Abstract
Sleep disturbance and cognitive dysfunction are two domains of impairment during inter-episode bipolar disorder. Despite evidence demonstrating the importance of sleep for cognition in healthy and sleep-disordered samples, this link has been minimally examined in bipolar disorder. The present study tested the association between insomnia-related sleep disruptions and cognitive dysfunction during inter-episode bipolar disorder. Forty-seven participants with bipolar disorder and a comorbid insomnia diagnosis (BD-Insomnia) and 19 participants with bipolar disorder without sleep disturbance in the last six months (BD-Control) participated in the study. Two domains of cognition were assessed: working memory and verbal learning. Insomnia-related sleep disruptions were assessed both categorically (i.e., insomnia diagnosis) and dimensionally (i.e., total wake time, total sleep time, total wake time variability, and total sleep time variability). Hierarchical linear regressions, adjusting for participant age, demonstrated that insomnia diagnosis did not have an independent or interactive effect on cognition. However, regardless of insomnia diagnosis, greater total sleep time variability predicted poorer working memory and verbal learning performance. Further, following sleep treatment, a reduction in total wake time predicted improved working memory performance and a reduction in total sleep time variability predicted improved verbal learning performance. These findings raise the possibility that sleep disturbance may contribute to cognitive dysfunction in bipolar disorder and highlight the importance of treating sleep disturbance in bipolar disorder.
Keywords: Bipolar disorder, cognition, insomnia, inter-episode phase, verbal learning, working memory
1. Introduction
Bipolar disorder is one of the 10 most disabling conditions worldwide (World Health Organization, 2001) and has a lifetime prevalence ranging from 0.4 – 2.4% (Merikangas et al., 2011). Individuals with bipolar disorder continue to experience substantial impairment during periods identified as neither depressive nor manic, a phase referred to as the inter-episode phase (Judd et al., 2003; MacQueen et al., 2003; Robb et al., 1997). Sleep disturbance and cognitive dysfunction (World Health Organization, 2001) are two important domains of impairment during the inter-episode phase, each contributing to functional impairment and reduced quality of life (e.g., Harvey et al., 2005; Robinson et al., 2006). Despite considerable literature demonstrating a link between sleep and cognition in healthy and sleep-disordered samples, the association between sleep and cognition remains under-examined in inter-episode bipolar disorder.
Seventy percent of individuals with bipolar disorder report clinically significant sleep problems during the inter-episode phase (Harvey et al., 2005) and over half (55%) meet diagnostic criteria for insomnia (Harvey et al., 2005). Insomnia is defined by subjective difficulty falling asleep, staying asleep, or waking up too early, despite adequate opportunity to sleep, with associated daytime impairment or distress (American Psychiatric Association, 2013). Insomnia is also associated with short sleep duration (Vgontzas et al., 2010; Vgontzas et al., 2009) and night-to-night variability in sleep behaviors (Buysse et al., 2010; Frankel et al., 1976). Fragmented sleep, shortened sleep duration, and elevated variability in sleep behaviors are also observed during inter-episode bipolar disorder, regardless of insomnia diagnosis (Eidelman et al., 2010; Geoffroy et al., 2014; Gruber et al., 2009; Jones et al., 2005; Kanady et al., 2015; Millar et al., 2004; Ritter et al., 2012).
Cognitive dysfunction is also common during inter-episode bipolar disorder. Although many cognitive processes are impaired during the inter-episode phase, some of the largest effect sizes have been found for performance on tasks of working memory (Robinson et al., 2006) and verbal learning (Robinson et al., 2006). Working memory is a system for temporarily storing and managing information so that it is easily accessible and can be utilized to carry out a task (Baddeley, 1992). Verbal learning is the process of acquiring, retaining, and recalling verbal material (Ausubel, 1963).
Previous research has demonstrated a relationship between sleep disruption and cognitive dysfunction in healthy and insomnia samples. In healthy populations, sleep deprivation prior to learning is associated with a 40% reduction in the ability to learn new material (Yoo et al., 2007) and impairs performance across a variety of tasks including working memory (Alhola and Polo-Kantola, 2007; Chee and Choo, 2004; Lim and Dinges, 2010) and verbal learning (e.g., Drummond et al., 2000). Some studies report that insomnia is associated with objective cognitive dysfunction (Edinger et al., 2009; Schneider et al., 2004; Varkevisser and Kerkhof, 2005; Varkevisser et al., 2007) while other studies reveal no such relation (Orff et al., 2007; Varkevisser et al., 2007). Mixed findings may be due to specific insomnia-related sleep disruptions underlying associations with cognition. Indeed, greater wake after sleep onset (Blackwell et al., 2006; Blackwell et al., 2014; Naismith et al., 2010; Wilckens et al., 2016) and longer sleep onset latency (Blackwell et al., 2006; Luik et al., 2015) are associated with greater cognitive deficits in older adults. Insomnia coupled with short sleep duration (<6 hours) also appears to have a greater impact on cognitive dysfunction when compared to insomnia with normal sleep duration and healthy sleep patterns (Fernandez-Mendoza et al., 2010). Only one study has examined the impact of sleep variability on cognition, (McCrae et al., 2012) revealing that night-to-night variability in sleep duration and total wake time did not predict performance on a processing speed or an executive functioning task in older adults.
Despite the evidence demonstrating an association between sleep and cognition in healthy and insomnia samples, the potential contribution of sleep disturbance to inter-individual variation in cognitive dysfunction during inter-episode bipolar disorder is under-characterized (see Boland and Alloy, 2013 for a review). Using a depression scale, one study demonstrated that individuals with bipolar disorder who demonstrate poorer cognitive performance on a neuropsychological battery report higher rates of insomnia compared to individuals with bipolar disorder who demonstrate intact cognitive performance (Volkert et al., 2015). Using post-hoc exploratory correlational analyses, a second study demonstrated an association between the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) rated daytime dysfunction and a working memory task (Boland et al., 2015). Another study demonstrated that poorer performance on measures of working memory, visual learning, and social cognition was associated with patient ratings of poor sleep quality and increased daytime sleepiness (Russo et al., 2015).
