Abstract
Ovariectomized rats that received previous administration of oestradiol in midlife display enhanced cognition and increased hippocampal levels of oestrogen receptor alpha (ERα) months after oestradiol treatment ended as compared to ovariectomized controls. Objectives of the current work were to investigate mechanisms by which ERα levels are maintained following midlife oestradiol exposure and the role of ERα in memory in aging females in the absence of circuiting oestrogens. Unliganded ERα has increased interaction with the ubiquitin ligase, C terminus of Hsc-70 interacting protein (CHIP) leading to increased degradation of the receptor. In our first experiment, we tested the hypothesis that midlife oestradiol exposure in ovariectomized rats results in decreased interaction between CHIP and hippocampal ERα, leading to increased levels of ERα. Middle-aged rats were ovariectomized and received oestradiol or vehicle implants. After 40 days, implants were removed. One month later, rats were killed and hippocampi processed for whole protein western blotting and co-immunoprecipitation, in which ERα was immunoprecipitated from lysate. As expected, ERα protein expression was increased in rats previously treated with oestradiol compared to vehicle-treated rats. In rats treated with oestradiol, there was a decrease in CHIP-ERα interaction, suggesting that previous oestradiol treatment reduces interaction, slowing degradation of ERα. In a second experiment, we determined the impact on memory of antagonism of ER in the absence of circulating oestrogens. Rats were ovariectomized and implanted with oestradiol capsules. Capsules were removed after 40 days. Rats received chronic i.c.v. infusion of ER antagonist, ICI 182,780 or aCSF vehicle and were tested on a spatial memory radial maze task. Rats treated with ICI 182,780 had significantly worse performance (more errors). These experiments provide evidence that previous midlife oestradiol treatment maintains hippocampal ERα by decreasing its interaction with CHIP and that activation of these receptors provides cognitive benefits in the absence of circulating oestrogens.
Keywords: oestradiol, memory, oestrogen receptor, degradation, hippocampus
Women spend approximately one-third of their lives post-menopause, a state characterized by a marked decrease in levels of circulating oestrogens and is associated with an increased risk of age-related dementias, including Alzheimer’s disease (1–4). Oestrogens, when administered during a critical period immediately following the cessation of ovarian function, is beneficial to cognition and hippocampal function (1,5–7). However, due to putative health-risks resulting from long-term exposure to oestrogens (8,9), current recommendations are that women use oestrogen therapy to treat menopausal symptoms for the shortest time possible.
The long-term implication for cognition and the brain of short-term, midlife oestrogen treatment is unclear. Results from several (10–13), but not all (14) studies indicate that short-term use of oestrogens following natural or surgical menopause can provide long-term cognitive benefits years after hormone treatment is terminated. Data from our lab using a rodent model of menopause also demonstrates long-term cognitive benefits resulting from short-term hormone treatment following ovariectomy. Ovariectomized, aged rats previously treated with 40 days of oestradiol, the primary oestrogen produced by the ovaries, in midlife show improved cognition on the hippocampal-dependent radial-arm maze task compared to ovariectomized, aged rats that were not exposed to oestradiol when tested one (15), 2.5 (16), and seven (16) months after termination of oestradiol exposure. Interestingly, we also found that rats previously treated with short-term, midlife oestradiol had increased levels of hippocampal oestrogen receptor alpha (ERα) at each of these time points when compared to ovariectomized rats that received no hormone treatment (15,16).
ERα, a classical nuclear receptor that acts as a transcription factor (17) is important for cognition in the aging brain. In Alzheimer’s patients, increased levels of ERα in the frontal cortex are associated with better cognition (18). Furthermore, ERα polymorphisms are associated with the onset of cognitive impairment in the elderly (19). Young adult, ovariectomized ERα knockout mice have impaired spatial memory in the absence of exogenously administered estrogens, an effect that is rescued by lenti-viral delivery of ERα to the hippocampus (20). Our lab has demonstrated that lenti-viral delivery of ERα to the hippocampus of aging female rodents, in the absence of circulating oestrogens, improves spatial cognition compared to ovariectomized rats that received vehicle (21). Collectively, these data from human and rodent models suggest that ERα can positively impact cognitive function in the absence of circulating oestrogens. There has yet to be a direct experimental test of the hypothesis that endogenous levels of ERα can impact memory in the absence of circulating oestrogens.
In addition to the question as to the functional impact for memory of lasting increased levels of hippocampal ERα following short-term midlife oestradiol exposure is the question of how ERα levels are maintained beyond the period of oestradiol exposure. Besides modification of ERα gene transcription, ERα levels can be impacted by changes in receptor degradation rate. Work in cell culture has demonstrated, in the absence of oestradiol, ERα is degraded by the E3 ubiquitin ligase, C terminus of Hsc70-interacting protein (CHIP; 22,23). When ERα is unliganded, it interacts with heat shock protein-90 (Hsp90) chaperone complexes, maintaining a competent conformation (24). If ERα is liganded, ERα dissociates from the chaperone complex and becomes transcriptionally active. However, if ERα remains unliganded, CHIP targets Hsp90 for proteasomal degradation, resulting in ubiquitination and degradation of ERα (22). In young adult rats, long-term oestrogen deprivation following ovariectomy resulted in increased interaction between CHIP and ERα, as demonstrated by co-immunoprecipitation (25). This increase in interaction was accompanied by decreased protein expression of ERα, an effect that was attenuated by administration of MG132, a proteasome inhibitor. From these data we hypothesize that midlife oestradiol treatment will impact the interaction between CHIP and ERα, protecting it from degradation even after hormone exposure has ended.
