology. 2014;193(7):3803–3815. doi: 10.4049/jimmunol.1400571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maejima T, Inoue T, Kanki Y, et al. Direct evidence for pitavastatin induced chromatin structure change in the KLF4 gene in endothelial cells. PloS one. 2014;9(5):e96005. doi: 10.1371/journal.pone.0096005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y-Z, Jiménez JM, Ou K, McCormick ME, Zhang L-D, Davies PF. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circulation research. 2014;115(1):32–43. doi: 10.1161/CIRCRESAHA.115.303883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Gong J, Maiti D, Vong L, Wu L, Schwarz JJ, Duh EJ. MEF2C ablation in endothelial cells reduces retinal vessel loss and suppresses pathologic retinal neovascularization in oxygen-induced retinopathy. The American journal of pathology. 2012;180(6):2548–2560. doi: 10.1016/j.ajpath.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacilotto N, Chouliaras KM, Nikitenko LL, et al. MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes & development. 2016;30(20):2297–2309. doi: 10.1101/gad.290619.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. The Journal of biological chemistry. 2010;285(10):7045–7055. doi: 10.1074/jbc.M109.079277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sörensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113(22):5680–5688. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 32.Nam D, Ni C-W, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. American journal of physiology. Heart and circulatory physiology. 2009;297(4):H1535–43. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brisset AC, Hao H, Camenzind E, Bacchetta M, Geinoz A, Sanchez J-C, Chaponnier C, Gabbiani G, Bochaton-Piallat M-L. Intimal smooth muscle cells of porcine and human coronary artery express S100A4, a marker of the rhomboid phenotype in vitro. Circulation research. 2007;100(7):1055–1062. doi: 10.1161/01.RES.0000262654.84810.6c. [DOI] [PubMed] [Google Scholar]
- 34.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer research. 2007;67(21):10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 35.Cuttano R, Rudini N, Bravi L, Corada M, Giampietro C, Papa E, Morini MF, Maddaluno L, Baeyens N, Adams RH, Jain MK, Owens GK, Schwartz M, Lampugnani MG, Dejana E. KLF4 is a key determinant in the development and progression of cerebral cavernous malformations. EMBO molecular medicine. 2015 doi: 10.15252/emmm.201505433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48(9):563–567. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116(11):1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 38.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. The Journal of biological chemistry. 2003;278(33):31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 39.Murphy PA, Hynes RO. Alternative splicing of endothelial fibronectin is induced by disturbed hemodynamics and protects against hemorrhage of the vessel wall. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(9):2042–2050. doi: 10.1161/ATVBAHA.114.303879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jørgensen HF. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circulation research. 2016;119(12):1313–1323. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straub AC, Butcher JT, Billaud M, Mutchler SM, Artamonov MV, Nguyen AT, Johnson T, Best AK, Miller MP, Palmer LA, Columbus L, Somlyo AV, Le TH, Isakson BE. Hemoglobin α/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(12):2594–2600. doi: 10.1161/ATVBAHA.114.303974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clifford PS, Ella SR, Stupica AJ, Nourian Z, Li M, Martinez-Lemus LA, Dora KA, Yang Y, Davis MJ, Pohl U, Meininger GA, Hill MA. Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(12):2889–2896. doi: 10.1161/ATVBAHA.111.236570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandow SL, Gzik DJ, Lee RMKW. Arterial internal elastic lamina holes: relationship to function? Journal of anatomy. 2009;214(2):258–266. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: connections that deliver the message. Physiology. 2014;29(4):242–249. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saddouk FZ, Sun L-Y, Liu YF, Jiang M, Singer DV, Backs J, Van Riper D, Ginnan R, Schwarz JJ, Singer HA. Ca2+/calmodulin-dependent protein kinase II-γ (CaMKIIγ) negatively regulates vascular smooth muscle cell proliferation and vascular remodeling. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015 doi: 10.1096/fj.15-279158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavin B, Gómez M, Pello OM, Castejon B, Piedras MJ, Saura M, Zaragoza C. Nitric oxide prevents aortic neointimal hyperplasia by controlling macrophage polarization. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(8):1739–1746. doi: 10.1161/ATVBAHA.114.303866. [DOI] [PubMed] [Google Scholar]
- 47.Anggrahini DW, Emoto N, Nakayama K, Widyantoro B, Adiarto S, Iwasa N, Nonaka H, Rikitake Y, Kisanuki YY, Yanagisawa M, Hirata K-I. Vascular endothelial cell-derived endothelin-1 mediates vascular inflammation and neointima formation following blood flow cessation. Cardiovascular research. 2009;82(1):143–151. doi: 10.1093/cvr/cvp026. [DOI] [PubMed] [Google Scholar]
- 48.Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 49.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 50.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352(6283):353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Degenhardt K, Singh MK, Aghajanian H, Massera D, Wang Q, Li J, Li L, Choi C, Yzaguirre AD, Francey LJ, Gallant E, Krantz ID, Gruber PJ, Epstein JA. Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nature medicine. 2013;19(6):760–765. doi: 10.1038/nm.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aghajanian H, Choi C, Ho VC, Gupta M, Singh MK, Epstein JA. Semaphorin 3d and semaphorin 3e direct endothelial motility through distinct molecular signaling pathways. The Journal of biological chemistry. 2014;289(26):17971–17979. doi: 10.1074/jbc.M113.544833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nature reviews. Cancer. 2008;8(8):632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 54.Movassagh H, Shan L, Halayko AJ, Roth M, Tamm M, Chakir J, Gounni AS. Neuronal chemorepellent Semaphorin 3E inhibits human airway smooth muscle cell proliferation and migration. The Journal of allergy and clinical immunology. 2014;133(2):560–567. doi: 10.1016/j.jaci.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Yamaji M, Mahmoud M, Evans IM, Zachary IC. Neuropilin 1 is essential for gastrointestinal smooth muscle contractility and motility in aged mice. PloS one. 2015;10(2):e0115563. doi: 10.1371/journal.pone.0115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pellet-Many C, Frankel P, Evans IM, Herzog B, Jünemann-Ramírez M, Zachary IC. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochemical Journal. 2011;435(3):609–618. doi: 10.1042/BJ20100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo H-F, Li X, Parker MW, Waltenberger J, Becker PM, Vander Kooi CW. Mechanistic basis for the potent anti-angiogenic activity of semaphorin 3F. Biochemistry. 2013;52(43):7551–7558. doi: 10.1021/bi401034q. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.