The objective of the present study was to examine the association between sleep and cognition during inter-episode bipolar disorder using standard measurement methods and a therapeutic manipulation of sleep. The first aim was to examine whether insomnia diagnosis and subjective insomnia-related sleep disruptions – in particular total wake time (TWT), total sleep time (TST), total wake time variability (TWTvar), and total sleep time variability (TSTvar) – have an independent or interactive effect on working memory and verbal learning performance during the inter-episode phase. Based on the mixed findings of previous research, we tested two competing hypotheses: (1) there would be a main effect of insomnia diagnosis and an interactive effect whereby insomnia diagnosis and greater subjective TWT, shorter TST, greater TWTvar, and/or greater TSTvar in the insomnia group would predict poorer cognitive performance, versus (2) greater TWT, shorter TST, greater TWTvar, and/or greater TSTvar would independently predict poorer cognitive performance, regardless of insomnia diagnosis.
The second aim was to determine if working memory and verbal learning performance improves following a form of cognitive behavior therapy for insomnia modified specifically for bipolar disorder (CBTI-BD; Harvey et al., 2015). We hypothesized that participants with bipolar disorder who demonstrated an improvement in the subjective sleep parameters of interest (i.e., TWT, TST, TWTvar, and TSTvar) following CBTI-BD would show a related improvement in cognitive performance relative to a control psychoeducation treatment condition.
2. Materials and Methods
2.1. Participants
Forty-seven adults with bipolar disorder and a comorbid insomnia diagnosis (BD-Insomnia) and 19 adults with bipolar disorder without sleep disturbance in the last six months (BD-Control) participated in the study. Individuals were eligible if they (a) met DSM-IV-TR criteria (American Psychiatric Association, 2000) for bipolar disorder, type I; (b) were inter-episode as defined by a score of 24 or less on the Inventory of Depressive Symptomatology, Clinician Rating (IDS-C; Rush et al., 1996), a score of 12 or less on the Young Mania Rating Scale (YMRS; Young et al., 1978), and not meeting DSM-IV-TR criteria for depression, mania, or hypomania in the month preceding the clinical interview; (c) were at least 18 years old; (d) reported English fluency; and (e) were on a stable medication regimen (i.e., no changes in the dosage or frequency of medication use) for at least four weeks prior to enrollment in the study as side effects are more likely early in treatment than with continued use (Ketter and Wang, 2002).
Exclusion criteria for all participants included: (a) an alcohol and/or substance use diagnosis within the past three months; (b) a current post-traumatic stress disorder; (c) an active or progressive neurodegenerative disease or physical illness; (d) evidence of sleep apnea, restless legs syndrome, or periodic limb movements during sleep; (e) employment as an overnight shift worker in the last three months; (f) current suicidal risk/homicidal risk (g) attempted suicide within the past 6 months; (h) and pregnancy and/or breast-feeding mothers.
BD-Insomnia participants met criteria for current insomnia. Insomnia was defined as a subjective report of difficulty falling asleep (>30 minutes), difficulty maintaining sleep (wake after sleep onset >30 minutes), and/or waking up too early (early morning awakening >30 minutes), with associated daytime complaints, at least three times a week for at least one month despite adequate opportunity to sleep (American Academy of Sleep Medicine, 2005; American Psychiatric Association, 2013; Edinger et al., 2004). To enhance generalizability and feasibility, we did not exclude participants based on sleep medication and/or sleep aid use.
Participants in the BD-Control group were excluded if they endorsed a clinically significant sleep disorder (e.g., insomnia, hypersomnia, delayed sleep phase, sleep apnea, restless leg syndrome, periodic leg movement, etc.) in the last six months and/or if they reported difficulty falling asleep (>30 minutes), difficulty maintaining sleep (wake after sleep onset >30 minutes), and/or waking up too early (early morning awakening >30 minutes) at least three times in the last month. Sleep medications and sleep aids, including hypnotic use, off-label prescriptions, over-the-counter sleep aids, and alcohol and/or marijuana use with the intention of promoting sleep, were also exclusionary.
2.2. Measures
2.2.1. Clinical Measures
The Structured Clinical Interview for DSM-IV (SCID; First et al., 1995) is a validated semi-structured clinical interview to assess DSM-IV-TR Axis I disorders (Skre et al., 1991, Williams et al., 1992). Diagnostic reliability using the SCID in this study was exceptional (Harvey et al., 2015).
Young Mania Rating Scale (YMRS; Young et al., 1978)
The YMRS is an 11-item measure of manic symptom severity (Young et al., 1978).
Inventory of Depressive Symptomatology, Clinician Rating (IDS-C; Rush et al., 1996)
The IDS-C is a 30-item measure of depressive symptom severity (Rush et al., 1996). Sleep items were removed from IDS-C total scores.
Pharmacotherapy Tracking Log (Harvey et al., 2015)
A Pharmacotherapy Tracking Log was used to record medication name and dosage.
2.2.2. Sleep Measures
Insomnia Severity Index (ISI)
The ISI is a well-validated, seven-item measure of insomnia severity in the past week (Bastien et al., 2001; Morin and Barlow, 1993; Morin et al., 2011).
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a well-validated measure of sleep quality in the past month (Buysse et al., 1989). A global sleep quality score of greater than or equal to five is considered indicative of clinically significant sleep disturbance (Rush et al., 1996).
Duke Structured Interview for Sleep Disorder (DSISD)
The DSISD is a reliable and valid semi-structured interview used to assess DSM-IV-TR (American Psychiatric Association, 2000), ICSD-2 (American Academy of Sleep Medicine, 2005), and research diagnostic criteria for sleep disorders (Edinger et al., 2009). Diagnostic reliability using the DSISD in this study was exceptional (Harvey et al., 2015).