The objective of the current study was twofold. First, we aimed to elucidate mechanisms contributing to the long-term maintenance of hippocampal ERα following termination of short-term, midlife oestradiol treatment. Second, we aimed to determine the impact of endogenous ERα on cognition in the absence of circulating oestrogens. In our first experiment, we tested the hypothesis that prior, midlife oestradiol treatment maintains ERα in the aging female hippocampus by decreasing the interaction between ERα and the E3 ubiquitin ligase, CHIP. Rats were ovariectomized and treated with either vehicle or oestradiol capsules for 40 days. Hormone capsules were removed and one month later rats were sacrificed and hippocampi processed for co-immunoprecipitation of ERα and subsequent western blotting for ERα-associated CHIP as well as western blotting for total protein levels of ERα and CHIP. In the second experiment, we tested the hypothesis that antagonism of ER in the aging female brain impairs spatial cognition in the absence of circulating oestrogens. Rats were trained on the radial-arm maze, a hippocampal-dependent spatial memory task, ovariectomized, and treated with oestradiol capsules to optimize levels of ERα. Hormone capsules were removed and chronic i.c.v. infusion of vehicle or the anti-oestrogen, ICI 182,780, began. Spatial memory testing began one week after the initiation of drug infusion.
Materials and Methods
Experiment 1
Subjects
Twelve middle-aged female Long-Evans hooded rats, retired breeders (~11 months of age), were purchased from Harlan Sprague Dawley Inc. Animal care was in accordance with guidelines set by the National Institute of Health Guide for the Care and Use of Laboratory Animals), and the Institutional Animal Care and Use Committees of Tulane University approved all procedures. Rats were housed individually in a temperature-controlled vivarium under a 12-h light, 12-h dark cycle and had unrestricted access to food and water. See Figure 1A for an overview of experimental timeline.
Figure 1.
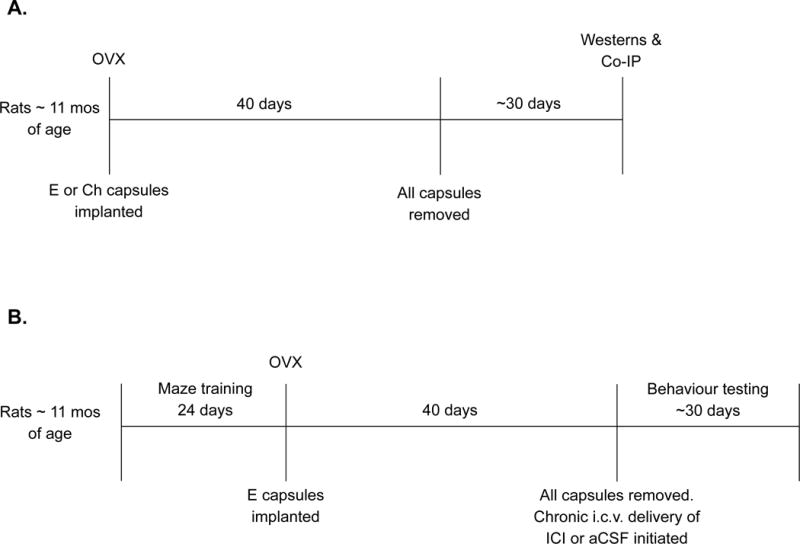
(A) Experiment 1 timeline. (B) Experiment 2 timeline. OVX, ovariectomy. E, oestradiol. Ch, Cholesterol. Co-IP, co-immunoprecipitation. ICI, ICI 182,780
Ovariectomy and hormone treatment
Rats were ovariectomized while under anesthesia induced by injection of ketamine (100 mg/kg ip; Bristol Laboratories, Syracuse, NY) and xylazine (7 mg/kg ip; Miles Laboratories, Shawnee, KS) and implanted with 5-mm SILASTIC brand capsules (0.058 in. inner diameter and 0.077 in. outer diameter; Dow Corning, Midland, MI) on the dorsal aspect of their necks. Capsules contained either 25% 17β-oestradiol (Sigma-Aldrich, St. Louis, MO) diluted with cholesterol (Previous E; n = 6) or 100% cholesterol vehicle (Previous Ch; n = 6). We reported previously that implants of these dimensions and oestradiol concentrations maintain blood plasma oestradiol levels in middle-age retired breeders at approximately 37 pg/mL (26), which falls in the physiological range. The ovariectomy model to induce cessation of ovarian function during the chronological equivalent of middle-age is a commonly used model of menopause. Whereas female rats undergo some of the same processes of reproductive aging as women, including cessation of reproductive cycles and loss of fertility, rats differ from humans in that middle-age is characterized by high levels of estrogens that are maintained for long periods (27).
Termination of hormone treatment
Forty days after ovariectomy and hormone capsule implantation, rats were anesthetized with ketamine and xylazine and all capsules were removed. Visual inspection confirmed their integrity.
Tissue dissection
Approximately 30 days after capsules were removed and oestradiol treatment had been terminated in the Previous E group, rats were killed by decapitation under anesthesia induced by ketamine and xylazine. This time-point is consistent with previous work in which we examined levels of hippocampal ERα 30 days following termination of oestradiol treatments in ovariectomized, aging rats (15). Hippocampi were dissected on ice, quick-frozen on dry ice, and stored at −80°C until processing.
Tissue processing
Hippocampal tissue was homogenized in 10 μL/mg IP Lysis/Wash Buffer included in the Pierce Classic Immunoprecipitation Kit (Pierce, Rockford, IL) then centrifuged at 13,000 × g for 10 min. Protein concentration of supernatant was determined using the Bradford Protein Assay Kit (Pierce). Half of the supernatant was used for total protein western blotting, while the other half was further processed for co-immunoprecipitation.
