Abstract
Context
Biogenic amines such as 5-hydroxy-indole acetic acid (5HIAA) the main metabolite of serotonin or metanephrines (catecholamines metabolites) are used as biomarkers of neuroendocrine tumours.
Objective
To re-evaluate the recommendations for urinary sampling (preservatives, diet, drugs, etc.) as many of the reported analytical interferences supporting these recommendations are related to obsolete assays.
Methods
Bibliographic analysis of old and modern assays concerning preservation, extraction, assay and interferences.
Results
5HIAA may degrade as soon as urine is excreted. Thus, acids as preservatives (hydrochloric or acetic acid) have to be immediately added. Care should be taken not to decrease the pH under 2. Urine preservative for metanephrine assays is not mandatory. Diets including serotonin-, tryptophan- and dopamine-rich foods have to be avoided depending on the biomarkers investigated (bananas, plantain, nuts, etc.). Tryptophan-rich over-the-counter formulas have to be prohibited when 5HIAA has to be assayed. Acetaminophen may interfere with electrochemical detection depending on high-pressure liquid chromatography (HPLC) parameters. No interference is known with mass spectrometric assays but with the one described for metanephrines determination. Some drugs interfere however with serotonin and catecholamines secretion and/or metabolism (monoamine oxidase inhibitors, serotonin or dopamine recapture inhibitors, etc.).
Conclusion
Revisited recommendations are provided for the diet, the drugs and the preservatives before HPLC coupled with electrochemical and mass spectrometry assays.
Keywords: 5-hydroxy indole acetic acid, metanephrines, assay, neuroendocrine tumours
Introduction
Endocrine and neuroendocrine tumours diagnosis is based on the convergence of clinical, imaging and biological arguments. Tumour secretory markers are often hormones (or their metabolites) that are secreted by normal tissue as well as tumoural cells although frequently in an abnormal way (different level, loss of negative feed-back, unusual molecular form, etc.). Many hormones or metabolites can be assayed in serum or plasma. Most assays are now modern immunoassays, mass spectrometry assays or electrochemical assays. Although prone to disadvantages such as cumbersomeness, incomplete collection and analytical matrix effect, urine collection provides an integrated profile of the daily filtration of secreted hormone or metabolites. Thus, 24h urine sampling is still useful in endocrine tumours. Whatever the assays undertaken, pre-analytical steps are crucial to assure the relevance of the analysis. More specifically, exploration of urinary excretion requires some attention paid to sampling conditions: hormone or metabolite preservation in urine during the collection and transport, control of diet and environmental conditions, control of drugs altering hormone concentrations (secretion, recapture, metabolism, etc.), control of drugs altering the analyte assay, etc. Many endocrine-related assays in urine have been set up in the early days of modern endocrinology and relied on pioneer work on biochemical colorimetric or fluorimetric analysis. Obviously, techniques have changed replacing most of these assays by modern alternatives that are simultaneously more specific and more sensitive. However, in our clinical practice as well as in recently published international consensus about neuroendocrine tumours or pheochromocytomas or paragangliomas, we have encountered recommendations for urine sampling that were either clearly obsolete or not otherwise supported by bibliographic references (Supplementary Data, see section on supplementary data given at the end of this article). We thus collected these – often historical – recommendations and tried to sort out the ones that are still valid and those that are obsolete. We will try in this review to weed out the most out-dated recommendations for urine sampling considering the use of electrochemical or mass spectrometric detections coupled to high-pressure liquid chromatography (HPLC) and excluding colorimetric and fluorimetric assays. We will consider the preservation of analytes in urine, diet-related issues and drug-related issues. We will not discuss the level of diagnosis performed to quantify these analytes as this was covered in many publications.
Urinary metabolite of serotonin, 5 hydroxy-indole acetic acid
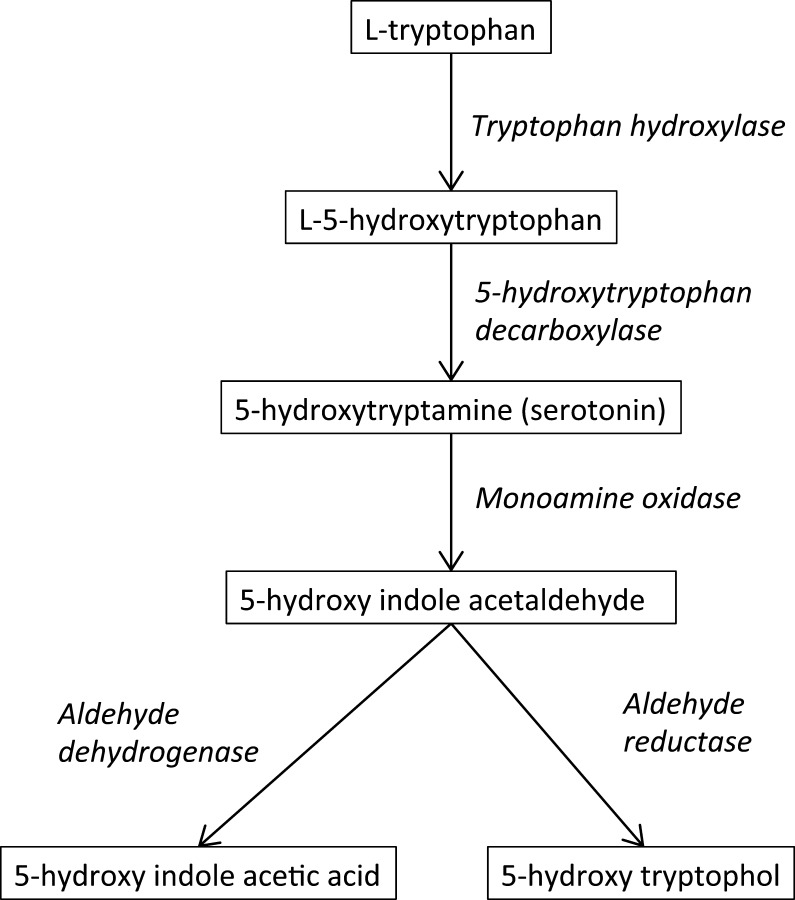
Because neuroendocrine cells may secrete serotonin, its main metabolite 5 hydroxy-indole acetic acid (5HIAA), or serotonin itself, may be used as tumour secretory markers to investigate and monitor patients with carcinoid syndrome (1, 2, 3, 4). Serotonin (5-hydroxytryptamin) is a monoamine neurotransmitter derived from the amino acid l-tryptophan via a 2-step enzymatic pathway (Fig. 1) (5). About 10% of serotonin is located in the neurons of the central nervous system. The remaining 90% is located in the enterochromaffin cells (5, 6). The 5HIAA is produced in the liver and excreted by the kidney.