Sleep Diary
Sleep diaries are a standard and well-validated daily self-report measure of sleep (Buysse et al., 2006). Average TWT, TST, TWTvar, and TSTvar were derived from sleep diaries. TWT was calculated by adding sleep onset latency (SOL; the amount of time it took each participant to fall asleep), wake after sleep onset (WASO; the number of minutes spent awake in bed after initial sleep onset), and early morning awakenings (EMA; minutes spent awake in the morning before getting out of bed that day) for each night and then averaging across the seven days. TST for each night was calculated by subtracting SOL, WASO, and EMA from total time in bed and then averaging across the week. TWTvar and TSTvar were calculated by using the root mean squared successive difference (RMSSD) due to the ability of RMSSD to detect night-to-night changes in TWT and TST across the week (e.g., Straus et al., 2015). The following formula was used to examine night-to-night variability of TWT and TST:
Neuropsychological Tests
Working Memory: The N-Back Task and Digit Span Task
The N-Back tests the effect of working memory load on performance (Cohen et al., 1997; Owen et al., 2005). Participants are presented with a sequence of letters and are required to provide a motor response whenever a specific letter repeats itself n-steps earlier in the sequence. A computerized N-Back was administered incorporating four conditions: 0-back, 1-back, 2-back, and 3-back. Participants completed three blocks for each condition. Blocks were presented in a random order. D-prime (d’) scores were calculated by subtracting the z-score for number of false positives from the z-score of number of correct hits for each N-back condition.
The digit span task is derived from the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV; Wechsler, 2008) and assesses the load capacity of working memory. Participants are required to repeat a dictated series of digits forward and a different series of digits backward. Both forward and backward digit span begin with two digits and keep increasing in length and difficulty. Total number of correct sequences were calculated for forward and backward digit span.
Verbal Learning: The Verbal Learning Task
A computerized verbal learning task shown to be sensitive to the effects of sleep loss was administered (Drummond et al., 2000). This task alternates between four experimental and five baseline blocks, starting and ending with a baseline block. Six unrelated words are presented during each block. Participants are told not to memorize the baseline words. Participants are instructed to actively memorize the experimental words for later testing. A total of 24 experimental words are presented. Immediately following word presentation, participants were asked to write down as many words that they could remember from the experimental blocks (immediate recall condition). Approximately 30 minutes later, participants were asked to do the same thing (delayed recall condition). D-prime (d’) scores were calculated by subtracting the z-score for number of false positives from the z-score of number of correct hits for both the immediate recall and delayed recall conditions.
2.3. Procedure
This study was carried out in accordance with the latest version of the Declaration of Helsinki. All study procedures were approved by the Committee for Protection of Human Subjects at the University of California, Berkeley. Participants in the BD-Insomnia group were recruited as part of a larger NIMH-funded treatment study (R34MH080958). Participants in the BD-Control group were recruited separately. All participants were recruited through Internet advertisements and flyers distributed to psychiatric clinics in the community.
Participants first completed a phone screen to establish preliminary eligibility. Participants were then invited for a pre-treatment clinical assessment. Participants completed a daily sleep dairy during the week prior to the clinical assessment. During the clinical assessment, written informed consent was obtained and clinician- and self-report measures were collected (e.g., SCID, DSISD, ISI, PSQI). Medication use was established using the Pharmacotherapy Tracking Log (Harvey et al., 2015). Bipolar diagnoses were confirmed using the Structured Clinical Interview for Axis I Disorders (SCID; First et al., 1995). The IDS-C and YMRS, in addition to the information obtained during the SCID, were used to establish a current inter-episode state. In the BD-Insomnia group, the DSISD (Edinger et al., 2009) and sleep diaries were used to establish insomnia diagnoses. In the BD-Control group, the DSISD and sleep diaries were used to screen for the presence of a sleep disorder in the last six months.
Approximately one week after the pre-treatment clinical assessment, participants in the BD-Insomnia group returned to the lab for their pre-treatment cognitive assessment. Participants in the BD-Control group were given a lunch break after the clinical assessment and were asked to return to the lab that afternoon for their cognitive assessment. The rationale for completing the pre-treatment clinical assessment and pre-treatment cognitive assessment during the same day for the BD-Control group was to reduce participant burden, as these participants were not returning to the lab for sleep treatment. During the pre-treatment cognitive assessment, participants completed the N-back, digit span, and verbal learning tasks. Cognitive assessments were always initiated between 3:00 PM and 4:00 PM in order to control for circadian differences that have been shown to influence cognition (e.g., Dijk et al., 1992).
BD-Insomnia participants were randomized to one of two treatment conditions: CBTI-BD or a Psychoeducation (PE) control condition (Harvey et al., 2015). Please refer to Harvey et al., 2015 for specifics about the treatment conditions. Following eight weekly sessions of treatment, participants returned to the lab for a post-treatment assessment. Post-treatment assessment procedures were identical to the pre-treatment clinical and cognitive assessments. In order to eliminate possible confounding variables (e.g., fatigue, exposure to tasks, circadian effects, etc.), all measures were completed in the same order as the pre-treatment assessment, different versions of the cognitive tasks were administered, and the post-treatment assessment was also conducted between 3:00 PM and 4:00 PM. BD-Insomnia participants completed sleep diaries for the duration of the study.
2.4. Analysis Plan
2.4.1. Preliminary Data Analysis
Working memory and verbal learning composite scores were calculated and served as the dependent variables for the study aims. For the first aim, the working memory composite score was derived from the pre-treatment d’ scores for each N-back condition (0-back, 1-back, 2-back, and 3-back) and forward and backward digit span z-transformations. These 6 scores were then averaged to create a working memory composite, which served as one of the dependent variables for aim one. A separate composite score was calculated for verbal learning performance by averaging pre-treatment d’ scores for immediate recall and delayed recall. This verbal learning composite score served as the second dependent variable (e.g., Neuchterlein et al., 2008; Nuechterlein et al., 2008) for the first aim. For the second aim, we calculated working memory and verbal learning composite scores from the post-treatment assessment using the same procedure described above. Change scores were then calculated by subtracting pre-treatment working memory and verbal learning composite scores from post-treatment working memory and verbal learning composite scores. The change score for working memory and verbal learning served as the dependent variables for aim two.