Western blotting sample preparation
Samples were diluted 1:1 with Laemmli Sample Buffer (Bio-Rad; Hercules, CA) mixed with 350 mM D,L-dithiothreitol, boiled for 5 min, and stored at -80°C.
Co-immunoprecipitation sample preparation
Supernatant containing 1 mg of protein was incubated overnight with 2 μg anti-ERα (H184; #sc7202, Santa Cruz; Santa Cruz, CA). Samples were then incubated for 1 h with Protein A/G beads. Following incubation, samples were eluted by non-reducing electrophoresis buffer and 20 mM DTT and boiled for 5 min followed by centrifugation at 1,000 × g. Elution was collected for western blotting.
Electrophoresis and western blotting
For total protein samples obtained from each rat, 22 μg of total protein were loaded and separated at 250 V on 7.5% SDS-PAGE gels (Bio-Rad) for 60 min in order to probe for ERα and CHIP protein levels. For samples in which we immunoprecipitated ERα, 15 μL of each sample were loaded and separated as described above in order to probe for ERα-associated CHIP protein levels. Molecular weight markers (Kaleidoscope, Bio-Rad) were included with each run. We have previously verified our procedures when probing for ERα using western blotting (5). Because multiple bands are evident in brain homogenate, uterus samples, which yield a single band in the area of interest, are included as positive controls for ERα. Proteins were transferred to nitrocellulose membranes at 100 V for 30 min. Membranes were blocked with 5% nonfat dry milk in 0.1% Tween/1 X Tris-buffered saline (TTBS) at room temperature for 1 h. Following block, membranes were incubated with primary antibody overnight at 4°C in 1% nonfat dry milk-TTBS. Primary antibodies used were for ERα (H184, rabbit polyclonal, 1:750; Santa Cruz) and CHIP (C3B6, rabbit polyclonal, 1:2000; #2080 Millipore). Blots were washed three times for 15 min each with TTBS and incubated with 5% nonfat dry milk containing secondary antibody conjugated to horseradish peroxidase for 1.5 h at room temperature. Secondary antibodies used were goat anti-rabbit IgG (ERα; 1:40,000, CHIP; 1:10,000; Santa Cruz) and for co-immunoprecipitated samples Clean-Blot IP Detection Reagent was used (1:2000; Pierce). Blots were washed again three times for 15 min each and incubated with the chemiluminescent substrate SuperSignal West Femto for 5 min (Fisher Scientific) and exposed to film (Kodak Biomax MR) for varying durations to capture optimal signal intensity. To verify there were no differences in initial loading of protein into wells, the loading control, β-actin was used. Blots that were previously probed for ERα and CHIP were washed and stripped with stripping buffer (RestorePlus Western Blot; Fisher Scientific) for 15 min at 37°C. Blots were then blocked and incubated with primary antibody for β-actin (mouse monoclonal, 1:15,000; Santa Cruz) overnight at 4°C in TTBS. Blots were washed three times for 15 min each with TTBS and incubated in 5% nonfat dry milk containing goat antimouse IgG (1:10,000; Santa Cruz) conjugated to horseradish peroxidase for 1.5 h at room temperature, washed, and detected by chemiluminescence. Films were imaged using MCID Core imaging software (InterFocus Imaging Ltd., Cambridge, England), and optical density × area was measured for bands of interest. Mean values for western blots were calculated from the previous cholesterol control samples. Values represent the percentage relative to the average control value.
Hormone treatment and ovariectomy efficacy
To confirm endocrine status, daily vaginal smears were collected by lavage during the final week of hormone treatment. Smears of ovariectomized, cholesterol-treated rats were characterized by a predominance of leukocytes, while smears of ovariectomized, oestradiol-treated rats were characterized by a predominance of cornified and nucleated epithelial cells indicating hormone treatment was effective. Two rats (1 Previous E and 1 Previous Ch) were excluded from the experiment because analysis of vaginal smears indicated the possibility that their hormone capsules had been switched. At the time rats were killed, right uterine horns were extracted and weighed to verify ovariectomy. All rats presented with atrophied uteri, indicating successful ovariectomy. Final number of rats included in the experiment was 10 (5 per group).
Statistical analyses
All data were analyzed by independent samples t-tests.
Experiment 2
Subjects
Seventeen middle-aged female Long-Evans hooded rats, retired breeders (~11 months of age), were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN). Animal care was conducted as described in Experiment 1. See Figure 1B for an overview of experimental timeline.
Maze training
Procedures were as previously described (16). One week after arrival, rats were trained on the radial-maze task, a test of spatial working memory. Rats were placed on diets and weighed daily to maintain body weights at 85–90% of pre-surgery weights and trained to obtain food rewards (Froot Loops; Kellogg Co., Battle Creek, MI) from the arms of an eight-arm radial maze purchased from Lafayette Instruments (Lafayette, IN). To begin a trial, a rat was placed in the center compartment in a pseudorandom orientation and had access to all eight arms. Arm choices were recorded by an observer seated in a fixed location approximately 1 m away from the maze. An arm choice was scored if the rat traversed halfway down an arm. Rats were allowed to choose arms in any order until all arms were visited or 5 min elapsed. Each animal received one trial per day across 24 days of acquisition.
Ovariectomy and hormone treatment
Following radial maze acquisition, rats were ovariectomized and implanted with oestradiol capsules. All rats received oestradiol capsules to optimize levels of ERα before antagonism of the receptor (15). Rats were trained on the radial maze acquisition task once per week to retain performance levels until testing (see below).
