Abstract
Introduction
Noise-induced hearing loss (NIHL) due to industrial, military, and recreational noise exposure is a major, but also potentially preventable cause of acquired hearing loss. For the United States it is estimated that 26 million people (15% of the population) between the ages of 20 and 69 have a high-frequency NIHL at a detriment to the quality of life of the affected individuals and great economic cost to society.
Areas covered
This review outlines the pathology and pathophysiology of hearing loss as seen in humans and animal models. Results from molecular studies are presented that have provided the basis for therapeutic strategies successfully applied to animals. Several compounds emerging from these studies (mostly antioxidants) are now being tested in field trials.
Expert opinion
Although no clinically applicable intervention has been approved yet, recent trials are encouraging. In order to maximize protective therapies, future work needs to apply stringent criteria for noise exposure and outcome parameters. Attention needs to be paid not only to permanent NIHL due to death of sensory cells but also to temporary effects that may show delayed consequences. Existing results combined with the search for efficacious new therapies should establish a viable treatment within a decade.
Keywords: Antioxidants, hair cells, magnesium, N-acetylcysteine, neurotrophins, noise trauma, oxidative stress, synaptopathy, vasoconstriction
1. Introduction
At least since the introduction of modern warfare and the industrial revolution, exposure to noise has been recognized as a potential cause of hearing impairment. Today, approximately 5% of the world’s population suffer from noise-induced hearing loss (NIHL) acquired from industrial occupations, military duty and combat, and recreation and leisure activities. This makes NIHL the most frequent occupational disease in the US and probably worldwide1.
Normal human conversation is conducted at around 60 decibels (dB), a logarithmic unit of sound pressure level (SPL), and the dynamic range of our auditory systems enables us to process sounds at both much lower and higher levels. However, beginning at 85 dB (~300 times the energy level of 60 dB) long or repeated exposures may result in a notable loss of hearing. This level of 85 dB and above includes some everyday sounds, for example, music on personal listening devices or emanating from small machinery such as lawnmowers. Chainsaws and other power tools may produce sound intensities of 120 dB and above, potentially causing immediate hearing damage. Firearms which are owned by around 60 million Americans can produce 140–170 dB of impact noise which, without the proper use of ear protectors, would lead to certain damage of the inner ear.
In addition to NIHL, acquired hearing loss can be of numerous other etiologies, such as the use of ototoxic medications, bacterial or viral ear infections, head injuries, and the aging process. Importantly, these effects may overlap and thereby confound investigations in both animal models and patients. In this review we will first discuss basic mechanisms underlying NIHL in well-defined experimental models as such studies provide the basis for protective strategies. We will then review progress towards a clinical management which is promising although a viable treatment to prevent or attenuate noise trauma is yet on the horizon. A comparison of this summary with an assessment written six years ago will illuminate progress made and problems still unresolved2.
2. Pathology
2.1 Pathophysiology
The classical functional measurement of NIHL in humans is an audiogram reading of auditory thresholds. By this measure, noise-induced hearing loss (NIHL) can be divided into two subtypes, temporary and permanent hearing loss that each have specific cochlear pathology. If a noise-induced elevation of thresholds recovers completely to pre-exposure levels within hours or days, the subject experiences a temporary threshold shift (TTS). Without recovery, a permanent threshold shift (PTS) ensues which is related to the intensity and frequency of exposure. The human audiogram of noise trauma is often characterized by a sharp dip between 3 kHz and 6 kHz, within the range of speech frequency. PTS therefore generally affects speech perception and, in extreme exposure situations, may include auditory damage up to complete deafness3.
2.2 Pathology of hair cells and auditory nerve
Auditory processing in the cochlea depends on the integrity of the sensory hair cells - inner hair cells (IHC) and outer hair cells (OHC) - and the auditory nerve connecting them to higher centers. Animal studies suggest that TTS does not involve major overt pathology to these structures. Temporary swelling, fusion, or distortion of stereocilia, however, may impair mechanotransduction necessary for hair cell depolarization4, 5 and reversible excitotoxic effects have been detected at IHC synapses in the form of swelling and retraction of dendrites6, 7. More recently, studies in a mouse model have uncovered delayed manifestations of neural degeneration of IHC synaptic ribbons and accelerated spiral ganglion cell (SGC) loss after TTS, despite the early reversibility of threshold shifts and the absence of appreciable hair cell loss8, 9. This observation has challenged the traditional view that degeneration of SGCs occurs exclusively as a consequence of sensory cell loss. Although longitudinal data on the impact of TTS on humans is lacking, we may soon begin to realize the impact of repeated recreational noise exposure on age-related hearing impairment.
Aside from the possibility of direct mechanical damage to the eardrum and the inner ear by high-intensity impulse noise10, a characteristic feature of PTS is loss of cochlear hair cells (fig.1) and loss of IHC synaptic ribbons11. The afferent terminals of IHCs become swollen and degenerate, followed by the degeneration of the afferent fibers and SGCs. Additionally, spiral ganglion neurons display a subset of swollen satellite cells. The extent of cell damage may increase for days or weeks after the cessation of the noise exposure while degenerations of nerve fibers tends to proceed on a much slower time scale of months (in experimental animals) to years (in humans). Since sensory hair cells in mammals do not regenerate, the loss is irreversible and—at the moment and the near future—not curable.
Figure 1.
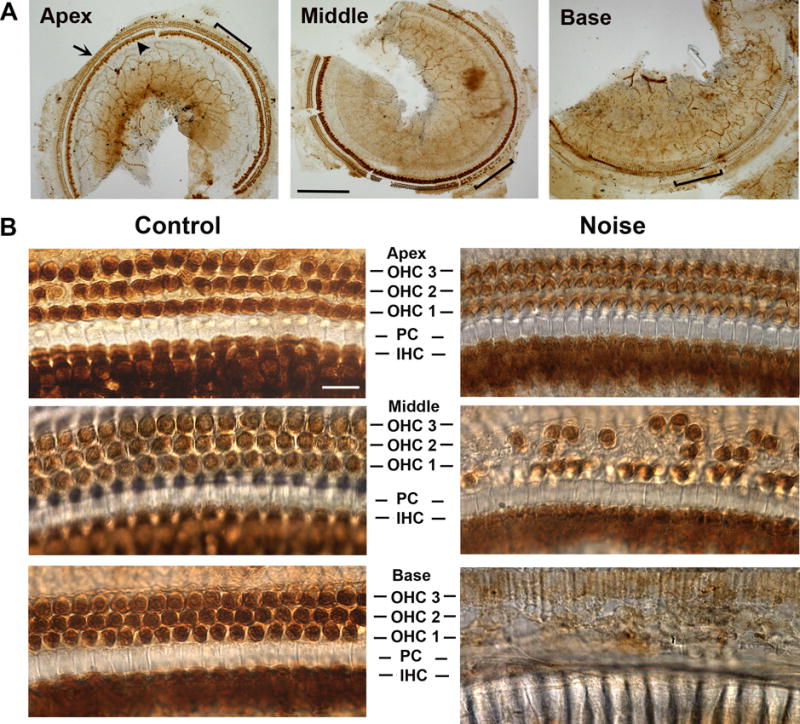
Hair cell loss in the cochlea of 12-week-old male CBA/J mice exposed to noise calibrated to yield PTS (broadband noise at 108 dB SPL for 2 h) assessed two weeks after the exposure. (a) A surface preparation of the cochlea was immunolabeled for Myosin VII and then stained with DAB. Images were taken along the entire length of the cochlear epithelium from the apex to the base. Staining outlines the outer hair cells (arrow in panel ‘Apex’) and the region of inner hair cells (arrowhead). The bracketed areas are shown in higher magnification in panel B (‘Noise’). Scale bar = 100 μm. (b) The comparison of corresponding images on the left and right illustrates the base-to-apex pattern of hair cell loss. Controls without noise exposure reveal three rows of intact outer hair cells (OHC), one row of inner hair cells (IHC) and pillar cells (PC). Following noise exposure, hair cells remain largely intact in the apex, sporadic losses occur in the middle segment, and complete destruction of the hair cells is evident in the base. Scale bar = 10 μm.