Inclusion of potential covariates was determined using established methods. Bivariate correlations and independent sample t-tests were used to establish the associations between cognitive outcome variables and baseline sociodemographic/clinical features. Continuous variables with a correlation coefficient of greater than 0.30 and categorical variables with an effect size of greater than 0.30 were included as covariates in subsequent analyses (e.g., Pocock et al., 2002). Based on these analyses, age was included as a covariate for all primary data analyses.
Independent sample t-tests and chi-squared tests assessed baseline differences in demographic, clinical, and sleep features across the BD-Insomnia and BD-Control groups.
2.4.2. Primary Analysis Plan
All statistical analyses were performed with IBM SPSS Statistics, Version 22. Hierarchical linear regressions examined the two aims of the study. All continuous independent variables were mean centered. Group status was dummy coded (Aim one: 0=BD-Control, 1=BD-Insomnia; Aim two: 0=PE, 1=CBTI-BD). Group-by-sleep interaction terms were calculated by multiplying dummy coded group status by mean-centered continuous insomnia variables.
For aim one, hierarchical linear regressions tested the extent to which insomnia diagnosis and subjective insomnia-related sleep disruptions have an independent or interactive effect on working memory and verbal learning performance. Using a stepwise approach, age was introduced in the first level, group status and continuous insomnia-related sleep variables in the second level, and interaction terms in the third level for each hierarchical linear regression.
BD-Insomnia participants who completed the post-treatment cognitive assessment were included in the analyses for the second aim (Total N = 38; CBTI-BD: N = 20; PE: N = 18). Change scores were calculated for sleep and cognitive outcome variable so that all positive change scores indicated an improvement in sleep and cognition. Hierarchical linear regressions tested the extent to which treatment group and improvement in subjective sleep parameters following treatment had an effect on improvement in working memory and verbal learning performance. Using a stepwise approach, age was introduced in the first level, treatment group and change score for each sleep variable were introduced in the second level, and interaction terms were introduced in the third level for each hierarchical linear regression.
3. Results
3.1. Preliminary Data Analyses
3.1.1. Demographic, Clinical, and Sleep Characteristics
Participant characteristics are reported in Table 1. The two groups did not differ on any demographic characteristic with the exception of age; the BD-Control group was significantly younger than the BD-Insomnia group (p<0.01). No significant group differences were observed for manic symptom severity (YMRS), duration of bipolar disorder, or number of lifetime mood episodes. The BD-Insomnia group reported greater depressive symptom severity (IDS-C, p<0.05). The BD-Insomnia group also reported higher rates of antidepressant and hypnotic use (p<0.001), but lower rates of mood stabilizer use (p<0.001) than BD-Control group. BD-Control participants were more frequently medication-free than BD-Insomnia participants (p<0.05). The BD-Insomnia group had greater insomnia severity (ISI, p<0.001), poorer sleep quality (PSQI, p<0.001), and less sleep continuity as assessed by the sleep diary (i.e., TWT = p<0.01, number of awakenings = p<0.05, and sleep efficiency = p<0.01). Average bedtime, average wake time, duration of early morning awakenings, TST, and all the sleep variability variables did not differ between groups.
Table 1.
Baseline Demographic and Clinical Characteristics of the BD-Insomnia Group and BD-Control Group
| BD-Insomnia(n=47) | BD-Control(n=19) | Statistical Test | |
|---|---|---|---|
|
| |||
| Demographic Information | |||
| % Female | 63.0% | 65.2% | χ2 (1) = 0.04 |
| Age (mean years ± SD) | 36.76 ± 11.23 | 30.74 ± 10.06 | t (64) = 2.22* |
| Education (mean years ± SD) | 15.67 ± 4.39 | 15.03 ± 5.70 | t (64) = 0.49 |
| Ethnicity | |||
| % Hispanic or Latino | 12.78% | 13.04% | χ2 (1) = 0.25 |
| % Not Hispanic or Latino | 85.11% | 86.96% | χ2 (1) = 0.06 |
| Race | |||
| % American Indian/Alaska Native | 1.85% | 0.00% | χ2 (1) = 0.43 |
| % Asian | 9.26% | 4.35% | χ2 (1) = 0.25 |
| % African American | 11.11% | 13.04% | χ2 (1) = 0.06 |
| % Caucasian | 66.67% | 52.17% | χ2 (1) = 1.42 |
| % Bi-racial | 7.40% | 14.39% | χ2 (1) = 1.73 |
| % Declined to Answer | 3.70% | 0.00% | χ2 (1) = 0.88 |
| Mood Variables | |||
| IDS-C (mean score ± SD) | 8.26 ± 6.90 | 5.17 ± 4.20 | t (64) = 1.99* |
| YMRS (mean score ± SD) | 3.81 ± 3.24 | 3.13 ± 3.11 | t (64) = 0.85 |