Termination of hormone treatment and initiation of drug treatment
Forty days after ovariectomies and hormone capsule implantation, rats were anesthetized with ketamine and xylazine and all capsules were removed. Visual inspection confirmed their integrity. Rats were then placed into a stereotaxic frame. An incision was made in the scalp and fascia that overlie the skull. A hole was drilled in the skull and cannulae (Brain Infusion Kits, Alzet; Cupertino, CA) were lowered through the hole to the appropriate depth (to the right lateral ventricle located −0.3 mm AP, +1.2 mm ML, and −4.5 mm DV relative to Bregma; (28) and anchored to the skull with screws and dental acrylic. Cannulae were connected to Alzet osmotic minipumps by vinyl tubing that delivered aCSF vehicle (Tocris; Ellisville, MO) or the oestrogen receptor antagonist ICI 182,780 (200 nM in 0.2% DMSO) (Sigma; St. Louis, MO) diluted in vehicle at a rate of 0.25 μl/h. ICI 182,780 prevents ER dimerization and suppresses ER-mediated transcription (29). All pumps were implanted s.c. in the nape of the neck and cannulae were inserted after the pumps began pumping. Approximately half of the rats received osmotic minipumps containing aCSF vehicle (aCSF, n = 8), and half received ICI 182,780 (ICI, n = 9).
Behavioural testing
Rats were allowed approximately one week to recover from surgeries before being tested on the radial-arm maze. Rats were re-trained in the maze for two days using the same acquisition protocol as described above. Performance of all rats was at pre-surgery levels. Behavioural testing consisted of daily delay trials in the radial maze during which various delays (1 min, 30 min, 2.5 h, 4 h, 5 h, 6 h) were imposed between the fourth and fifth arm choices to increase memory load. Delays increased memory load and required that rats remember over an extended period of time which arms had already been visited. After each fourth arm choice, the animal was removed from the maze and put in a holding cage in a separate room for delays. Then the animal was returned to the maze until the four remaining, still baited arms had been visited or until 5 min had elapsed. Arm choice accuracy was measured by the mean number of retroactive and proactive errors for each delay. A retroactive error is the first (and only the first) reentry into an arm visited before the delay. A proactive error is the reentry into an arm visited post delay. Rats were given one day of habituation to a one-minute delay trial. Subsequently, two daily trials were conducted for each increasingly longer delay beginning with a one-minute delay.
Cannulae placement confirmation
Coronal sections (20 μM) were taken from the right hemisphere of each brain, thaw-mounted onto gelatinized slides, stained with 0.5% cresyl violet and microscopically examined for verification of cannula placement. All animals received correct cannula placement.
Hormone treatment and ovariectomy efficacy
Two procedures were conducted to confirm endocrine status as in Experiment 1. Vaginal smears conducted during the final week of hormone treatment confirmed endocrine status in all rats. At the time of sacrifice, all rats presented with atrophied uterine horns, confirming success of ovariectomies.
Statistical analyses
Arm-choice accuracy data (number of retroactive and proactive errors) from each delay was averaged across the two days of testing and analyzed by two-way ANOVA (treatment × delay) with repeated measures on delay. Separate two-way ANOVAs across short (1 min, 1 hr, and 2.5 hr) and long (4 hr, 5 hr, 6 hr) delays were also conducted to investigate any differences that may be apparent only as memory load increased (30) because in our previous work the effect of manipulating hippocampal ERα levels via viral vectors on retroactive errors was evident across long, but not short delays (21).
Results
Experiment 1
Total protein western blotting
ERα
As illustrated in Figure 2A, previous exposure to oestradiol in aging, ovariectomized rats increased total protein levels of ERα in the hippocampus compared to cholesterol vehicle-treated rats, consistent with previous results (15,16). As in our previous work (15, 16), western blotting for ERα using the H184 antibody revealed multiple bands in brain homogenate. Therefore, rat uterus is used as a positive control to identify the band of interest. A band of ERα-like immunoreactivity at approximately 66 kDa was revealed in rat uterus and a band of the same molecular weight was revealed in brain samples. In addition, a single band of interest at approximately 43 kDa was detected on immunostaining for the loading control, β-actin. There was a significant effect of treatment on levels of ERα (t(8) = −2.533, p = .035) and no effect of treatment on levels of β-actin.
Figure 2.
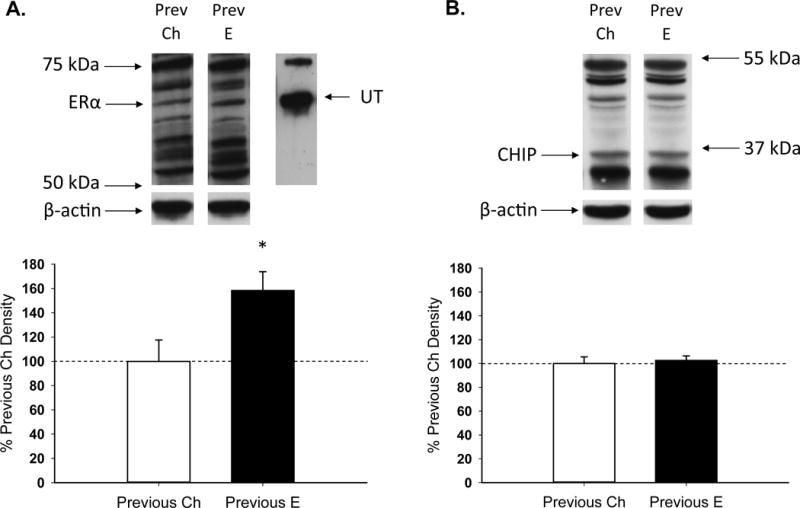
Effects of previous treatment with oestradiol on total ERα and C terminus of Hsc70-interacting protein (CHIP) levels in the hippocampus in aging, ovariectomized rats. Middle-aged rats were ovariectomized and implanted with either oestradiol (Previous E) or cholesterol vehicle (Previous Ch) capsules for 40 days. Hormone capsules were removed and one month later rats were killed. Hippocampi were processed for total protein western blotting. Western blot data showing the effects of treatments on hippocampal protein levels of (A) ERα, using uterus (UT) as a positive control to confirm band of interest, and (B) CHIP. Mean density (±SEM) expressed relative to Previous Ch control group. Representative blot images for ERα or CHIP and the loading control β-actin are shown in insets above the graph. * P<.05.