Figure 1.
l-Tryptophan is metabolised by tryptophan hydroxylase, a rate-limiting step for serotonin synthesis. Monoamine oxidase occurs at 2 subtypes MAO-A and MAO-B with a widespread occurrence in the brain and in the periphery. They show differences in substrate specificities and sensitivities to inhibitors.
Serotonin may be assayed either in its free state in the plasma or bound in platelets. 5HIAA can also be assayed in plasma but its daily excretion in urine is still the more classically recommended assay (1, 2, 7, 8). We will not consider here the respective level of diagnosis performance of serotonin vs 5HIAA determination. Strikingly, although consensus and review publications sometimes mention that most modern 5HIAA assays, if not all, provide recommendations for urine collection irrelative to modern assays. We will also mention new causes of false-positive causes of increased 5HIAA excretion.
5HIAA assays
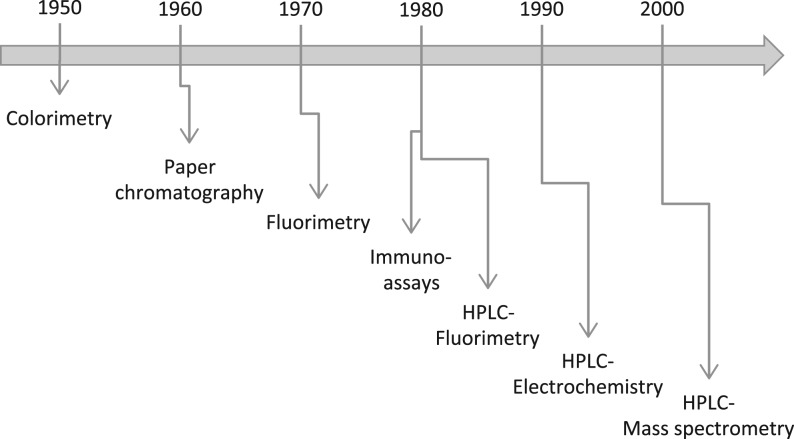
Historically, since these initial publications about 5HIAA and neuroendocrine tumours, assays have dramatically changed (Fig. 2). Assays in the 1950s were initially colorimetric (9, 10). Fluorimetric assays and gas chromatographic assays appeared in the 1960s (11, 12) followed in the 1980s by HPLC-fluorimetric assays (13, 14, 15) and immunoassays (16, 17).
Figure 2.
Approximate chronology of the use of routine 5HIAA assays.
Today, the most common assays use HPLC coupled to electrochemical detection (18, 19, 20). Recently, liquid chromatography coupled to mass spectrometry has been used for the detection and quantification of urinary as well as serum 5HIAA (21, 22, 23). Obviously, these last 5HIAA assays after HPLC improved both specificity and sensitivity. Since the biochemical and biophysical reactions involved are very different, cross-reacting substances in colorimetric assays or fluorimetric assays have no systematic deleterious consequences on electrochemical detection or mass spectrometry. Conversely, new drugs may interfere with these modern assays especially with electrochemical detection.
Urine preservation
Common sense advice for urine collection has to be given: collection in clean and closed plastic containers and complete collection of urine during a 24-h period. With no treatment, 5HIAA tends to degrade during and after a 24-h urine collection. To avoid an underestimation of its concentration preservatives have to be added to urine. Ideally, the pH should be lowered around 3 as early work stated that in an alkaline solution 5-HIAA is degraded. Hence, it is recommended to lower the pH of urine (usually with hydrochloric acid) but not lower than 3 as very low pH increases 5HIAA degradation (24).
Historically boric or acetic acid may be added to acidify urine. Acetic acid was erroneously described as inefficient as a preservative as, in fact, it caused problems with a one particular colorimetric assay. When acidified and kept at 4°C, 5HIAA is stable for more than one month (24). Various laboratories propose alternatives to acidification although published results are scarce. A useful list of preservatives for many analytes has been proposed (25). These alternatives to strong acids or boric acid (recently classified harmful and toxic for reproduction (26)) are especially useful for protecting patients and professional from the preservatives themselves. Preservatives such as Na2CO3 (although increasing pH) or NaHSO3 or EDTA are safe and easy to manipulate, and are recommended by some laboratories such as the Mayo Clinics (25). However, they have to be tested for each laboratory analytical procedure to ascertain both their real efficiencies and their absence of impact on the chosen assay. This is particularly important as solid-phase extraction of 5HIAA from urine may depend on pH. There is no difference of preservation when the pH is lowered to 3 or 1.5 with hydrochloric acid (our unpublished results). However, obviously to decrease pH to 1.5 a larger volume of acid has to be added. We also found that adding acetic acid as a preservative was not practical because the volume necessary to lower the pH to 3 resulted in an important dilution of urine. Despite being safe to manipulate, we ruled out Na2CO3 as it prevented 5HIAA degradation only partly. Although some laboratories recommend protecting 5HIAA from light, we were not able to find many published data about this point. It has been related that as 5HIAA solution changes colour with light, a chemical reaction may occur (27). Although many recommendations advise to keep samples in the dark, we did not find any effect of light when hydrochloric acid was added as a preservative (unpublished results). Many laboratories also recommend keeping urine at 4°C during collection with the preservative. To our knowledge, there is no published data reporting the degradation of 5HIAA in acidified urine at room temperature: once urine was acidified, there were minor differences (5%) between room temperature and 4°C (unpublished results). Conversely, when a delay before analysis is unavoidable, it is safe to recommend refrigerating acidified urine samples.