| Illness Duration (mean years ± SD) | 15.19 ± 9.97 | 11.44 ± 7.41 | t (64) = 1.46 |
| # Mood Episodes | 11.93 ± 13.61 | 7.06 ±5.38 | t (64) = 1.47 |
| # Manic Episodes (mean ± SD) | 5.55 ± 6.27 | 3.00 ± 2.11 | t (64) = 1.68 |
| # Depressive Episodes (mean ± SD) | 6.38 ± 8.37 | 4.06 ± 3.89 | t (64) = 1.13 |
| Psychotropic Medication Use | |||
| % Mood Stabilizers | 17.24% | 60.87% | χ2 (1) = 14.73*** |
| % Antidepressants | 53.44% | 13.04% | χ2 (1) = 10.30*** |
| % Antipsychotics | 65.52% | 43.48% | χ2 (1) = 3.52 |
| % Anxiolytics | 0.00% | 4.35% | χ2 (1) = 2.49 |
| % Hypnotics | 60.34% | 0.00% | χ2 (1) = 23.76*** |
| % Anticonvulsant | 56.90% | 4.35% | χ2 (1) = 15.81*** |
| % Stimulants | 12.07% | 0.00% | χ2 (1) = 3.14 |
| % No medications | 5.17% | 21.74% | χ2 (1) = 4.89* |
| Sleep Variables | |||
| ISI (mean score ± SD) | 18.28 ± 4.31 | 3.83 ± 3.73 | t (64) = 14.00*** |
| PSQI (mean score ± SD) | 10.59 ± 5.05 | 3.43 ± 2.15 | t (64) = 6.53*** |
| Sleep Diaries | |||
| BT (mean ± SD) | 0:11 ± 1.62 | 0:44 ± 1.41 | t (64) = 0.83 |
| WT (mean ± SD) | 8:17 ± 2.06 | 8:36 ± 1.24 | t (64) = 0.69 |
| TWT (mean mins. ± SD) | 98.03 ± 59.05 | 57.12 ± 37.94 | t (64) = 3.05** |
| NWAK (mean number ± SD) | 0.11 ± 2.16 | 0.93 ± 0.69 | t (64) = 2.49* |
| TST (mean mins. ± SD) | 433.39 ± 92.97 | 464.71 ± 67.68 | t (64) = 1.46 |
| SE (mean percentage ± SD) | 81.52 ± 10.26 | 89.00 ± 7.31 | t (64) = 3.16** |
| BT Variability (mean mins. ± SD) | 92.97 ± 52.97 | 70.99 ± 49.63 | t (64) = 1.70 |
| WT Variability (mean mins. ± SD) | 113.14 ± 87.50 | 75.51 ± 28.45 | t (64) = 1.92 |
| TWT Variability (mean mins. ± SD) | 76.10 ± 66.49 | 48.03 ± 44.23 | t (64) = 1.82 |
| TST Variability (mean mins. ± SD) | 128.45 ± 77.84 | 100.56 ± 74.28 | t (64) = 1.44 |
| SE Variability (mean mins. ± SD) | 13.48 ± 11.68 | 9.52 ± 9.14 | t (64) = 1.42 |
Note.
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
BT=bedtime; WT=wake time; TWT=total wake time; NWAK=number of nocturnal awakenings; TST=total sleep time; SE=sleep efficiency.
3.2. Primary Data Analyses
Regression models for aim one are presented in Table 2. Insomnia diagnosis was not a significant predictor of verbal learning or working memory performance. Independent of insomnia diagnosis, greater TSTvar predicted poorer verbal learning (p=0.002) and poorer working memory performance (p=0.004). No interactions were significant.
Table 2.
Working Memory and Verbal Learning Performance Predicted by Insomnia Diagnosis, Insomnia-related Sleep Disruptions, and Group BY Insomnia-related Sleep Disruptions, Adjusting for Participant Age
| Working Memory
|
Verbal Learning
|
|||||
|---|---|---|---|---|---|---|
| B | SE | Beta | B | SE | Beta | |
| Step 1: Participant Characteristics | ||||||
| Age | −0.03 | 0.01 | −0.46*** | −0.05 | 0.02 | −0.35** |
| Step 2: Insomnia Diagnosis and Insomnia-Related Sleep Disruptions | ||||||
| R2 Change | 0.21** | 0.16 | ||||
| Group (BD-Insomnia vs. BD-Control) | 0.52 | 0.01 | 0.16 | 0.27 | 0.44 | 0.08 |
| TWT | −0.01 | 0.01 | −0.17 | −0.01 | 0.01 | −0.05 |
| TST | −0.01 | 0.01 | −0.13 | −0.01 | 0.01 | −0.09 |
| TWTvar | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.21 |
| TSTvar | −0.01 | 0.01 | −0.37** | −0.01 | 0.01 | −0.39** |
| Step 3: Insomnia Diagnosis (Group) BY Insomnia-Related Sleep Disruption Interaction | ||||||
| R2 Change | 0.01 | 0.02 | ||||
| Group X TWT | 0.01 | 0.01 | 0.08 | 0.01 | 0.02 | 0.21 |
| Group X TST | 0.01 | 0.01 | 0.14 | −0.01 | 0.01 | −0.18 |
| Group X TWTvar | 0.01 | 0.01 | 0.05 | −0.01 | 0.01 | −0.33 |
| Group X TSTvar | −0.01 | 0.01 | −0.10 | 0.01 | 0.01 | 0.01 |
Values reported are unstandardized coefficients (B), standard error of B (SE), standardized regression coefficients (Beta) with significance of t and R2 changes with significance of F.
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
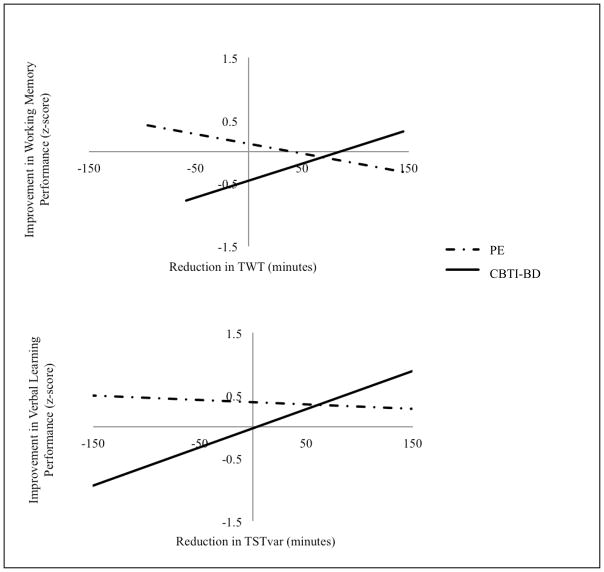
Regression models for aim two are presented in Table 3. Treatment group (CBTI-BD vs. PE) was not a significant predictor of improved cognitive performance. There was a main effect of TST when examining change in verbal learning performance; a decrease in TST was associated with an improvement in verbal learning (p=0.05). There were two significant interactions. Treatment group by reduction in TWT predicted improved working memory performance (p=0.05) and treatment group by reduction in TSTvar predicted improved verbal learning performance (p<0.03). Interaction plots are presented in Figure 1. Follow-up bivariate correlation analyses for each treatment group demonstrated that in the CBTI-BD group, a reduction in TWT was associated with improved working memory performance (r=0.30 vs. PE: r=−0.34) and a reduction in TSTvar was associated with improved verbal learning performance (r=0.45 vs. PE: r=−0.07).