CHIP
As illustrated in Figure 2B, previous exposure to oestradiol in aging, ovariectomized rats had no effect on total protein levels of CHIP in the hippocampus. The antibody recognized additional bands of unknown origin and densiometric analyses of these bands did not reveal any difference between groups. There was no effect of treatment on levels of CHIP or β-actin.
Co-immunoprecipitation
Co-immunoprecipitation of ERα
As illustrated in Figure 3, previous exposure to oestradiol in aging, ovariectomized rats decreased the amount of ERα-associated CHIP in the hippocampus. Western blots revealed a band of CHIP-like immunoreactivity at approximately 37 kDa in lysate from which ERα was immunoprecipitated. There was a significant effect of treatment (t(8) = 4.490, p = .002)
Figure 3.
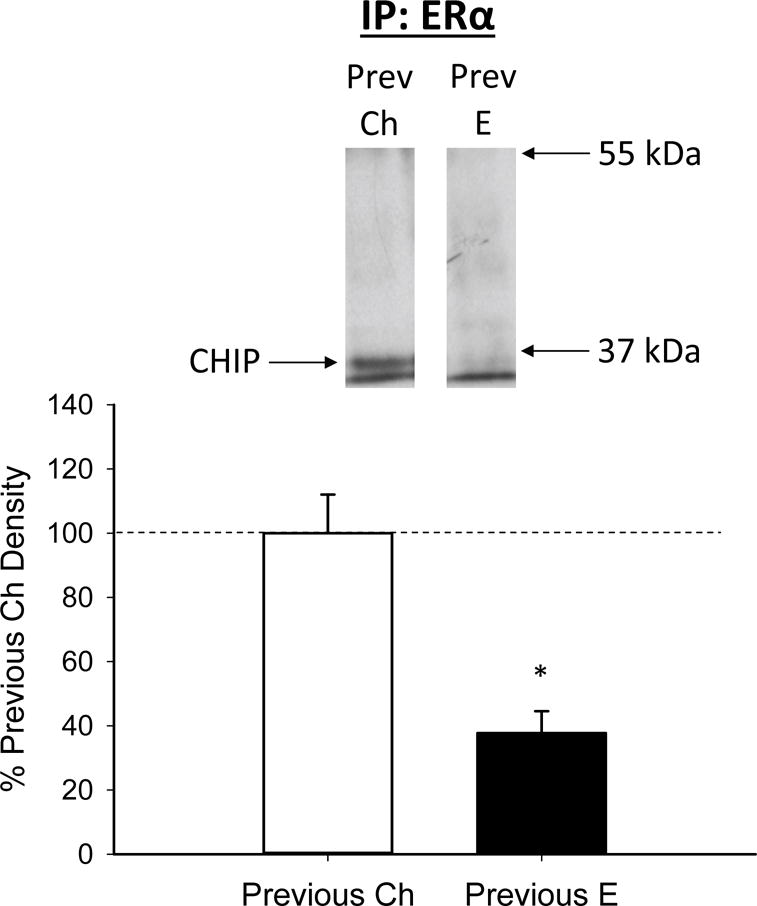
Effects of previous treatment with oestradiol on interaction between ERα and C terminus of Hsc70-interacting protein (CHIP) in the hippocampus in aging, ovariectomized rats. Middle-aged rats were ovariectomized and implanted with either oestradiol (Previous E) or cholesterol vehicle (Previous Ch) capsules for 40 days. Hormone capsules were removed and one month later rats were killed. Hippocampi were processed for co-immunoprecipitation, in which ERα was immunoprecipitated from lysate and resultant sample was probed for the ubiquitin ligase, CHIP. Western blot data showing the effects of treatments on protein levels of CHIP that is associated with ERα. Mean density (±SEM) expressed relative to Previous Ch control group. Representative blot images for CHIP are shown in insets above the graph.
Experiment 2
Total retroactive errors
Two-way ANOVA conducted across all delays revealed no main effect of delay or treatment (Figure 4A). As illustrated in Figure 4B, two-way ANOVA conducted on the three shortest delays revealed no effect of treatment, but there was a significant effect of treatment across the three longest delays (F(1,15) = 8.97, p = .009) indicating administration of ICI 182,780 resulted in decreased accuracy as memory load increased. There were no main effects of delay on either the three shortest delays or the three longest delays. There were no interactive effects of delay and treatment for either the three shortest delays or the three longest delays.
Figure 4.