Both animal models and human studies agree on hair cells as a target of noise trauma. Schuknecht’s study of temporal bones of human subjects12 noted that loss of OHCs at the basal turn of the cochlea was the most prominent change, while loss of IHCs was limited. However, with increasing noise intensity, destruction of IHCs followed11. In contrast to the vulnerability of sensory hair cells, supporting cell structures remain generally well-preserved unless under severe noise conditions.
2.3 Pathology in non-sensory tissues
Vasoconstriction and capillary loss in the spiral ligament, as well as decreased strial blood flow, have been detected in noise-exposed animals13, 14. These changes may transiently alter the endocochlear potential, elevating auditory threshold shifts and contributing to TTS or PTS. Severe exposures may cause permanent degeneration of the fibrocytes of the spiral limbus and spiral ligament, of which type II and IV fibrocytes are the most vulnerable11. Additionally, the stria vascularis may swell acutely and subsequently shrink due to loss of intermediate and marginal cells.
3. Molecular mechanisms of neurosensory damage
Essentially all information on the molecular events underlying NIHL comes from animal experimentation. The use of different animal species and noise conditions have provided a plethora of mostly consistent results, but at times also observations that are difficult to reconcile or to categorize as causal or epiphenomenal. The following sections will summarize the most prominent current hypotheses with a focus on those that have given rise to attempts at therapeutic protection.
3.1 Oxidative stress and stress-induced pathways
The mitochondrial electron transport chain is considered a major source of reactive oxygen species (ROS) produced by all tissues including those of the cochlea. Under normal physiological conditions, 1–2% of molecular oxygen is reduced to superoxide, a free radical that can be converted to less toxic hydrogen peroxide by mitochondrial superoxide dismutase (SOD2) or proceed in a reaction chain to even more detrimental compounds such as the hydroxyl radical. Increased ROS generation in cochlear fluids and tissues, including in OHCs and the stria vascularis, by traumatic noise is well documented and other free radicals in the form of reactive nitrogen species (RNS) derived from nitric oxide (NO) are also present15–17. ROS may persist for 7–10 days and spread apically from the basal end of the organ of Corti, thus widening the area of damage well after an exposure has been terminated. Likewise, peroxynitrite (ONOO-), generated by the combination of NO and superoxide, has been found in the cochlea several days after noise exposure, underscoring the case for multiple oxidants contributing to hair cell death. The delayed spread of injury is an important feature of NIHL as it might provide a window of opportunity for post-exposure intervention and containment of the extent of hearing loss.
On the other hand, moderate noise exposure increased ROS in OHCs only marginally thus prompting autophagy, a cellular homeostatic response17. The autophagy marker LC3 was upregulated in OHCs of CBA/J mice, and the fact that only a TTS but no permanent damage was observed, suggested autophagy as a protective mechanism against NIHL. However, excessive oxidative stress, as under PTS-noise conditions, overwhelmed the beneficial potential of autophagy in OHCs and led to OHC death and NIHL17.
Cell death in the cochlea displays features of both apoptosis and necrosis as well as necroptosis, a necrotic-like process. This assessment is based on morphological and molecular features where apoptotic cell death is actively regulated and characterized by condensed nuclei with activation of multiple cell death pathways that are primarily regulated by caspases18–20. In addition, noise exposure causes the release of cytochrome C and the translocation of endonuclease G in apoptotic OHCs19–21. Swollen nuclei, a marker of necrotic cell death, in OHCs after noise exposure indeed suggest involvement of necrosis18. Recent studies have added necroptosis to this spectrum in which signaling pathways like that of the receptor-interacting protein (RIP) kinases are induced, followed by caspase activation. Caspases 3, 8, and 9 have been found in OHCs with condensed nuclei after noise exposure18, 22. While an intervention into a cell death sequence may attenuate noise trauma, pathway interactions add a layer of complexity: inhibition of noise-induced apoptosis shifts the prevalence of OHC death to necrosis18.
3.2 Calcium overload
Another consequence of noise exposure is an increase in free Ca2+ in OHCs immediately after acoustic overstimulation to which both entry through ion channels and liberation from intracellular stores might contribute23,24. Influx through channels may be linked to increased endolymphatic calcium levels which increased 50-fold in the noise-exposed guinea pig. This generates a high concentration gradient driving calcium into sensory cells through voltage-gated calcium channels (VGCCs) or mechanoelectrical transduction (MET) channels24. Noise exposure at 110 dB to guinea pigs increased free calcium in isolated OHCs followed by OHC death and hearing loss while a non-pathologic sound level of 75 dB did not alter intracellular calcium levels25. Noise trauma induced by a 130 dB exposure was likewise accompanied by a sustained 60% increase in free calcium in guinea pig OHCs and a decrease in cochlear microphonics.
Calcium overload can trigger cell death in any cell type by the activation of specific calcium-dependent pathways. In addition, in IHC it can cause synaptopathy by stimulating the excessive release of glutamate neurotransmitter. The over-activation of glutamate receptors on the post-synaptic terminals can cause excitotoxicity and swelling of the nerve terminals, resulting in anatomical and functional deficits.
An involvement of calcium channels is corroborated by the fact that channel blockers attenuate NIHL. The L-type is the predominant VGCC in IHCs but is also present in OHCs26 and under normal conditions a block of this channel reduces the calcium content of guinea pig IHCs and OHCs27. Furthermore, several L-type channel inhibitors (diltiazem, verapamil, nicardipine, and nimodipine) reduce the amount of hair cell loss and auditory threshold shifts following noise exposure in female ddY mice28. Guinea pig OHCs can likewise be protected from acute noise damage by delivery of diltiazem29. T-type channel blockers also offer protection. After noise exposure of C57BL/6 mice at 110 dB trimethadione protects against both TTS and PTS hearing loss and hair cell death, while ethosuximide protects against PTS30.
3.3 Mitochondrial pathways
Mitochondrial dysfunction has been speculated to be the major mechanism of NIHL and involved in other acquired hearing pathologies as well31. Indispensible as the cellular energy factory they are also the major site of ROS formation. As discussed in Section 3.1, noise exposure causes ROS generation in cochlear tissues and inner ear fluids, and oxidative damage is observed in sensory cells. A related issue is transient cellular energy depletion after noise exposure.
A major energy expenditure of cochlear tissues is the maintenance of ionic gradients across cell membranes. In order to sustain the necessary level of ATP, cochlear blood flow must provide an adequate supply of oxygen and nutrients. However, high-intensity noise exposure decreases capillary blood flow and causes local vasoconstriction32, 33. The resulting ischemia reduces ATP levels within the inner ear, including the lateral wall structures which are responsible for upholding the endocochlear potential19, 34–36. Consequently, a reduction of ATP levels in the cochlea is associated with noise-induced permanent hearing loss19, 34, 35. Conversely, maintenance of ATP levels by supplying creatine as an energy source attenuates temporary and permanent NIHL in guinea pigs37. Creatine kinase, the enzyme required for the utilization of creatine as an ATP buffer, is abundant in the stria vascularis; hence, creatine treatment may maintain the endocochlear potential by supplying ATP for ionic pumps38.
A potential pathway from ATP depletion to cell death may involve the downstream actions of adenosine monophosphate-activated protein kinase (AMPK). As a homeostatic energy sensor AMPK reacts to negative fluctuations of the AMP:ATP ratio by switching off energy-consuming activities and turning on energy-generating pathways39. While the activation of AMPK to phospho-AMPK initially is an adaptive response to cellular stress, its sustained activation in a noise-dependent manner has a detrimental effect on sensory hair cells40, similar to responses in other cellular stress models, including ischemia-reperfusion, hypoxia, and stroke41, 42. The prolonged elevation of phospho-AMPK activates c-Jun N-terminal kinase (JNK), upregulates pro-apoptotic Bim and subsequent apoptotic cell death pathways known to be involved in NIHL18, 43, 44. Consequently, inhibition of AMPK activation by siRNA silencing or the specific pharmacological inhibitor dorsomorphin attenuates NIHL and cochlear synaptopathy40. In addition, silencing LKB1, a kinase upstream of AMPKα, also protects against these pathologies40.