Table 3.
Improvements in Working Memory and Verbal Learning Performance Predicted by Treatment Group, Reductions in TWT, Increase in TST, Reductions in TWTvar, and Reductions in TSTvar, Adjusting for Participant Age
| Improvement in Working Memory
|
Improvement in Verbal Learning
|
|||||
|---|---|---|---|---|---|---|
| B | SE | Beta | B | SE | Beta | |
| Step 1: Participant Characteristics | ||||||
| Age | 0.01 | 0.01 | 0.13 | −0.01 | 0.02 | −0.05 |
| Step 2: Treatment Group and Change in Insomnia-related Sleep Disruptions | ||||||
| R2 Change | 0.05 | 0.27 | ||||
| Treatment Group (CBTI-BD vs. PE) | −0.28 | 0.28 | −0.19 | −0.30 | 0.45 | −0.11 |
| Reduction in TWT | 0.01 | 0.01 | 0.14 | 0.01 | 0.01 | 0.01 |
| Increase in TST | 0.01 | 0.02 | 0.06 | −0.01 | 0.01 | −0.38* |
| Reduction in TWTvar | −0.01 | 0.01 | −0.02 | 0.01 | 0.04 | 0.01 |
| Reduction in TSTvar | −0.01 | 0.01 | −0.26 | 0.01 | 0.01 | 0.40* |
| Step 3: Treatment Group BY Change in Insomnia-Related Sleep Disruptions Interactions | ||||||
| R2 Change | 0.15 | 0.22* | ||||
| Treatment Group X Reduction in TWT | 0.02 | 0.01 | 0.90* | −0.01 | 0.01 | −0.31 |
| Treatment Group X Increase in TST | −0.01 | 0.01 | −0.22 | −0.01 | 0.01 | −0.36 |
| Treatment Group X Reduction in TWTvar | −0.01 | 0.01 | −0.45 | −0.01 | 0.01 | −0.14 |
| Treatment Group X Reduction TSTvar | −0.01 | 0.01 | −0.15 | 0.01 | 0.01 | 0.66* |
Values reported are unstandardized coefficients (B), standard error of B (SE), standardized regression coefficients (Beta) with significance of t and R2 changes with significance of F.
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Figure 1.
Improved Cognitive Performance Predicted by Treatment Group by Improvement in Sleep Interactions. Interaction plots demonstrating changes in cognitive performance predicted by treatment group (PE vs. CBTI-BD) by changes in sleep interactions.
TST= total sleep time; TWT= total wake time; PE= psychoeducation treatment condition; CBTI-BD= cognitive behavioral therapy for insomnia for inter-episode bipolar disorder.
4. Discussion
The overarching goal of the present study was to examine the impact of insomnia diagnosis and subjective insomnia-related sleep disruptions on working memory and verbal learning performance during inter-episode bipolar disorder. Results from the first aim demonstrated a main effect of TSTvar. More specifically, greater TSTvar predicted poorer working memory and verbal learning performance, regardless of insomnia diagnosis. Insomnia diagnosis did not predict working memory or verbal learning performance. This is consistent with the literature demonstrating no differences in objective cognitive performance when comparing an insomnia sample to healthy sleepers (Drummond et al., 2013; Orff et al., 2007; Varkevisser et al., 2007). Instead, results suggest that there are certain sleep disruptions common to insomnia and the inter-episode phase of bipolar disorder – more specifically inconsistent TST across the week – that are related to cognition. Further, these results are the first to demonstrate an association between TSTvar and cognition and suggest that future studies should include measures of TSTvar when examining associations between sleep and cognition.
Consistent with our hypothesis, results from the second aim demonstrated that an improvement in sleep was associated with an improvement in cognition following CBTI-BD. More specifically, following CBTI-BD, (a) reduction in TWT was associated with an improvement in working memory and (b) reduction in TSTvar was associated with an improvement in verbal learning. These results demonstrate that not only is baseline TSTvar associated with impaired cognition, but stabilization of TSTvar and TWT is also related to improvement on cognitive tasks. Taken together, the results from both aims suggest that TWT and TSTvar may be two possible pathways underlying cognitive dysfunction in bipolar disorder. Moreover, these results further highlight the importance of treating sleep disturbance during the inter-episode phase.
There were several surprising and noteworthy results. First, results the second aim demonstrated that a decrease in TST following CBTI-BD and PE was associated with an improvement in verbal learning (p=0.05). One possible explanation is that studies of habitual sleep suggest a U-shaped association between TST and cognition whereby receiving fewer than 6 hours of sleep nightly or more than 9 hours of sleep nightly are both associated with cognitive impairment (Ferrie et al., 2011; Kronholm et al., 2009). Given the prevalence of hypersomnia and long TST during inter-episode bipolar disorder (Kaplan et al., 2011; Kaplan et al., 2015; Ritter et al., 2012), it is possible that this result is being driven by long-sleepers (>9 hours per night). More specifically, in long-sleepers, receiving less sleep may be beneficial for cognitive performance. There were too few long-sleepers in the BD-Insomnia group to test this hypothesis (N=5), but notably the long-sleepers had the largest decrease in TST (61.9 minutes fewer) and the largest improvement in verbal learning (z-score increase of 0.67) at the post-treatment assessment A second noteworthy finding was that there were no significant between group differences for variability of sleep diary parameters (see Table 1). This suggests that circadian and sleep instability is inherent to bipolar disorder, regardless of insomnia diagnosis (Levenson and Frank, 2010; Ng et al., 2015).