Diet-related issues
5HIAA is a metabolite issued from serotonin metabolism, and the latter is synthetized from the essential amino acid tryptophan (Fig. 2). Serotonin is mainly metabolised by monoamine oxidase in the liver and the lungs. Thus, diets rich in tryptophan (28) or serotonin (29) may increase 5HIAA urinary excretion. This point has been clearly established since it was believed that serotonin might be a remedy for constipation (30)!
Bananas and plantains were the first-described serotonin-rich fruits (30, 31, 32, 33); kiwi and pineapple have also high contents of serotonin (32, 33). Many (walnut and hickory families) but not all (chestnuts and pistachios) nuts have high serotonin contents. Almond, cashews and macadamia nuts have intermediate contents. For example, walnuts contain twenty-fold more serotonin than bananas (34). Tomatoes and some varieties of plums and avocados have moderate serotonin contents (<5 µg/g) (32, 33). Strawberries, cranberries, raspberries, grapes, oranges apples and pears have apparently no detectable levels of serotonin (33). Eggplants were in an early study (10) reported to be rich in serotonin but not in a later one (32) suggesting that the former study used a less specific assay for 5HIAA determination.
Serotonin-rich fruits are also usually tryptophan rich although nuts have only twice the tryptophan contents of bananas (34). Seeds and nuts and beans are also usually tryptophan rich. Animal-derived products such as meat, milk, cheese may also have elevated tryptophan contents. The USDA provides a large Nutrient Database including one about tryptophan food content (35). It must be noted that all the ingested tryptophan is not found in urine as 5HIAA as tryptophan may be used as a structural amino acid and not solely as a precursor of serotonin. The publications reporting a follow-up of urinary excretion of 5HIAA usually claim a return to basal level two days after the end of the diet enriched either in tryptophan or serotonin (36).
Nowadays, many over-the-counter formulas contain tryptophan either alone or in combination with other compounds including 5-hydroxytryptophan. It is difficult to precisely indicate the subsequent increase of urinary 5HIAA because of the various tryptophan dosages available. These formulas should be discontinued before any biological investigation of neuroendocrine tumours as they can cause up to tenfold increase of urinary 5HIAA (37). Of note, the effect of dietary intake of 5 hydroxy-tryptophan on 5HIAA excretion seems very variable between individuals (37, 38). Serotonin is hardly present in over-the-counter formulas since it does not significantly cross the blood brain barrier and that most of these formulas target the brain alleging neuropsychological benefits. Lastly, dietary NaCl restriction increases 5HIAA excretion (39).
We encountered recommendations forbidding natural vanilla and vanillin during 5HIAA collection. We found no publication supporting this view and, conversely, Odink and coworkers reported no influence of vanilla on 5HIAA excretion (40).
Drugs interfering with serotonin metabolism
Serotonin is a major neurotransmitter involved in nearly all neuronal pathways involving human behavioural processes despite being produced by about one million neurons only (5). Because of this critical role, many drugs in psychiatric treatments target serotonin pathways. Furthermore, drugs targeting other pathways interfere with serotonin pathways because of its ubiquitous role. Several classes of drugs directly target the serotonin system: antidepressant, antipsychotic, anxiolytic, antiemetic, antimigraine drugs. Amongst these drugs, monoamine oxidase inhibitors and inhibitors of serotonin reuptake at the synaptic level should modify 5HIAA excretion. However, the effects may not be so clear as not all monoamine oxidase-inhibiting hydrazines have the same effect (41), suggesting that there may be some confusion of causes between the effects of the drugs on neurotransmitter pathways and interferences in earlier assays. Subsequently, however, many such drugs have been prohibited during 5HIAA collection for neuroendocrine tumours evaluation. For instance, the serotonin receptor antagonist risperidone mildly but significantly increases plasma 5HIAA (42). In rats, a reserpine analogue clearly increases 5HIAA excretion but the dosages far exceeded those used in humans (43). In rats or mice, many drugs either locally or generally administered alter 5HIAA concentrations in some brain structures: diazepam, phenobarbital, piperazine, methamphetamine… (44, 45, 46, 47). In humans, desipramine and zimeldine mildly reduced 5-HIAA concentrations in the cerebrospinal fluid (CSF) (48). Isoniazid given for depression was also suspected of modifying 5HIAA concentrations (49). This was probably because it was erroneously thought to be a monoamine oxidase inhibitor (50).
To summarize, to our knowledge, no report has been published about a major modification of 5HIAA urinary excretion in humans under psychiatric treatments. Of note, it must be kept in mind that some psychiatric diseases may also be associated to a modified serotonin metabolism. For instance, some autistic subjects display mildly increased 5HIAA excretion (51). In psychotic-depressed subjects, 5HIAA excretion was linked to insomnia and interest (52). Various studies of patients with depression reported differences in 5HIAA concentrations in CSF although they did not report urinary 5HIAA levels. 5HIAA concentrations in CSF, serum or urine are not modified by schizophrenia (53).
Thus, although possible, there is no hard data showing that these psychiatric drugs are really responsible for false diagnosis of neuroendocrine tumours. Of course, it may be because their discontinuation was often recommended! It may also be because the amount of serotonin involved is low and concerns ‘only’ one million neurons compared to the much larger mass of serotonin in the neuroendocrine cells of the gut. Thus, although these drugs, and the disease they were prescribed for, may not be completely devoid of effect on 5HIAA excretion, it is difficult to rapidly and/or easily stop the treatments if they are necessary for psychiatric diseases. Should 5HIAA excretion under such treatment be over the upper limit of reference values, one should then consider interrupting the current psychiatric treatment.