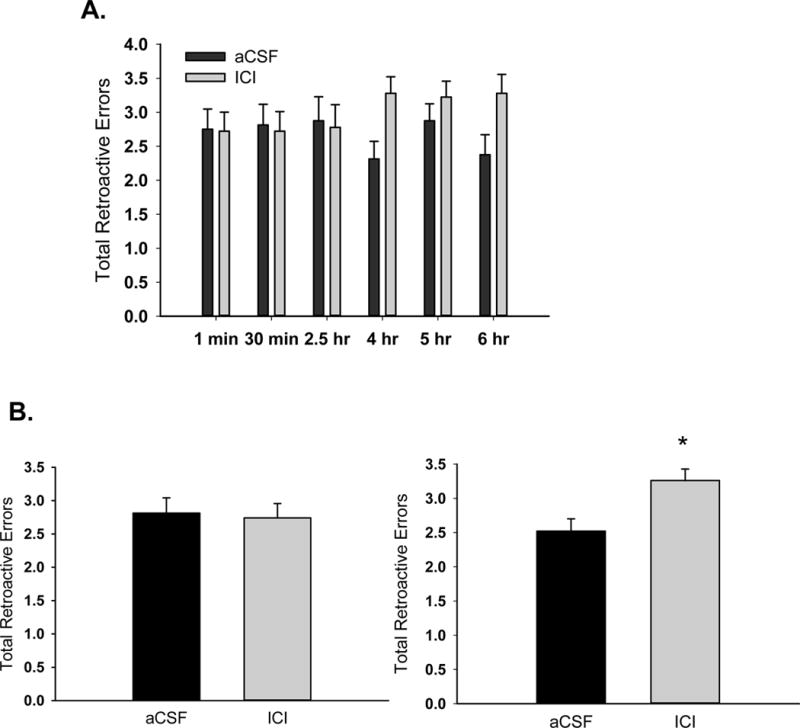
Effects of antagonism of brain estrogen receptor on total retroactive errors in aging, ovariectomized rats. Middle-aged rats were ovariectomized and implanted with oestradiol capsules. After 40 days all capsules were removed and chronic i.c.v. delivery of aCSF vehicle or the estrogen receptor antagonist, ICI 182,780 (ICI) was initiated and continued for the duration of testing on a radial-arm maze task. (A) Retroactive errors across various delays that were imposed between the fourth and fifth arm choices. (B) Mean retroactive errors averaged across short delays (1 min, 30 min, and 2.5 hr; left) and long delays (4 hr, 5 hr, 6 hr; right). *P<.05.
Total proactive errors
Two-way ANOVA conducted across all delays revealed no main effect of delay or treatment (Figure 5A). Two-way ANOVAs conducted on the three shortest delays and the three longest delays revealed no main effect or interactive effect of delay or treatment (Figure 5B).
Figure 5.
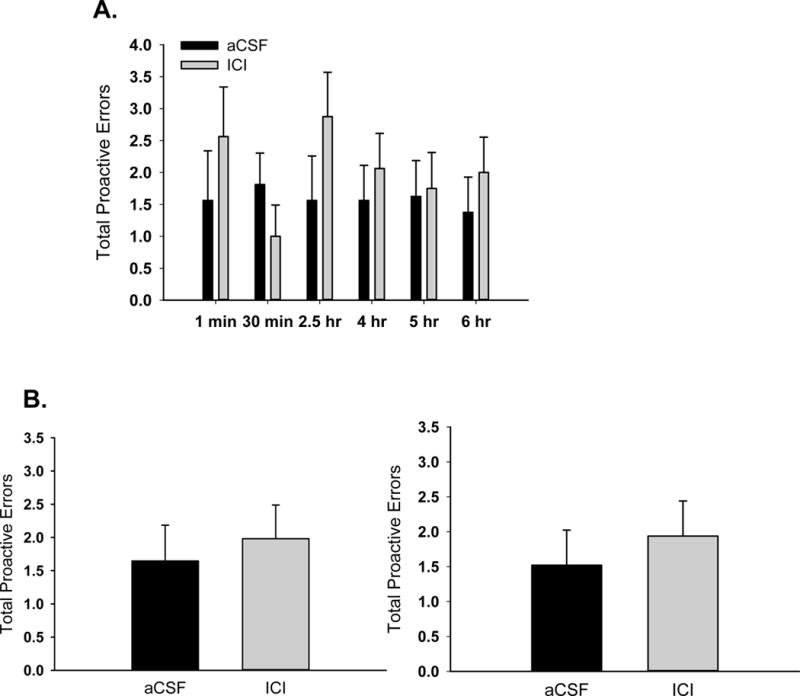
Effects of antagonism of brain estrogen on total proactive errors in aging, ovariectomized rats. Middle-aged rats were ovariectomized and implanted with oestradiol capsules. After 40 days all capsules were removed and i.c.v. delivery of aCSF vehicle or the estrogen receptor antagonist, ICI 182,780 (ICI) was initiated and continued for the duration of testing on a radial-arm maze task. (A) Proactive errors across various delays that were imposed between the fourth and fifth arm choices. (B) Mean proactive errors averaged across short delays (1 min, 30 min, and 2.5 hr; left) and long delays (4 hr, 5 hr, 6 hr; right).
Discussion
The results of the current experiment reveal a mechanism involving CHIP-mediated degradation underlying the maintenance of hippocampal ERα levels in aging females following the termination of transient, midlife oestradiol treatment. The implications of this decreased degradation of ERα are evident in our results demonstrating that action at oestrogen receptors is beneficial to cognition in the absence of circulating oestrogens. In the first experiment, we examined the interaction between ERα and CHIP, an ubiquitin ligase, approximately one month following termination of a 40-day period of oestradiol treatment. As shown previously (15,16), aging, ovariectomized rats that had previous exposure to exogenous oestradiol had increased levels of ERα in the hippocampus as compared to ovariectomized controls that had never been treated with oestradiol. There was no effect of previous oestradiol treatment on total levels of CHIP. There was, however, decreased interaction between CHIP and ERα, as demonstrated by co-immunoprecipitation procedures in which we immunoprecipitated ERα and immunoblotted for CHIP. These results suggest previous oestradiol treatment provides extended protection to ERα from CHIP-mediated degradation. Results from our second experiment, in which antagonism of ER in ovariectomized, aging females negatively impacted hippocampal-dependent memory, demonstrate the importance of brain ER to memory in the absence of circulating oestrogens. Aging, ovariectomized rats that received administration of the ER antagonist, ICI 182,780 displayed a delay-dependent decrement in memory for the location of food rewards in a maze as compared to controls. Together, these data support the hypotheses that transient, midlife oestradiol treatment following ovariectomy maintains hippocampal ERα long-term by decreasing CHIP-mediated degradation and that activation of ERα is associated with cognitive benefits in the absence of circulating estrogens.