Results from this study should be interpreted in light of several limitations. First, we did not adjust for the use of psychotropic medications. Notably, side effects of psychotropic medications (e.g., cognitive impairment) often wear off or diminish as people continue on a medication course (Ketter and Wang, 2002) and participants in this study were required to be on a stable medication regimen for at least four weeks prior to study enrollment. We highlight that research on medication-free bipolar samples is unrepresentative and lacks generalizability (Phillips et al., 2008). Second, it is possible that the BD-Insomnia group and BD-Control completed the sleep diaries with differing accuracy due to differences in sleep perception as a result of having a psychiatric and/or insomnia diagnosis (e.g., sleep state misperception that can occur in insomnia and/or mental health conditions (Bliwise et al., 1993; Edinger and Krystal, 2003; Gonzalez et al., 2013)). Future studies should consider utilizing adjunctive prospective objective measures of sleep such as actigraphy to address some limitations of subjective measures. Third, this is a preliminary study and thus, we did not adjust for multiple comparisons. Ideally, results from this study will be used to inform future studies that are sufficiently powered. Fourth, results from this study may have been strengthened by comparing cognitive performance in the BD-Insomnia group to an insomnia sample and cognitive performance in the BD-Control group to a healthy control sample. Future studies should consider utilizing this study design.
Despite these limitations, this study contributes to the literature by demonstrating that subjective insomnia-related sleep disruptions are associated with deficits in working memory and verbal learning performance during inter-episode bipolar disorder. Given the functional impairment associated with both sleep disturbance and cognitive dysfunction, this study further highlights the importance of treating sleep disturbance during the inter-episode phase of BD.
Acknowledgments
Role of Funding Source
This project was supported by a National Institute of Mental Health Grant No. R34MH080958 awarded to AGH
The authors would like to thank Jason Lee, Jillian Tessier, and Anita Satish for their contributions to data collection, Jillian Tessier for her contribution to the literature search, and Kerrie Hein for her continued support throughout the study.
Footnotes
Contributions
All authors were involved in the conception, drafting and final approval of this manuscript.
References
- Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatric disease and treatment. 2007;3(5):553. [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. American Acad. of Sleep Medicine; 2005. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual-text revision (DSM-IV-TRim, 2000) American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Ausubel DP. The psychology of meaningful verbal learning. 1963. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S, Ensrud KE, Song Y, Stone KL. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37(4):655. doi: 10.5665/sleep.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, Friedman L, Yesavage JA. Depression as a confounding variable in the estimation of habitual sleep time. Journal of Clinical Psychology. 1993;49(4):471–477. doi: 10.1002/1097-4679(199307)49:4<471::aid-jclp2270490403>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: toward an integrated examination of disorder maintenance and functional impairment. Clinical psychology review. 2013;33(1):33–44. doi: 10.1016/j.cpr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland EM, Stange JP, Adams AM, LaBelle DR, Ong ML, Hamilton JL, Connolly SL, Black CL, Cedeño AB, Alloy LB. Associations between sleep disturbance, cognitive functioning and work disability in Bipolar Disorder. Psychiatry research. 2015;230(2):567–574. doi: 10.1016/j.psychres.2015.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-lsrael S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep: Journal of Sleep and Sleep Disorders Research. 2006 doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Cheng Y, Germain A, Moul DE, Franzen PL, Fletcher M, Monk TH. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep medicine. 2010;11(1):56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. The Journal of Neuroscience. 2004;24(19):4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. Journal of sleep research. 1992;1(2):112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Drummond S, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural correlates of working memory performance in primary insomnia. Sleep. 2013;36(9):1307–1316. doi: 10.5665/sleep.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403(6770):655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- Edinger J, Wyatt J, Olsen M, Stechuchak K, Carney C, Chiang A, Krystal A, Lineberger M, Means M, Radtke R. Sleep. American Academy of Sleep Medicine; 2009. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening; pp. A265–A265. [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, McCall WV, Morin CM, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep medicine reviews. 2003;7(3):203–214. doi: 10.1053/smrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter-episode bipolar disorder. Journal of behavior therapy and experimental psychiatry. 2010;41(2):145–149. doi: 10.1016/j.jbtep.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, Vela-Bueno A, Ramos-Platon MJ, Sauder KA, Vgontzas AN. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34(5):565–573. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Frankel BL, Coursey RD, Buchbinder R, Snyder F. Recorded and reported sleep in chronic primary insomnia. Archives of General Psychiatry. 1976;33(5):615–623. doi: 10.1001/archpsyc.1976.01770050067011. [DOI] [PubMed] [Google Scholar]
- Geoffroy PA, Boudebesse C, Bellivier F, Lajnef M, Henry C, Leboyer M, Scott J, Etain B. Sleep in remitted bipolar disorder: a naturalistic case-control study using actigraphy. Journal of affective disorders. 2014;158:1–7. doi: 10.1016/j.jad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Tamminga C, Tohen M, Suppes T. Comparison of objective and subjective assessments of sleep time in subjects with bipolar disorder. Journal of affective disorders. 2013;149(1–3):363–366. doi: 10.1016/j.jad.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Wang PW, Brooks JO, Thase ME, Sachs GS, Ketter TA. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Journal of affective disorders. 2009;114(1):41–49. doi: 10.1016/j.jad.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Schmidt AD, Scarnà A, Semler C, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry. 2005;162(1):50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Soehner AM, Kaplan KA, Hein K, Lee J, Kanady J, Li D, Rabe-Hesketh S, Ketter TA, Neylan TC. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: A pilot randomized controlled trial. Journal of consulting and clinical psychology. 