Drugs described as interfering with various techniques of 5HIAA quantification
The drugs described hereafter have been historically forbidden in various recommendations about 5HIAA urinary collection. Guaifenesin (glyceryl guaiacolate ether) has been used as a cough suppressant and as an expectorant. Since 1970, it has been known to cause errors in laboratory determinations of 5HIAA using 1-nitroso-2-naphthol (54). However, this interference should have been eliminated in 1972 by using a modified colorimetric technique (55). Methocarbamol and mephenesin carbamate elicited false-positive tests for 5HIAA (56). This happened because their urinary metabolites produce a positive nitrosonaphthol reaction similar to the one elicited by Guaifenesin. l-DOPA also gave false 5HIAA results in colorimetric reaction (57). Homogentisic acid and/or gentisic acid produced an artefactual increase in 5HIAA determination using a colorimetric assay in a patient with alcaptonuria (57). Phenothiazine and some of its derivatives have been described as falsely reducing 5HIAA urinary excretion. This is due to an interference in the development of colour in the colorimetric assay (58). This problem was partly corrected in the 70s in a modified colorimetric assay (59). Aspirin gave false 5HIAA results in a colorimetric assay (57). An improved method corrected this interference (60). Antipyrin interference was however not eliminated from this colorimetric reaction. Aspirin also gave false 5HIAA results in a fluorimetric assay (61). Sulfasalazine, acetyl-5-aminosalicylic and mesalazine mainly used for the treatment of Crohn’s disease, ulcerative colitis and rheumatoid arthritis interfered with fluorescent assays (62). Naproxen falsely generated elevated 5HIAA urinary concentrations (63, 64). One of its metabolite reacted in a 5HIAA spectrophotometric assay (65).
Very importantly, for all the above-mentioned compounds, there is no indication of interference when using electrochemical or mass spectrometric assays.
Nowadays, paracetamol (a.k.a. acetaminophen, 4-hydroxy acetanilide), a very common drug, may frequently be taken in investigated patients. Paracetamol has been the origin of one of the most serious electrochemical interferences when using oxidase-based amperometric biosensors. It produced an interfering current that increased glucose readings in early glucose sensors (66). In fact, Paracetamol can be assayed by electrochemical detection (67). Thus, it has been described as a potential source of interference in electrochemical detection after HPLC separation of analytes such as 5HIAA, metanephrines and catecholamines (68) or vanilmandelic and homovanillic acids (69) or serotonin (70) depending on the analytical method used (mobile phase, column, etc.). It could interfere with the peak of internal standard leading to overestimation of its value and, thus, lowering the value of the 5HIAA in the sample. Quality control of the internal standard signal should prevent this error. Of note, Paracetamol could, at least in rats, inhibit an enzyme metabolising tryptophan thereby reducing urinary 5HIAA (71).
Urinary metabolites of catecholamines, metanephrines
To biologically diagnose pheochromocytomas or paragangliomas or neuroblastomas, various tumour-related markers might be assayed (72, 73, 74). Because these tumoural cells are from the same origin as the medullary adrenal, they may also secrete catecholamines and/or their methoxylated metabolites, a.k.a. metanephrines. Thus, catecholamines and metabolites may be used as tumour secretory markers either in plasma or urine.
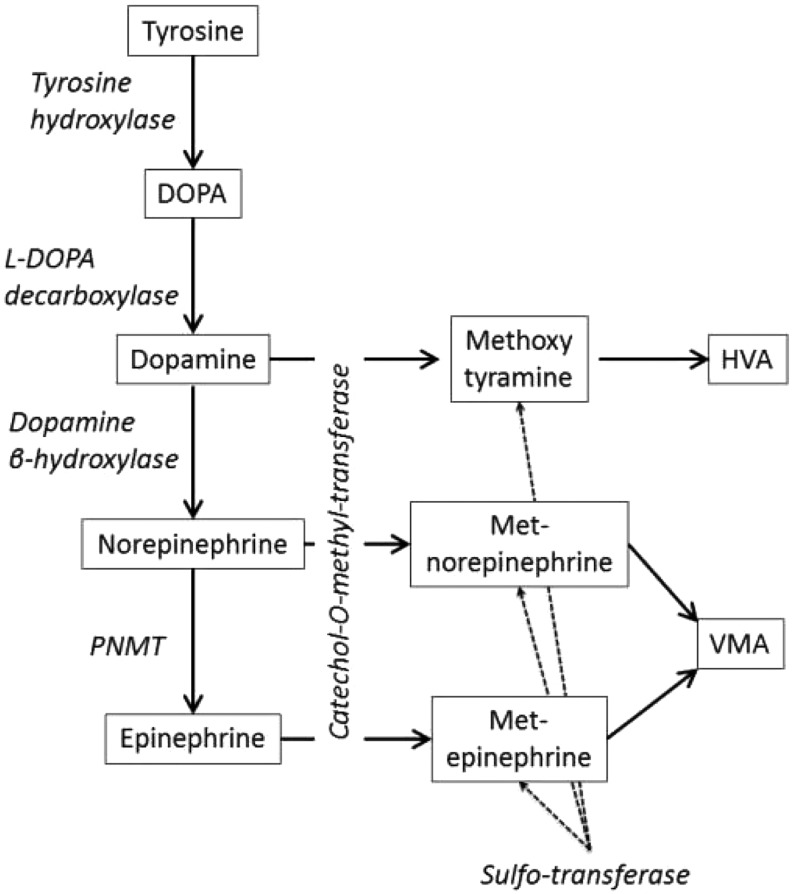
Catecholamines are hormones derived from the amino acid tyrosine via a multistep enzymatic pathway (Fig. 3). Part of circulating catecholamines comes from neurons and part comes from the adrenal medulla (75): adrenaline originates from the adrenal medulla and noradrenaline comes from the adrenal medulla and the sympathetic nerves (75). Physiologically, metabolites are produced in catecholamine-synthesizing tissues from leakage of storage vesicles as well as in the liver to be excreted by the kidney and to a lower extant liver (see reviews (75, 76, 77)). Most metanephrines in urine are either sulpho- or glucurono-conjugated; free metanephrines in the plasma have half-lives lower than five minutes. Although it simplifies the assays if free catecholamines are simultaneously assayed (78), there is no clinical argument (yet?) favouring the assay of free metanephrines in urine vs total metanephrines (although there are solid arguments in the plasma). Very importantly, in pheochromocytomas intratumoural metabolism generates metanephrines that are excreted by the tumour itself even though it does not excrete clear excess of catecholamines (76). This is why determining metanephrine levels either in urine or plasma is more clinically efficient than determining catecholamines level. Because of the latter and because they have been detailed elsewhere (79), we will not describe procedures for catecholamines collection as much detail as other analytes.
Figure 3.