4. Pharmaceutical intervention in animal models
The wide array of presumed mechanisms of NIHL has suggested the notion of a convergence of many factors leading to cell death. Consequently, a wide array of agents has been tested for their protective efficacy in animals, including antioxidants, adenosine receptor antagonists, calcium-channel blockers, NMDA receptor antagonists, and inhibitors of apoptotic signaling. This list is not nearly exhaustive. By the year 2005 at least 28 drugs had already been tested45.
4.1.1 Antioxidants and related compounds
Antioxidants, such as glutathione (GSH)46, 47, D-methionine48, ebselen49, resveratrol50, ascorbic acid51, 52, and water-soluble coenzyme Q1053, all attenuated NIHL in animal models when applied prior to noise exposure. Treatments up to 3 days after exposure were also able to attenuate NIHL to some degree, particularly A1 adenosine receptor agonists54, ferulic acid55, D-methionine48, or the combined administration of the ROS and RNS scavengers salicylate and trolox56. Among antioxidants, N-acetylcysteine (NAC) has probably been the most extensively evaluated for reducing noise trauma under a variety of conditions, animal models, and dosages57. The diverse experimental parameters preclude direct comparisons of individual studies and make it difficult to establish a single efficacious treatment modus58, 59. However, NAC has exhibited protective effects not only when given prior to noise60, 61 but it also rescued from NIHL after exposure62. In surprising contrast, some studies failed to see protection by NAC63, 64.
4.1.2 Neurotrophic factors
Another successful line of protection utilized neurotrophins but the efficacy of neurotrophic factors varied with the individual compound and the dose administered65–68. Direct injection of glial cell line-derived neurotrophic factor (GDNF) into the guinea pig cochlea provided protection in a dose-dependent manner, although the highest doses of GDNF actually increased susceptibility to noise65. The efficacy of GDNF may reside in its ability to reduce free radical generation, as well as modulate intracellular Ca2+ through induction of calcium binding proteins, and interference with apoptotic factors66.
4.1.3 Calcium-channel blockers
As outlined in more detail in Section 3.2, protection against calcium overload has successfully been employed to prevent NIHL. Blockade of L-type voltage-gated Ca2+ channels protected against NIHL in mice28 and in guinea pigs29, and blockade of T-type voltage-gated Ca2+ channels was effective in mice30. Also consistent with a contribution of calcium-mediated events in hair cell damage, application of the calcineurin inhibitor FK506 attenuated NIHL in guinea pigs69.
4.1.4 Vasodilators
Magnesium can exert multiple actions on a cell; it may reduce calcium influx and block apoptosis in hair cells, but can also limit ischemia by promoting vasodilation of cochlear arterioles70. In early experiments, noise caused significantly greater hearing loss in rats when fed a magnesium-deficient rather than a magnesium-enriched diet71. Additionally, long-term administration of magnesium after exposure to impulse noise improved hearing thresholds in guinea pigs70. Further, intense noise induced vasoconstriction via formation of the lipid peroxidation product 8-iso-PGF2α in the cochlea72, and this reduction of cochlear blood flow was reversed by a specific antagonist33, offering another approach for protection.
4.1.5 Glutamate antagonists
Also in the armamentarium against NIHL is regulation of glutamate excitotoxicity. Application of a glutamate antagonist reduced the dendritic damage from subsequent noise trauma73. An NMDA receptor antagonist, MK-801, also offered some protection against NIHL60, 74. Interestingly, magnesium, discussed above in the context of vasoconstriction, may also act on excitotoxic events as magnesium deficiency may lead to an increased release of glutamate via exocytosis and overstimulation of NMDA receptors on the auditory nerve75.
4.1.6 Steroid hormones
Steroids are extensively being used in clinical practice and their benefits and safety are well established. In animal models, direct administration of dexamethasone into the inner ear and intravenous administration of dehydro-epiandrosterone each lessened NIHL76, 77. However, the therapeutic time window was very short and another study did not confirm a positive effect of dexamethasone78. The hormone estradiol may also be involved in a protective circuit, acting through estrogen receptor (ER)β, as well as by interaction with BDNF. In support, the ERβ-selective agonist 2,3-bis(4-hydroxyphenyl)-proprionitrile attenuated noise trauma in mice while, conversely, ERβ knock-out mice had an enhanced sensitivity to noise exposure79.
4.1.7 Anti-apoptotic agents
Finally, therapy with anti-apoptotic agents is another potential strategy, and several animal studies document protection against or enhanced recovery from NIHL by blocking apoptotic cascades, such as the MAP kinase (MAPK)-c-Jun-N-terminal kinase (JNK) pathway. Local administration of a JNK-inhibitor into the inner ear protected against NIHL44, and round-window administration restored hearing when given as much as 12 hours after noise exposure80. Retinoic acid, which is an active metabolite of vitamin A and functions as a potent inhibitor of the JNK pathway, also protected from NIHL after oral administration to mice81. However, inhibition of apoptosis with the pan-caspase inhibitor ZVAD reduced hair cell apoptosis, but shifted the prevalence of OHC death to necrotic-like cell death. Conversely, administration of the necrosis inhibitor necrostatin-1 (Nec-1) diminished noise-induced RIP3 induction and necrotic hair cell death, but increased apoptotic hair cell death18, suggesting that prevention of NIHL requires a multi-pronged approach.
4.2 Limitations of animal studies
Although results from animal studies have been promising for the attenuation of NIHL, the discrepancies between evaluations of potential protectants in different studies (e.g. for N-acetylcysteine or dexamethasone) present an important caveat in the translation of such experiments into the clinic. Success and failure in animal studies may be owed to specific experimental conditions and animal species. As a case in point, noise-induced hair cell loss is frequency-related in humans and in some animal models, such as the guinea pig and chinchilla; however, in mice and rats, hair cell susceptibility follows a base-to-apex pattern where basal hair cells are much more easily damaged than the apical cells. Importantly, the properties of noise exposure vary considerably between studies in terms of intensity, duration, band width, and use of continuous or impulse noise. A given protective regimen may be suitable for one exposure paradigm but not for another and It would therefore be prudent for translational studies to confirm potential effects of protective compounds in at least two animal species and several noise conditions.
In addition, it is important to note that a physiologically significant impact on auditory performance in humans requires a shift of above 10 dB. A hearing loss or improvement in hearing below this threshold will have a marginal impact in everyday life, if any. Compounds providing “statistically significant” but small-scale protection might prove a principle, but it remains to be established in clinical trials whether such compounds would provide a physiologically relevant protection. It is therefore prudent for translational studies to confirm potential effects of protective compounds in at least two animal species and several noise conditions before going to clinical trials.
5. Human studies
The success of animal studies suggests that pharmacological protection against noise trauma is possible in principle. In the design of clinical trials on noise-induced hearing loss, however, there is an ethical question of exposing subjects to potentially damaging conditions, notably PTS. Therefore, some trials have evaluated potential protectants under TTS conditions with noise exposures thought to leave no permanent damage in the auditory system. However, new evidence from animal experimentation8 has raised the prospect that a seemingly innocuous exposure in youth may lead to an aggravated age-related hearing loss. Another approach to avoid an ethical dilemma are military or industrial settings where noise trauma due to machinery or combat operations was deemed inevitable despite hearing protection. Utilizing those two scenarios, several treatments have been tested prospectively on TTS and PTS, and post-traumatic on potential PTS. Finally, accidental noise trauma is another condition where treatments can be tested although a baseline audiogram or a control group might not be available.
5.1 Confounding variables in NIHL
Before we discuss success and failure of human trials it is important to stress that human trials might face confounding factors that might not be obvious in inbred animal species. Nevertheless, even in laboratory experiments different strains (of mice, mostly) react differently to noise exposure indicating that genetic variations influence the outcome of noise trauma and, by extension, of protective therapies. The nutritional state might likewise influence the severity of responses to noise, as might other disease or lifestyle variables.