2015;83(3):564. doi: 10.1037/a0038655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar disorders. 2005;7(2):176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Archives of General Psychiatry. 2003;60(3):261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- Kanady JC, Soehnera AM, Harvey AG. A Retrospective Examination of Sleep Disturbance across the Course of Bipolar Disorder. Journal of sleep disorders & therapy. 2015;4(2) doi: 10.4172/2167-0277.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KA, Gruber J, Eidelman P, Talbot LS, Harvey AG. Hypersomnia in inter-episode bipolar disorder: does it have prognostic significance? Journal of affective disorders. 2011;132(3):438–444. doi: 10.1016/j.jad.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KA, McGlinchey EL, Soehner A, Gershon A, Talbot LS, Eidelman P, Gruber J, Harvey AG. Hypersomnia subtypes, sleep and relapse in bipolar disorder. Psychological medicine. 2015;45(08):1751–1763. doi: 10.1017/S0033291714002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, Wang PW. In: Bipolar Disorder: A Clinician’s guide to biological treatments. Yatham LM, Kusumaker V, editors. Brunner-Routledge; New York, New York: 2002. [Google Scholar]
- Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. Journal of sleep research. 2009;18(4):436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- Levenson J, Frank E. Behavioral Neurobiology of Bipolar Disorder and its Treatment. Springer; 2010. Sleep and circadian rhythm abnormalities in the pathophysiology of bipolar disorder; pp. 247–262. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological bulletin. 2010;136(3):375. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Ikram MA, Tiemeier H. Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep medicine. 2015;16(7):850–855. doi: 10.1016/j.sleep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Marriott M, Begin H, Robb J, Joffe RT, Young LT. Subsyndromal symptoms assessed in longitudinal, prospective follow-up of a cohort of patients with bipolar disorder. Bipolar disorders. 2003;5(5):349–355. doi: 10.1034/j.1399-5618.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- McCrae CS, Vatthauer KE, Dzierzewski JM, Marsiske M. Habitual sleep, reasoning, and processing speed in older adults with sleep complaints. Cognitive therapy and research. 2012;36(2):156–164. doi: 10.1007/s10608-011-9425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Archives of general psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. Journal of affective disorders. 2004;80(2):145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Morin CM, Barlow DH. Insomnia: Psychological assessment and management. Guilford Press; New York: 1993. [Google Scholar]
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology. 2010 doi: 10.1177/0891988710363710. [DOI] [PubMed] [Google Scholar]
- Neuchterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frederick FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Ng TH, Chung KF, Ho FYY, Yeung WF, Yung KP, Lam TH. Sleep–wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep medicine reviews. 2015;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese PD, III, Frederick J, Gold JM. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Orff HJ, Drummond SP, Nowakowski S, Perlis ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. SLEEP-NEW YORK THEN WESTCHESTER- 2007;30(9):1205. doi: 10.1093/sleep/30.9.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human brain mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practiceand problems. Statistics in medicine. 2002;21(19):2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- Ritter PS, Marx C, Lewtschenko N, Pfeiffer S, Leopold K, Bauer M, Pfennig A. The characteristics of sleep in patients with manifest bipolar disorder, subjects at high risk of developing the disease and healthy controls. Journal of neural transmission. 2012;119(10):1173–1184. doi: 10.1007/s00702-012-0883-y. [DOI] [PubMed] [Google Scholar]
- Robb JC, Cooke RG, Devins GM, Young LT, Joffe RT. Quality of life and lifestyle disruption in euthymic bipolar disorder. Journal of Psychiatric Research. 1997;31(5):509–517. doi: 10.1016/s0022-3956(97)00030-7. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of affective disorders. 2006;93(1):105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychological medicine. 1996;26(03):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Russo M, Mahon K, Shanahan M, Ramjas E, Solon C, Purcell SM, Burdick KE. The relationship between sleep quality and neurocognition in bipolar disorder. Journal of affective disorders. 2015;187:156–162. doi: 10.1016/j.jad.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Fulda S, Schulz H. Daytime variation in performance and tiredness/sleepiness ratings in patients with insomnia, narcolepsy, sleep apnea and normal controls. Journal of sleep research. 2004;13(4):373–383. doi: 10.1111/j.1365-2869.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the Structured Clinical Interview for DSM-III-R Axis I (SCID-I) Acta Psychiatrica Scandinavica. 1991;84(2):167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Straus LD, Drummond S, Nappi CM, Jenkins MM, Norman SB. Sleep Variability in Military-Related PTSD: A Comparison to Primary Insomnia and Healthy Controls. Journal of traumatic stress. 2015;28(1):8–16. doi: 10.1002/jts.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. Journal of Sleep Research. 2005;14(1):49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- Varkevisser M, Van Dongen HP, Van Amsterdam JG, Kerkhof GA. Chronic insomnia and daytime functioning: an ambulatory assessment. Behavioral sleep medicine. 2007;5(4):279–296. doi: 10.1080/15402000701557425. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, Fernández-Mendoza J, Bixler EO. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes. Diabetes care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert J, Kopf J, Kazmaier J, Glaser F, Zierhut K, Schiele M, Kittel-Schneider S, Reif A. Evidence for cognitive subgroups in bipolar disorder and the influence of subclinical depression and sleep disturbances. European Neuropsychopharmacology. 2015;25(2):192–202. doi: 10.1016/j.euroneuro.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-fourth. San Antonio, TX: The Psychological Corporation Google Scholar; 2008. [Google Scholar]
- Wilckens KA, Hall MH, Nebes RD, Monk TH, Buysse DJ. Changes in cognitive performance are associated with changes in sleep in older adults with insomnia. Behavioral sleep medicine. 2016;14(3):295–310. doi: 10.1080/15402002.2014.1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JBW, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B. The structured clinical interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Archives of general psychiatry. 1992;49(8):630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The World Health Report 2001: Mental health: new understanding, new hope. World Health Organization; 2001. [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nature neuroscience. 2007;10(3):385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]