Tyrosine is metabolised by a hydroxylase in dihydroxyphenylalanine (DOPA) that in turn is decarboxylated in dopamine. Dopamine can be hydroxylated in noradrenaline that in turn can be methylated by Phenylethanolamine N-Methyltransferase (PNMT) to form adrenaline. Dopamine, noradrenaline and adrenaline are inactivated by methylation forming metanephrines that can be sulphated or further metabolised by monoamine oxidases in homovanillic and vanillylmandelic acids (HVA and VMA, respectively).
Catecholamines and metabolites assays
The historical progress of catecholamines and metabolites assays followed the same course as 5HIAA assays did. For instance, the first chemical assays for catecholamines and metabolites were colorimetric (80) followed about fifty years later by fluorimetric assays (81). In the late 60s, radioenzymatic assays were developed (82).
Electrochemical assays after liquid chromatography were developed in the late 80s using the same principles as colorimetric assays i.e. the ability to be oxidized. A prior high-pressure chromatography allows the separation of the different amines (83). In some laboratories, gas chromatographic assays and immunoassays were developed but they are less convenient. More recently, liquid chromatography coupled to mass spectrometry has been used for the detection and quantification of urinary catecholamines (84). Most authors assay total metanephrines i.e. after acid hydrolysis or enzymatic treatment of conjugated metanephrines. The first metanephrines assays (85) were progressively improved (86, 87, 88) but remained prone to analytical interferences compared to the more recent electrochemical assays. Some analytical interference is still occurring with the latter (89). Similar to other amines, liquid chromatography coupled to mass spectrometry has been recently developed with very rare interferences (90, 91).
Urine preservation
Catecholamines and metabolites assays are very similar to 5HIAA assays and hence some technical recommendations can be identical. Complete urine collection should be performed in clean and closed plastic containers during a 24-h period.
Catecholamines are easily degraded unless preservatives such as HCl are added. However, in acidified urine, free catecholamines are less degraded but conjugated catecholamines are deconjugated. As the latter are in much higher concentrations, acidification may artefactually increase free catecholamines concentrations (92, 93). Thus, should catecholamines be required, moderate acidification is recommended (pH ≈ 4).
Urinary metanephrines are much less degraded with time than catecholamines at least within a week (94). However, at 30°C, the degradation is not nil but can be prevented by HCl (Na2–EDTA is less efficient) (93). Thus, it is safe to add HCl if urine is not to be brought immediately to the laboratory. One point may be worth mentioning is that HCl, even at room temperature, may hydrolyse a fraction of conjugated metanephrines. Should free (and not total) urinary metanephrines be assayed, it would probably be better not to add HCl to minimise the risk of artefactually increasing the free fraction of metanephrines.
Homovanillic and vanillylmandelic acids (HVA and VMA, respectively) are historically collected with the same preservatives as catecholamines although they are more stable.
Diet-related issues
Catecholamines (mainly, but not exclusively, dopamine) are present in a large variety of foods: fruits (especially bananas and plantains), nuts, tomatoes, beans, and cheeses (90, 95). These catecholamines are largely conjugated in the digestive tract by a sulphotransferase, SULT1A. Having a catecholamine-rich diet has then major consequences when total catecholamines are assayed in urine. Moreover, free noradrenaline and free dopamine are also increased by such a diet. Should catecholamines to be assayed be collected, it is then recommended to avoid theses dietary products. One point worth mentioning is the existence of xenobiotics inhibiting SULT1A. SULT1A inhibition does not create the catecholamines, but prevents catecholamine deactivation (96). This could increase catecholamines and possibly metanephrines level from dopamine of gut origin whether endogenous or exogenous.
Metanephrines have not been reported to be present in food but part of the catecholamines ingested is to be metabolised into metanephrines (and further in HVA and VMA). Subsequently, conjugated metanephrine and normetanephrine and methoxytyramine (both in urine and plasma) increase after a catecholamine-rich diet (90). Free metanephrine and normetanephrine are not modified by such a diet but free methoxytyramine is increased (90). This latest point is important when screening for paragangliomas. HVA is mainly a metabolite of dopamine. Thus, as previously said, dopamine-rich diets have to be avoided before its assay in urine. Although less dependent on dopamine-rich diet, VMA excretion is still influenced by catecholamine intake. As for neuroblastomas, VMA and HVA are often requested in the same samples, and it is simpler to avoid these diets before urine collection for both assays. Diets that include artificial vanilla flavouring have to be avoided for VMA determination in urine because VMA is a chemical precursor of vanillin in food industry (an early assay used to transform VMA in vanillin to assay the latter!).
One natural component of Indian curry, methoxyhydroxybenzylamine, has been described as causing interference in HPLC with electrochemical detection for the detection of the internal standard using a commercial kit for metanephrines determination (97).
Drugs interfering with catecholamine metabolism
Catecholamines are metabolised mainly by catechol-O methyl-transferase and aldehyde dehydrogenase (Fig. 3). Drugs inhibiting these enzymes such as entacapone, disulfiram and possibly metronidazole have then to be avoided before catecholamines or catecholamines metabolites assays. Alcohol is also degraded by aldehyde dehydrogenase, and it has been reported that alcohol intake may interfere with catecholamines metabolism especially in subjects with allelic variations such as those of Asian descent (98). Thus, it may be recommended to suppress large alcohol intake during urine collection for catecholamine metabolites determination.
It should be kept in mind that many drugs although not interfering with the assays of metanephrines or catecholamines do interfere with the secretion of catecholamines. Most of them are drugs given for hypertension (α1- and β-adrenoceptor blockers) or neurological diseases (monamine oxidase inhibitors, tricyclic antidepressants, noradrenaline synaptic uptake such as antidepressant or cocaine) (79, 99). Lastly, acutely stressful situations (congestive heart failure, hypernoradrenergic hypertension, shock, sepsis, panic disorder, etc.) but also chronic pathologies such as obstructive sleep apnoea and metabolic syndrome increase catecholamine secretion (for review (79)). These points have to be taken into account when investigating a possible pheochromocytoma even – or possibly despite – using the best assay available.
Drugs described as interfering with various techniques of catecholamines or metabolites quantification
Early colorimetric or fluorimetric assays exhibited many drug-elicited interferences (100, 101, 102, 103, 104, 105). These drugs should not cause interference with electrochemical or mass spectrometric assays.