5.1.1 Genetics
Genetic variations in human populations influence both the responses to noise exposure and the potential effectiveness of protection (see 5.2). Considering the likely mechanisms of NIHL, it is not surprising that susceptibility to noise is frequently linked to traits that regulate cochlear redox homeostasis. Polymorphisms influencing the outcome of noise exposure have been identified for:
Glutathione S-transferase (GST)
In a population of 58 steel factory workers those with GST null genotypes in GSTT1 and GSTM1, and GSTP1 suffered more noise-induced TTS during their shift82.
Superoxide dismutase 2 (SOD2; Mn-SOD)
Two studies have linked single nucleotide polymorphisms in this enzyme to enhanced NIHL. One study was based on a screen of 200 participants in Taiwan83. The other took audiometric data from 2400 Han Chinese workers and compared the genotypes of the 10% most susceptible and the 10% most resistant individuals84.
Heat shock proteins (HSP)
Single nucleotide polymorphisms in HSP70 were associated with enhanced susceptibility to noise in 349 Taiwanese workers85.
PCDH15 and MYH14
In contrast to GST, SOD and HSP, these genes are not directly involved in antioxidant and homeostatic defenses. PCDH is a member of the cadherin superfamily serving in stereocilia function and mutated in Usher Type IF. MYH14 is a non-muscle myosin heavy chain and mutations may cause autosomal dominant hearing loss. A gene association study in two independent populations, a Polish and a Swedish cohort, revealed a significant association of these two genes with susceptibility to NIHL86.
5.1.2 Nutrition and nutritional supplements
Adequate nutrition is a prerequisite for good health and coping with stress and disease. Preservation of hearing and resistance to noise are no exceptions. Data from the National Health and Nutrition Examination Survey87 documented a significant relationship between dietary quality and auditory sensitivity at high frequencies. In addition, the data suggested a benefit of healthy diets for a noise-exposed population.
Specifically, an adequate supply of vitamins appears essential. Several studies found positive correlations between better auditory thresholds and self-reported intake of vitamin C, vitamin E, retinol (vitamin A analog), riboflavin (vitamin B2), niacin (vitamin B3). Also, diets high in beta-carotene, magnesium, or lycopene (a carotenoid antioxidant) are associated with better preservation of hearing88–90. Conversely, vitamin deficiencies might aggravate noise-induced hearing loss and tinnitus. Army personnel with NIHL or NIHL with tinnitus had lower vitamin B12 levels than normal hearing subjects91.
5.1.3 Smoking
Smoking is another controllable parameter that may influence NIHL. Smokers have a higher risk of developing high-frequency hearing loss than non-smokers with a similar occupational noise exposure92, 93. The interaction may be related to a dual insult to mitochondria, since animal experiments have documented that exposure to cigarette smoke leads to the generation of oxidative stress by affecting mitochondrial function94.
5.2 Pharmacological protection
Of the myriad compounds effective in protecting animals from noise trauma, only a few have been tested in human trials. Most of these studies have employed drugs related to antioxidant defenses and cell death pathways, some of which have already been established as beneficial in other medical contexts. While the results are not uniformly positive, evidence points to the potential to attenuate NIHL.
5.2.1 N-acetyl cysteine
Prospective study on TTS in a leisure setting
An early study with N-acetylcysteine (NAC) could not confirm its efficacy. Thirty-two young attendees of a night club received either 900 mg oral NAC or a placebo. After two hours at a noise level of 93–103 dBA the resulting TTS was small and similar in both groups95.
Prospective study of TTS in an industrial setting
A double-blind cross-over study with 53 workers employed at a steel manufacturing plant (daily noise levels 88.4–89.4 dB) found “significantly reduced TTS” in the groups receiving 1200 mg NAC per day for the 14-day trial period96. However, the difference in TTS was not significant in the population overall but emerged when the participants were subdivided based on the genetic polymorphisms of glutathione S-transferase (GST; see section 5.1.1). Only the subgroup with null genotypes in both GSTT1 and GSTM1 had profited from NAC. Even then, the protection was not remarkable (3.1 ± 3.1 dB on placebo versus 1.2 ± 3.6 dB on NAC, at 3, 4, and 6 kHz). The result can be taken as a suggestion of a potential benefit of NAC but also points to genetics as an important modulator of noise trauma.
Prospective study on TTS in a military setting
More complex auditory functions than only audiogram or otoacoustic emissions were included in an analysis of hearing in military personnel after a shooting exercise and revealed protection of the cochlea by NAC97. Thresholds were minimally elevated by shooting. However, psycho-acoustical modulation transfer functions (thresholds for brief tones in modulated noise) revealed a highly significant decrease in cochlear non-linearity after the noise exposure in 23 control subjects while no changes were seen in the NAC-group of 11 subjects. The NAC treatment consisted of 4× 200 mg NAC, taken at various times after exposure.
Prospective study on potential PTS in a military setting
Likewise, a randomized trial of 566 military subjects on weapons training had differentiated outcomes depending on the analyses used98. There were no differences in the primary outcomes, threshold shifts and percent of adverse events, between the placebo group and NAC (a total daily dose of 2700 mg NAC for each of the first 13 days of training followed by three days of 1800 mg). However, additional analyses revealed significant differences in threshold shifts when handedness (trigger hand) of the shooters was taken into account.
Blast trauma
It is informative in this context to compare NIHL to blast trauma and the resulting mild traumatic brain injury (mTBI), a frequent injury in combat. The sequelae of blast trauma are highly complex and hearing loss may only be one of its manifestations. Due to the unpredictability of battlefield explosions, blast injuries can only be addressed after the trauma has occurred. In a double-blind, placebo-controlled study in an active war zone99 NAC treatment was begun within 24 hours after an injury. Following a 4-gram loading dose, subjects were given 4 g NAC daily for 4 days, then 3 g daily, leading to a significantly improved resolution of symptoms associated with blast exposure mTBI such as dizziness, hearing loss, headache, memory loss, sleep disturbances, and neurocognitive dysfunction at a 7-day endpoint.
5.2.2 Magnesium
Magnesium has long drawn attention for human trials given the reports on noise-induced and Mg-attenuated vasoconstriction (see sections 2.3 and 4) and its relative non-toxicity.
Prospective studies on PTS in military settings
As early as 1987 a significant correlation was found between noise-induced hearing loss and serum magnesium concentrations in 24 air force pilots71. As a consequence, daily oral magnesium supplementation of 167 mg Mg-aspartate was tested in a double-blind placebo-controlled fashion during a 2-months period of military training71, 100. During this time, the recruits were exposed to 420 firearm shots each at a level of ~140 dB after accounting for attenuation by ear plugs. The incidence of PTS (>25 dB at 4 kHz/6 kHz and or 8 kHz) was approximately twice as high in the placebo group than in the Mg group (11%). It is important to note that the degree of PTS was low in subjects with high serum Mg2+ levels and higher in subjects with low serum Mg2+ levels, regardless of treatment. This observation again points to the influences of genetics and physiology (here the tendency of hypomagnesemia) on susceptibility to noise trauma.
Prospective study on TTS in a military setting
A subsequent study by the same group100 tested the efficacy of magnesium supplementation on 20 volunteers who were exposed for 10 days monaurally to 90 dB SL white noise for 10 minutes per day. In the magnesium-supplemented group, higher Mg2+ blood levels were associated with some protection from TTS. However, the correlation was relatively small (r = 0.36) and serum Mg2+ levels in placebo subjects were not reported.
5.2.3 Other agents
‘Supra-physiological’ vitamin B12
As discussed in section 5.1.2, Vitamin B12 is one of the nutrients that influence auditory performance and sensitivity to noise. In a prospective double-blind study on potential TTS101, 20 subjects were injected cyanocobalamin or placebo for 10 days. Threshold shifts were assessed 1 hour after a 10-minute monaural exposure to a continuous narrowband noise masker centered at 3 kHz at 112 dB SPL. The vitamin treatment achieved blood vitamin B12 concentrations that were up to 10-fold higher than the normal range and afforded significant protection. It is not clear how vitamin B12 interacts with noise exposure; it may be involved in stabilizing neural activity, possibly reducing the excitatory effects of excess noise stimulation.