The current results provide a mechanism for the long-term maintenance of ERα levels in the hippocampus following midlife oestradiol treatment by demonstrating that there is a decrease in interaction between the ubiquitin ligase CHIP and ERα. Mechanisms underlying this decreased interaction between ERα and CHIP are unclear but could involve novel mechanisms by which ERα is phosphorylated in the absence of circulating estrogens maintaining ERα in a transcriptionally active state and thereby preventing interaction with CHIP. For example, in MCF7 cells that were transfected with a phosphomimetic ERα serine 118 (S118) plasmid, but not cells transfected with phosphomimetic ERα-S104/106 or ERα-S167, ERα was protected from ligand-dependent degradation (31), which occurs via a unique ubiquitin ligase (23). Because phosphorylation of ERα is protective against ligand-dependent degradation, we hypothesize that activation of ERα via phosphorylation at S118 could protect ERα from CHIP-mediated, ligand-independent degradation as well.
Phosphorylation of ERα in the absence of ovarian oestrogens can occur via ligand-independent mechanisms by growth factors, including insulin-like growth factor 1 (IGF-1). IGF-1 receptors and ERα co-localize in the CA1 of the hippocampus (32) and are both decreased in the aging rat brain (33). Activation of IGF-1 receptors leads to activation of either ERK/MAPK or AKT/PI3K pathways (34), both of which can activate ERα-mediated transcription in the absence of ligand, via phosphorylation of ERα (35) including at site S118 (36,37). Our lab has demonstrated that antagonism of IGF-1 receptors beginning after termination of midlife oestradiol treatment in ovariectomized rats reverses the increase in hippocampal levels of ERα induced by the previous oestradiol treatment (15). Results suggest that short-term oestradiol treatment in midlife permanently alters communication between the IGF-1 system and ERα, leading to increased ERα levels, even when there are no circulating ovarian or exogenous oestrogens present. This altered communication could increase second-messenger cascades and phosphorylation of ERα, specifically at S118, offering a potential mechanism for protection of ERα from CHIP-mediated degradation.
Besides ligand-independent mechanisms, in the absence of circulating oestrogens, locally produced neuro-oestrogens could activate ERα. Proteins necessary for oestradiol synthesis are expressed in the hippocampus (38,39) and inhibition of aromatase, the enzyme involved in the final step of oestradiol synthesis, decreases oestradiol production in adult hippocampal neurons in vitro (40). Rapid actions, including activation of the ERK/MAPK pathway, at membrane-associated ERα may be initiated by hippocampal-derived oestradiol. Data from our lab demonstrate, along with increased levels of ERα, there is also increased activation of ERK/MAPK in the hippocampus following short-term oestradiol treatment in ovariectomized females (15). Viral vector infusion of ERα into the hippocampus of female rodents also increases ERK/MAPK in the absence of circulating oestrogens, suggesting that the ERK/MAPK pathway plays a role in the cognitive benefits of ERα following ovariectomy and short-term, midlife oestradiol treatment (21).
Increasing ERα levels via viral vector infusion to the hippocampus results in cognitive benefits in the absence of circulating oestrogens (20,21). However, to date, the importance of endogenous hippocampal ERα in the absence of circulating oestrogens has not been directly tested. In our second experiment, we found that action at endogenous ERα enhances cognitive function in ovariectomized aging rats, as administration of ICI 182,780, an ER antagonist, resulted in poorer memory performance. The importance of ERα on memory has also been demonstrated in humans. In elderly men and women, performance on the Modified Mini-Mental Examination was negatively correlated with single nucleotide polymorphisms in the gene encoding for ERα (19). Additionally, increased levels of wild-type ERα, but not ERβ, in the frontal cortex was associated with better cognitive performance in Alzheimer’s disease patients (18). Together, these data support the hypothesis that ERα can have positive impacts in the aging brain and on cognitive function in the absence of circulating oestrogens.
Whereas ICI 182,780, the ER antagonist used in the current study, antagonizes both ERα and ERβ (41), it is more potent at inhibiting activity at ERα than ERβ (42). Previously, our lab found that ERα, but not ERβ, protein expression was increased in the hippocampus following previous short-term oestradiol treatment in ovariectomized, aging females (16). Furthermore, ERβ knockout animals showed enhanced cognition on a hippocampal-dependent task, compared to both ERα knockout and wild-type controls (43). Thus, results from the current study combined with results from previous work in our lab and others support the hypothesis that activation at ERα, but not ERβ, is necessary for enhanced hippocampal-dependent memory in the absence of circulating oestrogens.
In addition to decreased CHIP-mediated degradation, there may be other mechanisms involved in maintaining ERα levels in the absence of circulating oestrogens. For example, increased transcription of ESR1, the gene encoding for ERα could be a direct result of increased phosphorylation of ERα. In young adult mice ovariectomized and implanted with oestradiol capsules, there was an increase in ERα gene expression in the hippocampus compared to rats that did not receive oestradiol (44). Furthermore, mRNA expression of hippocampal ESR1 positively correlated with better performance on the radial-arm maze. We are currently investigating the role of transcription in the absence of circulating oestrogens following midlife oestradiol treatment.