Nowadays, as previously mentioned, paracetamol may interfere with electrochemical detection including the assays of catecholamines and metanephrines (68). Methenamine, a drug used in the prevention and treatment of urinary tract infections, altered the signal of the internal standard thus eliciting erroneously elevated metanephrines level (106). We described an interference with normetanephrine determination due to an antihypertensive drug, urapidil in an HPLC with electrochemical detection assay (107). Amoxicillin given in two paediatric patients caused interference in a commercial normetanephrine assay (108). Another interference in the quantification of normetanephrine has been reported, in a mass spectrometry assay, because of a metabolite of methylenedioxy-methamphetamine a.k.a. ecstasy (109). Indeed, even mass spectrometry may present interference mainly with internal standard detection. In patients taking l-DOPA for Parkinson disease, 3-O-methyldopa, the methoxylated metabolite of the drug, might cause interference with measurements of methoxytyramine (110). Ibuprofen has been reported to interfere with the detection of HVA by gas chromatography with flame ionization detection after extraction and derivatization (111).
Conclusion
Modern assays for the determination of the excretion of urinary 5HIAA and metanephrines are electrochemical or mass spectrometry assays. Molecules are detected after HPLC to sort the molecules in both techniques. Electrochemical quantification is very sensitive but interferences may occur (e.g. paracetamol). Mass spectrometry now achieves high sensitivity along with its high specificity (although for a higher cost). Subsequently, some ancient recommendations for urine collection are thus obsolete.
Although our purpose here was not to compare urine vs plasma samplings, it must be noted that because of these increases of specificity and sensitivity, assays in the plasma have been described for 5HIAA, metanephrines and methoxytyramine, VMA and HVA (72, 73, 112, 113, 114, 115). It is possible that these plasma assays will supplant urine assays for tumour assessment. Yet these assays have not all been tested for the diagnosis of (neuro)endocrine tumours. It is important to note that although blood sampling during a consultation is very convenient compared to 24-h urine collection, it must be proven clinically par with urine collection. As the techniques for plasma assays are either electrochemistry or mass spectrometry, the recommendations will probably be close to those described for modern urine assays: diet, sample preservation, associated drugs, etc. Such recommendations have, for instance, been proposed for plasma metanephrines and methoxytyramine (90, 116, 117).
Meanwhile, in many countries, laboratories cannot invest immediately in the up-to-date analytical equipment to transform all their urine assays into plasma assays. Thus, for assays in urine, pre-analytical critical considerations are necessary to insure relevant data and conditions for evaluating urinary excretion have to be rigorous (Table 1). First, a complete 24-h urine collection coupled with good preservation has to be provided especially for 5HIAA determination (pH 3–4). Failure to insure these points may obviously result in false-negative tests. Secondly, oral intake of precursor amino acid such as tryptophan and of the amines themselves such as serotonin or dopamine has to be limited. Some specific fruits (bananas, plantains, nuts) beans and some cheeses as well as tryptophan-rich formulas should be prohibited two days before urine collection depending on the analysis to be done. Thirdly, paracetamol may have to be avoided in some electrochemical assays. Fourthly, drugs involved in serotonin or catecholamines metabolism or specific clinical circumstances such as stress have to be taken into account when analysing the results of a screening test. Interrupting psychiatric drugs involved in serotonin metabolism may prove necessary.
Table 1.
Main elements of urine collection for 5HIAA, metanephrines or VMA-HVA determination.
| Analyte | Diet | Preservative | Drugs | Analytical interferences* |
|---|---|---|---|---|
| 5HIAA | Banana, plantain, pineapple, kiwi, plums, walnut, hickory nut | Yes (HCl) | Inhibitors of monoamine oxidase and of serotonin reuptake; serotonin receptor antagonist | Paracetamol |
| Tryptophan-containing supplements | ||||
| Metanephrines | Banana, plantain nuts, tomatoes, beans | Not mandatory | Sympathomimetics, adrenergic receptor blockers, monamine oxidase inhibitors, noradrenaline uptake inhibitors, tricyclic antidepressants | Paracetamol, methenamine, urapidil, amoxicillin, buspirone, mesalamine sulphasalazine |
| VMA-HVA | Banana, plantain nuts, tomatoes, beans; Artificial vanilla flavour (VMA) | Yes (HCl) | Sympathomimetics, adrenergic receptor blockers, monamine oxidase inhibitors, noradrenaline uptake inhibitors, tricyclic antidepressants? | Paracetamol |
If necessary, drug-free collections may be necessary, and repeated sampling may be used because of the known variable metabolisms and diet intakes.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
The authors wish to thank their colleagues from the Groupe de Biologie Spécialisée of the Société Française de Médecine Nucléaire for sharing their own experience and recommendations.