Anti-apoptotic agents
AM-111, an anti-apoptotic cell-permeable JNK ligand, was tested on 11 subjects that had sustained exposure to firecrackers102. The victims were treated within 24 hours or less in a double-blind, randomized parallel-dose trial in which two different dosages were administered intratympanically. There was no placebo group for ethical reasons of withholding a potentially beneficial treatment. Thirty days after treatment there was no difference between the two dosage groups. However, their overall recovery was judged to have exceeded a spontaneous recovery as would have been observed (based on clinical experience) in untreated patients following acute noise trauma. By the same argument, analysis of hearing recovery rates on a patient-by-patient basis suggested that AM-111 had a marked therapeutic effect in at least two cases.
Prednisolone & piracetam
Prednisolone, a corticosteroid primarily used in anti-inflammatory therapy and piracetam, a widely approved nootropic agent and presumed cognitive enhancer, were injected into 52 subjects who had been exposed to gunshot impulse noise at military training sessions103. Placebos were not administered but groups were analyzed by onset of therapy after the trauma. Improvement of auditory thresholds correlated with the delay in treatment and ranged from a 69% recovery rate among patients treated within the first hour to a 13% recovery in the group with a 24-hour delay. The final threshold shifts were also significantly lower in the early treatment groups.
5.3 Currently registered clinical trials
In addition to the above studies, several clinical trials are currently underway or anticipated. Table 1 lists those registered on ClinicalTrials.gov, a service of the U.S. National Institutes of Health. The table is not necessarily complete as registration is not compulsory, for example, for trials in countries outside the United States. The treatments under investigation are generally again based on successful animal experimentation and results from previous trials. Six of the seven agents are antioxidants (see section 4). In addition, N-acetylcysteine and magnesium haven been the subject of field tests before, as has prednisolone (see section 5.2). Details of the studies such as subject selection, dosing regimen, exposure parameters and outcome measures can be accessed under the NCT identifier number.
Table 1.
Clinical Trials on Noise-induced Hearing Loss in the NIH Registry
| Compound(s) | Class | Sponsor | Study Phase & NCT Identifier | Scheduled Completion | Results |
|---|---|---|---|---|---|
| N-acetylcysteine | Antioxidant | National Taiwan University Hospital, Taipei, Taiwan | Phase 2 NCT00552786 | Completed March 2008 | Lin et al., Hear. Res. 269:42–47, 2010 |
| AuraQuell, ACEMg (Magnesium, ascorbate, α-tocopherol, β-carotene) | Antioxidants, vasodilator | University of Michigan, Ann Arbor, Michigan, USA | Phase 2NCT00808470 | Completed Dec 2013 | No results posted |
| SPI-1005 (ebselen) | Antioxidant | Sound Pharmaceuticals Inc, Seattle, Washington, USA | Phase 2 NCT01444846 | Completed March 2014 | No results posted |
| EPI-743 (p-benzoquinone analog) | Mitochondrial redox modulator | Edison Pharmaceuticals Inc, Mountain View, California, USA | Phase 2ANCT02257983 | Completed Feb 2016 | No results posted |
| d-methionine | Antioxidant | MetAmor Inc,Glen Gardner, New Jersey, USA | Phase 3NCT01345474 NCT02903355 | December 2017 | Recruiting |
| Zonisamide, methylprednisolone | Anticonvulsant, anti-inflammatory | Washington University School of Medicine, St. Louis, Missouri, USA | Phase 1/2NCT02049073 | January 2020 | Study not yet open |
| N-acetylcysteine plus magnesium | Antioxidant, vasodilator | University Hospital Antwerp, Antwerp, Belgium | Phase unknown NCT01727492 | Unknown | Unknown |
5.4 Unknown risks of pharmacological protection
While adequate nutrition is essential for the maintenance of auditory acuity (see section 5.1.2), chronic or excess consumption of antioxidant or vitamin supplements may not only be detrimental to hearing but may also be associated with an increased risk of cancer and mortality.
Vitamins A and D
Two population-based surveys with over 2000 participants each confirmed the generally positive effects of a balanced diet and vitamin intake but singled out high retinol (a vitamin A analog) intake88 and high serum levels of vitamin D89 as associated with worse hearing.
β-carotene, vitamin A, and vitamin E
Meta-analyses of clinical trials found β-carotene, vitamin A, and vitamin E correlated with increased overall mortality104, 105. In addition, β-carotene was found to be potentially associated with an increased risk of cancer in smokers106.
While these are important caveats in deciding the composition of therapeutic regimens, we might have to distinguish between acute treatment and chronic exposures. A short-term administration of antioxidants or vitamins should still be a safe protection against drug- and noise-induced hearing loss. However, the hazards of a chronic program of nutritional supplements remain unknown.
6. A new paradigm: The hidden hearing loss of cochlear synaptopathy
Some of the clinical studies discussed in section 5 noted defects in secondary outcome measures without significant threshold changes in the audiograms. Such deficits in auditory processing indicate that the measurement of thresholds is insufficient to detect subtle forms of cochlear damage. Such “hidden hearing loss”107 can represent faulty neural output and manifest, for example, as impaired speech perception. In both animals and humans, physiological evidence for hidden hearing loss can be gained by a detailed analysis of auditory brain stem response (ABR) to sound stimuli. While the ABR thresholds—just as the audiogram thresholds—may be normal, the stimulus-dependent amplitude of ABR waves and their latencies might be affected.
Recent studies using these measures have revealed deafferentation of hair cell-to-auditory nerve fiber connections in mice subjected to mild acoustic trauma that only resulted in a TTS8, 108. This pathology might represent an early event in response to noise and an additional contributing factor to NIHL, while not negating the importance of hair cell loss in permanent NIHL. Interestingly, the phenomenon of cochlear synaptopathy is not limited to NIHL but may also exist in aminoglycoside ototoxicity109 and age-related hearing loss110.
A potential mechanism of cochlear synaptopathy is suggested by experiments9, 111 demonstrating that the cell-derived neurotrophin NT-3 is crucial for the maintenance of adult cochlear hair cell synapses in the mouse. In specific support of a critical role in NIHL, overexpression of NT-3 promoted the regeneration of ribbon synapses and the recovery of auditory responses after acoustic trauma. Furthermore, local round-window delivery of NT-3 24 hours after noise trauma also regenerated cochlear synapses and function.
7. Conclusion
Animal models have given convincing insights into the pathology and mechanisms of NIHL. Calcium overload and mitochondrial dysfunction with reduced energy production and enhanced formation of free radicals might give rise to pathways of apoptosis, necrosis or necroptosis in auditory sensory cells. More subtle but potentially equally important injury can occur to hair cell-auditory nerve connections causing hidden hearing loss.
A therapeutic intervention into noise-induced hearing loss is possible. Several classes of drugs appear particularly effective (antioxidants, anti-apoptotic agents, steroids) and some of these have been and will be tested in industrial, military and leisure settings. Intriguingly, considerably more drugs than those currently listed in registered trials are suggested by animal studies. Compounds to be considered would include additional antioxidants mentioned in section 3.1, neurotrophic factors, calcium-channel blockers, or steroids which already have a place in the clinic. Whether such drugs will eventually be viable therapeutics against NIHL in humans remains to be established in further translational studies that would more rigorously test their efficacy, dosing, and the best route of administration.
Caveats also apply. First, a single treatment may not protect in all cases of NIHL but its efficacy may depend on the type, duration, and intensity of noise exposure. Second, genetics and the physiological (nutritional) state of subjects are confounding factors that are capable of influencing the outcome of therapeutic interventions and may necessitate a more targeted approach. Nevertheless, the outlook is promising and a viable clinical treatment should be available in the near future.