In conclusion, the current experiments provide evidence that maintenance and activation of endogenous ERα provides benefits for cognition in the absence of ovarian oestrogens. In addition, results provide a mechanism involving increased degradation as to how previous oestradiol treatment maintains ERα beyond the period of oestradiol exposure. These data add to a growing body of literature suggesting that action at ERα mediates lasting cognitive benefits resulting from short-term oestradiol treatment in midlife.
Acknowledgments
This study was supported by grant R01AG041374 from the National Institute on Aging (JMD). The authors thank Rachel Springer, Megan Fitzpatrick, and Erin MacDougal for assistance in behavioral training and testing.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats☆. Neurobiol Aging. 2000;21:107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 2.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 3.Henderson V, Watt L, Buckwalter JG. Cognitive skills associated with estrogen replacement in women with Alzheimer’s disease. Psychoneuroendocrinology. 1996;21:421–30. doi: 10.1016/0306-4530(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 4.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–21. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 5.Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol. 2009;21:640–7. doi: 10.1111/j.1365-2826.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- 6.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 7.Maki PM, Dennerstein L, Clark M, Guthrie J, LaMontagne P, Fornelli D, Little D, Henderson VW, Resnick SM. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–43. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Colditz G. Risk factors and hormone-receptor status: epidemiology, risk-prediction models and treatment implications for breast cancer. Nat Clin Pract Oncol. 2007;4:415–23. doi: 10.1038/ncponc0851. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Manson J, Hankinson S, Rosner B, Holmes M, Willett W, Colditz G. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027–32. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 10.Bagger Y, Tanko L, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. J North Am Menopause Soc. 2005;12:12–7. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Whitmer R, Quesenberry C, Zhou J, Yaffe K. Timing of hormone therapy and dementia: The critical window theory revisited. Ann Neurol. 2011;69:163–9. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, De Andrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 13.Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–9. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated Equine Estrogens and Global Cognitive Function in Postmenopausal Women. J Am Med Assoc. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 15.Witty C, Gardella L, Perez M, Daniel J. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: A role for insulin-like growth factor-I. Endocrinology. 2013;154:842–52. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- 16.Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- 17.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–62. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 18.Kelly J, Bienias J, Shah A, Meeke K, Schneider J, Soriano E, Bennett D. Levels of estrogen receptors α and β in frontal cortex of patients with Alzheimers disease: Relationship to Mini-Mental State Examination scores. Curr Alzheimer Res. 2008;5:45–51. doi: 10.2174/156720508783884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR. Estrogen receptor genotype and risk of cognitive impairment in elders: Findings from the Health ABC study. Neurobiol Aging. 2009;30:607–14. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16:1587–93. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witty C, Foster T, Semple-Rowland S, Daniel J. Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan M, Park A, Nephew KP. CHIP (carboxyl terminus of Hsc70-interacting protein) promotes basal and geldanamycin-induced degradation of estrogen receptor-alpha. Mol Endocrinol. 2005;19:2901–14. doi: 10.1210/me.2005-0111. [DOI] [PubMed] [Google Scholar]
- 23.Tateishi Y, Kawabe Y, Chiba T, Murata S, Ichikawa K, Murayama A, Tanaka K, Baba T, Kato S, Yanagisawa J. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. EMBO J. 2004;23:4813–23. doi: 10.1038/sj.emboj.7600472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–60. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Han D, Wang R, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A. 2011;108:E617–24. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2010;35:694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med. 2004;229:977–87. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1998 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 29.Robertson JFR. ICI 182,780 (Fulvestrant™) – the first oestrogen receptor down-regulator – current clinical data. Br J Cancer. 2001;85:11–4. doi: 10.1054/bjoc.2001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–73. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 31.Valley CC, Métivier R, Solodin NM, Fowler AM, Mashek MT, Hill L, Alarid ET. Differential Regulation of Estrogen-Inducible Proteolysis and Transcription by the Estrogen Receptor α N Terminus. Mol Cell Biol. 2005;25:5417–28. doi: 10.1128/MCB.25.13.5417-5428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardona-Gómez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–60. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 33.Sonntag W, Lynch C, Bennett S, Khan A, Thornton P, Cooney P, Ingram R, McShane T, Brunso-Bechtold J. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269–79. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- 34.Russo V, Gluckman P, Feldman E, Werther G. The insulin-like growth factor system and its pleiotropic functions in the brain. Endocrinol Rev. 2005;26:916–43. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 35.Hall J, Couse J, Korach K. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J B. 2001;276:36869–72. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 36.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science (80-) 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 37.Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–11. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- 38.Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: Co-localization with StAR and aromatase. J Neurochem. 2001;76:1879–86. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- 39.Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 40.Kretz O, Fester L, Wherenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune G. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–21. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Am Assoc Cancer Res. 1991;51:3867–73. [PubMed] [Google Scholar]
- 42.Wade CB, Robinson S, Shapiro Ra, Dorsa DM. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–42. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 43.Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC. Role of estrogen receptor α and β in preserving hippocampal function during aging. J Neurosci. 2013;33:2671–83. doi: 10.1523/JNEUROSCI.4937-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iivonen S, Heikkinen T, Puolivali J, Helisalmi S, Hiltunen M, Soininen H, Tanila H. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen alpha and beta mRNA levels in ovariectomized female mice. Neuroscience. 2006;137:1143–52. doi: 10.1016/j.neuroscience.2005.10.023. [DOI] [PubMed] [Google Scholar]