References
- 1.Haverback BJ, Sjoerdsma A, Terry LL. Urinary excretion of the serotonin metabolite, 5-hydroxyindoleacetic acid, in various clinical conditions. New England Journal of Medicine 1956. 255 270–272. ( 10.1056/NEJM195608092550605) [DOI] [PubMed] [Google Scholar]
- 2.Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncology 2015. 16 e435–e446. ( 10.1016/S1470-2045(15)00186-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zandee WT, Kamp K, van Adrichem RC, Feelders RA, de Herder WW. Limited value for urinary 5-HIAA excretion as prognostic marker in gastrointestinal neuroendocrine tumours. European Journal of Endocrinology 2016. 175 361–366. ( 10.1530/EJE-16-0392) [DOI] [PubMed] [Google Scholar]
- 4.Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, Pape UF, Perren A, Rindi G, Ruszniewski P, et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: biochemical markers. Neuroendocrinology 2017. [in press]. [DOI] [PubMed] [Google Scholar]
- 5.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annual Review of Medicine 2009. 60 355–366. ( 10.1146/annurev.med.60.042307.110802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 2007. 132 397–414. ( 10.1053/j.gastro.2006.11.002) [DOI] [PubMed] [Google Scholar]
- 7.Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen TT, Goldsmith SJ, Nutting C, Bushnell DL, Caplin ME, Yao JC. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors. Pancreas 2010. 39 753–766. ( 10.1097/MPA.0b013e3181ebb2a5) [DOI] [PubMed] [Google Scholar]
- 8.O’Toole D, Rindi G, Plockinger U, Wiedenmann B. ENETS consensus guidelines for the management of patients with rare metastases from digestive neuroendocrine tumors: rationale and working framework: introduction. Neuroendocrinology 2010. 91 324–325. ( 10.1159/000287272) [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane PS, Dalgliesh CE, Dutton RW, Lennox B, Nyhus LM, Smith AN. Endocrine aspects of argentaffinoma, with special reference to the use of urinary 5-hydroxyindoleacetic acid estimations in diagnosis. Scottish Medical Journal 1956. 1 148–155. ( 10.1177/003693305600100402) [DOI] [PubMed] [Google Scholar]
- 10.Udenfriend S, Titus E, Weissbach H. The identification of 5-hydroxy-3-indoleacetic acid in normal urine and a method for its assay. Journal of Biological Chemistry 1955. 216 499–505. [PubMed] [Google Scholar]
- 11.Korf J, Valkenburgh-Sikkema T. Fluorimetric determination of 5-hydroxyindoleacetic acid in human urine and cerebrospinal fluid. Clinica Chimica Acta 1969. 26 301–306. ( 10.1016/0009-8981(69)90383-0) [DOI] [PubMed] [Google Scholar]
- 12.Goodwin BL, Ruthven CR, Weg MW, Sandler M. A specific assay for urinary 5-hydroxyindole-3-acetic acid by gas chromatography. Clinica Chimica Acta 1975. 62 439–442. ( 10.1016/0009-8981(75)90097-2) [DOI] [PubMed] [Google Scholar]
- 13.Skrinska V, Hahn S. High-performance liquid chromatography of 5-hydroxyindole-3-acetic acid in urine with direct sample injection. Journal of Chromatography 1984. 311 380–384. ( 10.1016/S0378-4347(00)84733-7) [DOI] [PubMed] [Google Scholar]
- 14.Baars JD, van Haard PM, Lombarts AJ. Assay of urinary 5-hydroxyindole-3-acetic acid: two methodologies compared. Clinica Chimica Acta 1986. 158 173–178. ( 10.1016/0009-8981(86)90233-0) [DOI] [PubMed] [Google Scholar]
- 15.Brashear J, Zeitvogel C, Jackson J, Flentge C, Janulis L, Cantrell L, Schmidt B, Adamczyk M, Betebenner D, Vaughan K. Fluorescence polarization immunoassay of urinary 5-hydroxy-3-indoleacetic acid. Clinical Chemistry 1989. 35 355–359. [PubMed] [Google Scholar]
- 16.da Silva JMK, Mattar R, Colaúto C, Carrilho FJ. Quantification of urinary 5-hydroxyindoleacetic acid by in-house nitrosonaphthol reaction compared with nitrosonaphthol micro column chromatography and enzyme-linked immunosorbent assay. Jornal Brasileiro de Patologia e Medicina Laboratorial 2014. 30 210–214. ( 10.5935/1676-2444.20140017) [DOI] [Google Scholar]
- 17.Puizillout JJ, Delaage MA. Radioimmunoassay of 5-hydroxyindole acetic acid using an iodinated derivative. Journal of Pharmacology and Experimental Therapeutics 1981. 217 791–797. [PubMed] [Google Scholar]
- 18.Ponzio F, Jonsson G. A rapid and simple method for the determination of picogram levels of serotonin in brain tissue using liquid chromatography with electrochemical detection. Journal of Neurochemistry 1979. 32 129–132. ( 10.1111/j.1471-4159.1979.tb04519.x) [DOI] [PubMed] [Google Scholar]
- 19.Seyfried CA, Adam G, Greve T. An automated direct-injection HPLC-method for the electrochemical/fluorimetric quantitation of monoamines and related compounds optimized for the screening of large numbers of animals. Biomedical Chromatography 1986. 1 78–88. ( 10.1002/bmc.1130010206) [DOI] [PubMed] [Google Scholar]
- 20.Shihabi ZK, Scaro J. Liquid-chromatographic assay of urinary 5-hydroxy-3-indoleacetic acid, with electrochemical detection. Clinical Chemistry 1980. 26 907–909. [PubMed] [Google Scholar]
- 21.Tohmola N, Itkonen O, Sane T, Markkanen H, Joenvaara S, Renkonen R, Hamalainen E. Analytical and preanalytical validation of a new mass spectrometric serum 5-hydroxyindoleacetic acid assay as neuroendocrine tumor marker. Clinica Chimica Acta 2014. 428 38–43. ( 10.1016/j.cca.2013.10.025) [DOI] [PubMed] [Google Scholar]
- 22.Miller AG, Brown H, Degg T, Allen K, Keevil BG. Measurement of plasma 5-hydroxyindole acetic acid by liquid chromatography tandem mass spectrometry – comparison with HPLC methodology. Journal of Chromatography B 2010. 878 695–699. ( 10.1016/j.jchromb.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 23.de Jong WH, Graham KS, de Vries EG, Kema IP. Urinary 5-HIAA measurement using automated on-line solid-phase extraction-high-performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography B 2008. 868 28–33. ( 10.1016/j.jchromb.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 24.van Haard PM, Wielders JP, Wikkerink JB. Direct concurrent measurement of urinary vanillylmandelic acid, 5-hydroxyindoleacetic acid and homovanillic acid by HPLC. Three methodologies compared. Biomedical Chromatography 1987. 2 209–215. ( 10.1002/bmc.1130020508) [DOI] [PubMed] [Google Scholar]
- 25.Mayo Clinic Medical Laboratories. Urine Preservatives—Collection and Transportation for 24-Hour Urine Specimens, 2015. (available at: http://www.mayomedicallaboratories.com/it-mmfiles/Urine_Preservatives-Collection_and_Transportation_for_24-Hour_Urine_Specimens.pdf)
- 26.European Chemicals Agency. Boric Acid as a Substance of Very High Concern because of its CMR Properties, 2010. (available at: https://echa.europa.eu/documents/10162/d51fd473-40ec-4831-bc2d-6f53bdf9cbbe).