Expert opinion
The last decade has brought impressive progress in understanding NIHL and the feasibility of its prevention. However, in order to proceed and optimize potential treatments close attention must be paid to several open issues in both animal experimentation and translation.
Comparable test conditions and standards of protection must be established. Comparisons of studies from different laboratories are difficult because of variations in animal models, exposure and test parameters. Animal models primarily use continuous noise of different characteristics as the stimulus while in industrial, military, and recreational settings impulse noise predominates. Treatments efficacious against one form of noise might not protect against the other and prospective drugs should be tested for their efficacy under different conditions. Furthermore, pharmacokinetic evaluations are largely missing, making a compound’s efficacy (or lack thereof) and the extent to which noise trauma can be influenced difficult to quantify.
In this context, we should pay more attention to the notion of “significant” effects of protective drugs. A reduction of hearing loss of 5 or 10 dB can be statistically significant and prove a principle but would not improve the quality of life of the affected individual. More robust results are needed to warrant clinical trials.
Combination treatment may provide enhanced protection. It has rarely been tested whether protection might be more effective when a combination of agents were to target different molecular mechanisms. It might be promising, for example, to use complementary therapies to modulate oxidative stress as an early event with apoptosis/necrosis inhibitors to combat later sequels. Other combinations can be gleaned from the list of potentially protective agents that aim at diverse events such as excitotoxicity, blood flow, calcium overload, and neurotrophic or hormonal control mechanisms.
It is insufficient to rely on pure-tome audiograms as an outcome measure. Some forms of auditory damage (e.g., “hidden hearing loss”) will escape simple audiometric tests, and protective strategies may have to be subjected to more sophisticated audiological assessments. Some of the clinical studies discussed in section 5 already noted defects in secondary outcome measures without significant threshold changes in pure-tone audiograms.
We also must be more concerned about TTS which has in the past been considered innocuous. This assumption is now being called into question by the demonstration of cochlear synaptopathy in TTS with potential consequences for later accelerated hearing loss. We do not have data to know whether any magnitude of TTS will have late-life consequences or whether a threshold for late-life damage exists. Volunteers in TTS experiments have a right to know. The development of preventive or rescue treatment even for TTS is indicated.
Genetics of susceptibility to NIHL is a confounding variable and need to be tackled now that genetic screening has entered the analytical mainstream. As some clinical trials have already shown, protective therapies might be effective only in subsets of the population and treatments eventually will have to be tailored to genetic variants. Furthermore, genetic predisposition to noise trauma raises the question whether we have the ethical duty to exclude sensitive individuals from hazardous environments or test conditions.
Improved post-hoc treatments are essential. Most research so far has focused on intervention prior to exposure. However, the need for medications effective after trauma is obvious from the battlefield and also from recreational exposures when patients present with auditory problems after the fact. Both animal studies and field trials suggest that a “window of rescue” exists. Although there might be overlap with drugs that work prospectively, different drugs might be more efficacious for post-hoc rescue. This question has yet to be addressed.
Finally, compliance and safety might be an issue in long-term prevention. Compliance issues are well documented for the use of ear protectors and similar issues might arise for drug administration. Approaches need to be explored how individuals can best be motivated to take a daily pill and how compliance can be monitored. Safety of drugs has been established only for short treatments in both animals and humans but there is little information on the long-term effects even of common nutritional supplements. These need to be monitored, in particular in view of studies that suggest morbidity and mortality associated with excess vitamin intake.
Overall, the outlook for an intervention in NIHL is positive. Successful animal models and encouraging initial clinical trials have confirmed the proof-of-principle that NIHL can be prevented or at least attenuated. With attention to unresolved issues and fine-tuning of translational research, the next decade should see protective treatments enter the clinic.
Highlights.
The classical pathology of NIHL is destruction of auditory hair cells and degeneration of spiral ganglion cells.
Based on the molecular mechanisms of NIHL, therapeutic protection has successfully been developed in animal models.
Human trials to protect against noise trauma are promising but current results remain tentative.
Synaptopathy has emerged as an early event in NIHL and neurotrophins are potential rescue agents.
Acknowledgments
Funding
This paper is partly funded by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R01 DC009222) to S-H. Sha.
Footnotes
Declaration of Interest
J. Schacht is named as a co-inventor of ACEMg for treatment of hearing loss (US Patent 7,951,845 B2 to the University of Michigan), but is not involved in any trials testing this compound or any other commercial exploitation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Basner M, Babisch W, Davis A, et al. Auditory and non-auditory effects of noise on health. Lancet. 2014;383:1325–32. doi: 10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs. 2011;16:235–45. doi: 10.1517/14728214.2011.552427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bess FH, Humes L. Audiology: the fundamentals. 4th. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 4.Canlon B. The effect of acoustic trauma on the tectorial membrane, stereocilia, and hearing sensitivity: possible mechanisms underlying damage, recovery, and protection. Scand Audiol Suppl. 1988;27:1–45. [PubMed] [Google Scholar]
- 5.Canlon B, Miller J, Flock A, et al. Pure tone overstimulation changes the micromechanical properties of the inner hair cell stereocilia. Hear Res. 1987;30:65–72. doi: 10.1016/0378-5955(87)90184-5. [DOI] [PubMed] [Google Scholar]
- 6•.Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryngol. 1971;71:166–76. doi: 10.3109/00016487109125346. Classical paper on NIHL. [DOI] [PubMed] [Google Scholar]
- 7.Puel JL, Ruel J, Gervais d’Aldin C, et al. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–14. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- 8••.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. Seminal paper to draw attention to delayed effects of TTS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan G, Gomez-Casati ME, Gigliello AR, et al. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. eLife. 2014;3 doi: 10.7554/eLife.03564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu BH, Henderson D, Nicotera TM. Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res. 2006;211:16–25. doi: 10.1016/j.heares.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–68. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuknecht HF. Pathology of the ear. Cambridge, Mass: Harvard University Press; 1974. [Google Scholar]
- 13.Yamane H, Nakai Y, Takayama M, et al. The emergence of free radicals after acoustic trauma and strial blood flow. Acta Otolaryngol Suppl. 1995;519:87–92. doi: 10.3109/00016489509121877. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins JE., Jr The role of vasoconstriction in noise-induced hearing loss. Ann Otol Rhinol Laryngol. 1971;80:903–13. doi: 10.1177/000348947108000617. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita D, Jiang HY, Schacht J, et al. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–9. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 16•.Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–36. doi: 10.1159/000013846. One of the first pubications to demonstrate free radicals in noise trauma. [DOI] [PubMed] [Google Scholar]
- 17.Yuan H, Wang X, Hill K, et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Sign. 2015;22:1308–24. doi: 10.1089/ars.2014.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng HW, Chen J, Sha SH. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014;5:e1262. doi: 10.1038/cddis.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen FQ, Zheng HW, Hill K, et al. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J Neurosci. 2012;32:12421–30. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang WP, Henderson D, Hu BH, et al. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res. 2004;196:69–76. doi: 10.1016/j.heares.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita D, Miller JM, Jiang HY, et al. AIF and EndoG in noise-induced hearing loss. Neuroreport. 2004;15:2719–22. [PubMed] [Google Scholar]
- 22.Nicotera TM, Hu BH, Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol. 2003;4:466–77. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridberger A, Flock A, Ulfendahl M, et al. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. P Natl Acad Sci USA. 1998;95:7127–32. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda K, Kusakari J, Takasaka T. Ionic changes in cochlear endolymph of the guinea pig induced by acoustic injury. Hear Res. 1988;32:103–10. doi: 10.1016/0378-5955(88)90081-0. [DOI] [PubMed] [Google Scholar]
- 25.Zuo H, Cui B, She X, et al. Changes in Guinea pig cochlear hair cells after sound conditioning and noise exposure. J Occup Health. 2008;50:373–9. doi: 10.1539/joh.l8032. [DOI] [PubMed] [Google Scholar]
- 26.Brandt A, Striessnig J, Moser T. CaV1. 3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–40. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinrich UR, Maurer J, Mann W. Alteration of loosely bound calcium in the guinea pig organ of Corti after treatment with diltiazem as calcium channel blocker. Eur Arch Otorhinolaryngol. 1997;254:223–9. doi: 10.1007/BF00874093. [DOI] [PubMed] [Google Scholar]
- 28.Uemaetomari I, Tabuchi K, Nakamagoe M, et al. L-type voltage-gated calcium channel is involved in the pathogenesis of acoustic injury in the cochlea. Tohoku J Exp Med. 2009;218:41–7. doi: 10.1620/tjem.218.41. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich UR, Maurer J, Mann W. Ultrastructural evidence for protection of the outer hair cells of the inner ear during intense noise exposure by application of the organic calcium channel blocker diltiazem. ORL J Oto-rhinolary. 1999;61:321–7. doi: 10.1159/000027693. [DOI] [PubMed] [Google Scholar]
- 30.Shen H, Zhang B, Shin JH, et al. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hear Res. 2007;226:52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Böttger EC, Schacht J. The mitochondrion: a perpetrator of acquired hearing loss. Hear Res. 2013;303:12–9. doi: 10.1016/j.heares.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirk WS, Seidman MD. Cochlear vascular changes in response to loud noise. Am J Otol. 1995;16:322–5. [PubMed] [Google Scholar]
- 33.Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neuro-otol. 2003;8:207–21. doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- 34.Nagashima R, Yamaguchi T, Kuramoto N, et al. Acoustic overstimulation activates 5′-AMP-activated protein kinase through a temporary decrease in ATP level in the cochlear spiral ligament prior to permanent hearing loss in mice. Neurochem Int. 2011;59:812–20. doi: 10.1016/j.neuint.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Vlajkovic SM, Housley GD, Munoz DJ, et al. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 2004;126:763–73. doi: 10.1016/j.neuroscience.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Chen FQ, Zheng HW, Hill K, et al. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J Neurosci. 2012;32:12421–30. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minami SB, Yamashita D, Ogawa K, et al. Creatine and tempol attenuate noise-induced hearing loss. Brain Res. 2007;1148:83–9. doi: 10.1016/j.brainres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spicer SS, Schulte BA. Creatine kinase in epithelium of the inner ear. J Histochem Cytochem. 1992;40:185–92. doi: 10.1177/40.2.1313059. [DOI] [PubMed] [Google Scholar]
- 39.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–83. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 40.Hill K, Yuan H, Wang X, Sha SH. Noise-Induced Loss of Hair Cells and Cochlear Synaptopathy Are Mediated by the Activation of AMPK. J Neurosci. 2016;36:7497–510. doi: 10.1523/JNEUROSCI.0782-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCullough LD, Zeng Z, Li H, et al. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Zeng Z, Viollet B, et al. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–9. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J Neurosci Res. 2006;83:1564–72. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Van De Water TR, Bonny C, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug discovery today. 2005;10:1291–8. doi: 10.1016/S1359-6446(05)03561-0. [DOI] [PubMed] [Google Scholar]
- 46.Yamasoba T, Nuttall AL, Harris C, et al. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998;784:82–90. doi: 10.1016/s0006-8993(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 47.Ohinata Y, Yamasoba T, Schacht J, et al. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146:28–34. doi: 10.1016/s0378-5955(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 48.Campbell KC, Meech RP, Klemens JJ, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Pourbakht A, Yamasoba T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear Res. 2003;181:100–8. doi: 10.1016/s0378-5955(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 50.Seidman M, Babu S, Tang W, et al. Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg. 2003;129:463–70. doi: 10.1016/S0194-59980301586-9. [DOI] [PubMed] [Google Scholar]
- 51.McFadden SL, Woo JM, Michalak N, et al. Dietary vitamin C supplementation reduces noise-induced hearing loss in guinea pigs. Hear Res. 2005;202:200–8. doi: 10.1016/j.heares.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Heinrich UR, Fischer I, Brieger J, et al. Ascorbic acid reduces noise-induced nitric oxide production in the guinea pig ear. The Laryngoscope. 2008;118:837–42. doi: 10.1097/MLG.0b013e31816381ae. [DOI] [PubMed] [Google Scholar]
- 53.Fetoni AR, Piacentini R, Fiorita A, et al. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–16. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Wong AC, Guo CX, Gupta R, et al. Post exposure administration of A(1) adenosine receptor agonists attenuates noise-induced hearing loss. Hear Res. 2010;260:81–8. doi: 10.1016/j.heares.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Fetoni AR, Mancuso C, Eramo SL, et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169:1575–88. doi: 10.1016/j.neuroscience.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita D, Jiang HY, Le Prell CG, et al. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005;134:633–42. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 57.Bielefeld EC, Kopke RD, Jackson RL, et al. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol. 2007;127:914–9. doi: 10.1080/00016480601110188. [DOI] [PubMed] [Google Scholar]
- 58.Tamir S, Adelman C, Weinberger JM, et al. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J Occup Med Toxicol. 2010;5:26. doi: 10.1186/1745-6673-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talaska AE, Schacht J. Mechanisms of noise damage to the cochlea. Audiol Medicine. 2007;5:3–9. [Google Scholar]
- 60.Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966:265–73. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- 61.Wu HP, Hsu CJ, Cheng TJ, et al. N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hear Res. 2010;267:71–7. doi: 10.1016/j.heares.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 62.Fetoni AR, Ralli M, Sergi B, et al. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29:70–5. [PMC free article] [PubMed] [Google Scholar]
- 63.Hamernik RP, Qiu W, Davis B. The effectiveness of N-acetyl-L-cysteine (L-NAC) in the prevention of severe noise-induced hearing loss. Hear Res. 2008;239:99–106. doi: 10.1016/j.heares.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Davis RR, Custer DA, Krieg E, et al. N-Acetyl L-Cysteine does not protect mouse ears from the effects of noise. J Occup Med Toxicol. 2010;5:11. doi: 10.1186/1745-6673-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shoji F, Yamasoba T, Magal E, et al. Glial cell line-derived neurotrophic factor has a dose dependent influence on noise-induced hearing loss in the guinea pig cochlea. Hear Res. 2000;142:41–55. doi: 10.1016/s0378-5955(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 66.Shoji F, Miller AL, Mitchell A, et al. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146:134–42. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 67.Green SH, Altschuler RA, Miller JM. Cell Death and Cochlear Protection. In: Schacht J, Popper AN, Fay RR, editors. Auditory trauma, protection, and repair. New York: Springer; 2008. pp. 275–319. [Google Scholar]
- 68.Shibata SB, Osumi Y, Yagi M, et al. Administration of amitriptyline attenuates noise-induced hearing loss via glial cell line-derived neurotrophic factor (GDNF) induction. Brain Res. 2007;1144:74–81. doi: 10.1016/j.brainres.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 69.Minami SB, Yamashita D, Schacht J, et al. Calcineurin activation contributes to noise-induced hearing loss. J Neurosci Res. 2004;78:383–92. doi: 10.1002/jnr.20267. [DOI] [PubMed] [Google Scholar]
- 70.Abaamrane L, Raffin F, Gal M, et al. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2009;247:137–45. doi: 10.1016/j.heares.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Joachims Z, Babisch W, Ising H, et al. Dependence of noise-induced hearing loss upon perilymph magnesium concentration. J Acoust Soc Am. 1983;74:104–8. doi: 10.1121/1.389726. [DOI] [PubMed] [Google Scholar]
- 72•.Ohinata Y, Miller JM, Altschuler RA, et al. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–73. doi: 10.1016/s0006-8993(00)02733-5. This study links free-radical production by noise to vasoconstriction. [DOI] [PubMed] [Google Scholar]
- 73.Puel JL, D’Aldin CG, Saffiende S, et al. Excitotoxicity and plasticity of IHC-auditory nerve contributes to both temporary and permanent threshold shift. In: Axelsson A, Borchgrevink H, Hamernik RP, Hellstrom P-A, Henderson D, Salvi RJ, editors. Scientific Basis of Noise-Induced Hearing Loss. New York: Thieme; 1996. pp. 36–42. [Google Scholar]
- 74.Chen GD, Kong J, Reinhard K, et al. NMDA receptor blockage protects against permanent noise-induced hearing loss but not its potentiation by carbon monoxide. Hear Res. 2001;154:108–15. doi: 10.1016/s0378-5955(01)00228-3. [DOI] [PubMed] [Google Scholar]
- 75.Cevette MJ, Vormann J, Franz K. Magnesium and hearing. J Am Acad Audiol. 2003;14:202–12. [PubMed] [Google Scholar]
- 76.Takemura K, Komeda M, Yagi M, et al. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res. 2004;196:58–68. doi: 10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Tabuchi K, Murashita H, Tobita T, et al. Dehydroepiandrosterone sulfate reduces acoustic injury of the guinea-pig cochlea. J Pharmacol Sci. 2005;99:191–4. doi: 10.1254/jphs.scz050443. [DOI] [PubMed] [Google Scholar]
- 78.Bas E, Martinez-Soriano F, Lainez JM, et al. An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss. Acta Otolaryngol. 2009;129:385–9. doi: 10.1080/00016480802566279. [DOI] [PubMed] [Google Scholar]
- 79.Meltser I, Tahera Y, Simpson E, et al. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–70. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J, Ruel J, Ladrech S, et al. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol. 2007;71:654–66. doi: 10.1124/mol.106.028936. [DOI] [PubMed] [Google Scholar]
- 81.Shim HJ, Kang HH, Ahn JH, et al. Retinoic acid applied after noise exposure can recover the noise-induced hearing loss in mice. Acta Otolaryngol. 2009;129:233–8. doi: 10.1080/00016480802226155. [DOI] [PubMed] [Google Scholar]
- 82.Lin CY, Wu JL, Shih TS, et al. Glutathione S-transferase M1, T1, and P1 polymorphisms as susceptibility factors for noise-induced temporary threshold shift. Hear Res. 2009;257:8–15. doi: 10.1016/j.heares.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Chang NC, Ho CK, Wu MT, et al. Effect of manganese-superoxide dismutase genetic polymorphisms IVS3-23T/G on noise susceptibility in Taiwan. Am J Otolaryngol. 2009;30:396–400. doi: 10.1016/j.amjoto.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Liu YM, Li XD, Guo X, et al. SOD2 V16A SNP in the mitochondrial targeting sequence is associated with noise induced hearing loss in Chinese workers. Dis Markers. 2010;28:137–47. doi: 10.3233/DMA-2010-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang NC, Ho CK, Lin HY, et al. Association of polymorphisms of heat shock protein 70 with susceptibility to noise-induced hearing loss in the Taiwanese population. Audiol Neuro-otol. 2011;16:168–74. doi: 10.1159/000317119. [DOI] [PubMed] [Google Scholar]
- 86.Konings A, Van Laer L, Wiktorek-Smagur A, et al. Candidate gene association study for noise-induced hearing loss in two independent noise-exposed populations. Ann Hum Genet. 2009;73:215–24. doi: 10.1111/j.1469-1809.2008.00499.x. [DOI] [PubMed] [Google Scholar]
- 87.Spankovich C, Le Prell CG. Associations between dietary quality, noise, and hearing: data from the National Health and Nutrition Examination Survey, 1999-2002. Int J Audiol. 2014;53:796–809. doi: 10.3109/14992027.2014.921340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Spankovich C, Hood LJ, Silver HJ, et al. Associations between diet and both high and low pure tone averages and transient evoked otoacoustic emissions in an older adult population-based study. J Am Acad Audiol. 2011;22:49–58. doi: 10.3766/jaaa.22.1.6. Comprehensive summary of the relationship between diet and susceptibility to noise. [DOI] [PubMed] [Google Scholar]
- 89.Kang JW, Choi HS, Kim K, et al. Dietary vitamin intake correlates with hearing thresholds in the older population: the Korean National Health and Nutrition Examination Survey. Am J Clin Nutr. 2014;99:1407–13. doi: 10.3945/ajcn.113.072793. [DOI] [PubMed] [Google Scholar]
- 90.Choi YH, Miller JM, Tucker KL, et al. Antioxidant vitamins and magnesium and the risk of hearing loss in the US general population. Am J Otolaryngol. 2014;99:148–55. doi: 10.3945/ajcn.113.068437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shemesh Z, Attias J, Ornan M, et al. Vitamin B12 deficiency in patients with chronic-tinnitus and noise-induced hearing loss. Am J Otolaryngol. 1993;14:94–9. doi: 10.1016/0196-0709(93)90046-a. [DOI] [PubMed] [Google Scholar]
- 92.Mehrparvar AH, Mollasadeghi A, Hashemi SH, Sakhvidi MJ, Mostaghaci M, Davari MH. Simultaneous effects of noise exposure and smoking on OAEs. Noise Health. 2015;17:233–6. doi: 10.4103/1463-1741.160716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tao L, Davis R, Heyer N, et al. Effect of cigarette smoking on noise-induced hearing loss in workers exposed to occupational noise in China. Noise Health. 2013;15:67–72. doi: 10.4103/1463-1741.107159. [DOI] [PubMed] [Google Scholar]
- 94.Mannam P, Rauniyar N, Lam TT, et al. MKK3 influences mitophagy and is involved in cigarette smoke-induced inflammation. Free Radical Bio Med. 2016;101:102–15. doi: 10.1016/j.freeradbiomed.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Kramer S, Dreisbach L, Lockwood J, et al. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006;17:265–78. doi: 10.3766/jaaa.17.4.5. [DOI] [PubMed] [Google Scholar]
- 96.Lin CY, Wu JL, Shih TS, et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42–7. doi: 10.1016/j.heares.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Lindblad AC, Rosenhall U, Olofsson A, et al. The efficacy of N-acetylcysteine to protect the human cochlea from subclinical hearing loss caused by impulse noise: a controlled trial. Noise Health. 2011;13:392–401. doi: 10.4103/1463-1741.90293. [DOI] [PubMed] [Google Scholar]
- 98.Kopke R, Slade MD, Jackson R, et al. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear Res. 2015;323:40–50. doi: 10.1016/j.heares.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 99.Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PloS one. 2013;8:e54163. doi: 10.1371/journal.pone.0054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Attias J, Sapir S, Bresloff I, et al. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol Allied Sci. 2004;29:635–41. doi: 10.1111/j.1365-2273.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 101.Quaranta A, Scaringi A, Bartoli R, et al. The effects of ‘supra-physiological’ vitamin B12 administration on temporary threshold shift. Int J Audiol. 2004;43:162–5. doi: 10.1080/14992020400050022. [DOI] [PubMed] [Google Scholar]
- 102.Suckfuell M, Canis M, Strieth S, et al. Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Oto-laryngol. 2007;127:938–42. doi: 10.1080/00016480601110212. [DOI] [PubMed] [Google Scholar]
- 103.Psillas G, Pavlidis P, Karvelis I, et al. Potential efficacy of early treatment of acute acoustic trauma with steroids and piracetam after gunshot noise. Eur Arch Otorhinolaryngol. 2008;265:1465–9. doi: 10.1007/s00405-008-0689-6. [DOI] [PubMed] [Google Scholar]
- 104.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 105.Bjelakovic G, Nikolova D, Gluud C. Antioxidant supplements and mortality. Curr Opin Clin Nutr. 2014;17:40–4. doi: 10.1097/MCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 106.Druesne-Pecollo N, Latino-Martel P, Norat T, et al. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. 2010;127:172–84. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 107.Plack CJ, Barker D, Prendergast G. Perceptual consequences of “hidden” hearing loss. Trends Hear. 2014;18:1–11. doi: 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–23. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oishi N, Duscha S, Boukari H, et al. XBP1 mitigates aminoglycoside-induced endoplasmic reticulum stress and neuronal cell death. J Neurosci. 2015;6:e1763. doi: 10.1038/cddis.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–9. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111••.Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep. 2016;6:24907. doi: 10.1038/srep24907. Post-trauma recovery from noise-induced synaptopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]