- 27.Fornstedt N. Determination of 5-hydroxyindole-3-acetic acid in urine by high performance liquid chromatography. Analytical Chemistry 1978. 50 1342–1346. ( 10.1021/ac50031a038) [DOI] [PubMed] [Google Scholar]
- 28.Anderson JA, Ziegler MR, Doeden D. Banana feeding and urinary excretion of 5-hydroxyindoleacetic acid. Science 1958. 127 236–238. ( 10.1126/science.127.3292.236) [DOI] [PubMed] [Google Scholar]
- 29.Bowden K, Brown BG, Batty JE. 5-Hydroxytryptamine: its occurrence in cowhage. Nature 1954. 174 925–926. ( 10.1038/174925a0) [DOI] [PubMed] [Google Scholar]
- 30.Connell AM, Rowlands EN, Wilcox PB. Serotonin, bananas, and diarrhoea. Gut 1960. 1 44–47. ( 10.1136/gut.1.1.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crout JR, Sjoerdsma A. Catechol amine excretion after banana feeding. Journal of Pharmacy and Pharmacology 1959. 11 190–191. ( 10.1111/j.2042-7158.1959.tb12544.x) [DOI] [PubMed] [Google Scholar]
- 32.Feldman JM, Lee EM. Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid. American Journal of Clinical Nutrition 1985. 42 639–643. [DOI] [PubMed] [Google Scholar]
- 33.Huang X, Mazza G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. Journal of Chromatography A 2011. 1218 3890–3899. ( 10.1016/j.chroma.2011.04.049) [DOI] [PubMed] [Google Scholar]
- 34.Kema IP, Schellings AM, Meiborg G, Hoppenbrouwers CJ, Muskiet FA. Influence of a serotonin- and dopamine-rich diet on platelet serotonin content and urinary excretion of biogenic amines and their metabolites. Clinical Chemistry 1992. 38 1730–1736. [PubMed] [Google Scholar]
- 35.Agriculture USDo. National Nutrient Database for Standard Reference, Release 28, 2015. (available at: https://ndb.nal.usda.gov/ndb/)
- 36.Mashige F, Matsushima Y, Kanazawa H, Sakuma I, Takai N, Bessho F, Ohkubo A. Acidic catecholamine metabolites and 5-hydroxyindoleacetic acid in urine: the influence of diet. Annals of Clinical Biochemistry 1996. 33 43–49. ( 10.1177/000456329603300106) [DOI] [PubMed] [Google Scholar]
- 37.Joy T, Walsh G, Tokmakejian S, Van Uum SH. Increase of urinary 5-hydroxyindoleacetic acid excretion but not serum chromogranin. A following over-the-counter 5-hydroxytryptophan intake. Canadian Journal of Gastroenterology 2008. 22 49–53. ( 10.1155/2008/472159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallin ML, Mahmoud K, Viswanath A, Gama R. ‘Sweet Dreams’, ‘Happy Days’ and elevated 24-h urine 5-hydroxyindoleacetic acid excretion. Annals of Clinical Biochemistry 2013. 50 80–82. ( 10.1258/acb.2012.012041) [DOI] [PubMed] [Google Scholar]
- 39.Sharma AM, Schorr U, Thiede HM, Distler A. Effect of dietary salt restriction on urinary serotonin and 5-hydroxyindoleacetic acid excretion in man. Journal of Hypertension 1993. 11 1381–1386. ( 10.1097/00004872-199312000-00010) [DOI] [PubMed] [Google Scholar]
- 40.Odink J, Korthals H, Knijff JH. Simultaneous determination of the major acidic metabolites of catecholamines and serotonin in urine by liquid chromatography with electrochemical detection after a one-step sample clean-up on Sephadex G-10; influence of vanilla and banana ingestion. Journal of Chromatography 1988. 424 273–283. ( 10.1016/S0378-4347(00)81104-4) [DOI] [PubMed] [Google Scholar]
- 41.Kirberger E. Variable action of monoamine oxidase-inhibiting hydrazines on serotonin metabolism. Nature 1963. 197 1211–1212. ( 10.1038/1971211a0) [DOI] [PubMed] [Google Scholar]
- 42.Aymard N, Viala A, Clement MN, Jacquot M, Vacheron MN, Gauillard J, Caroli F. Long-term pharmacoclinical follow-up in schizophrenic patients treated with risperidone. Plasma and red blood cell concentrations of risperidone and its 9-hydroxymetabolite and their relationship to whole blood serotonin and tryptophan, plasma homovanillic acid, 5-hydroxyindoleacetic acid, dihydroxyphenylethyleneglycol and clinical evaluations. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2002. 26 975–988. ( 10.1016/S0278-5846(02)00218-X) [DOI] [PubMed] [Google Scholar]
- 43.Sreemantula S, Boini KM, Nammi S. Reserpine methonitrate, a novel quaternary analogue of reserpine augments urinary excretion of VMA and 5-HIAA without affecting HVA in rats. BMC Pharmacology 2004. 4 30 ( 10.1186/1471-2210-4-30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali SF, Newport GD, Slikker W., Jr Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Annals of the New York Academy of Sciences 1996. 801 187–198. ( 10.1111/j.1749-6632.1996.tb17441.x) [DOI] [PubMed] [Google Scholar]
- 45.Ge J, Barnes NM, Costall B, Naylor RJ. Effect of aversive stimulation on 5-hydroxytryptamine and dopamine metabolism in the rat brain. Pharmacology Biochemistry and Behavior 1997. 58 775–783. ( 10.1016/S0091-3057(97)00024-5) [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi C, Numakawa T, Odaka H, Ooshima Y, Kiyama Y, Manabe T, Kunugi H, Iwakura Y. IL-1 receptor-antagonist (IL-1Ra) knockout mice show anxiety-like behavior by aging. Neuroscience Letters 2015. 599 20–25. ( 10.1016/j.neulet.2015.05.019) [DOI] [PubMed] [Google Scholar]
- 47.Tokumo K, Tamura N, Hirai T, Nishio H. Effects of (Z)-3-hexenol, a major component of green odor, on anxiety-related behavior of the mouse in an elevated plus-maze test and biogenic amines and their metabolites in the brain. Behavioural Brain Research 2006. 166 247–252. ( 10.1016/j.bbr.2005.08.008) [DOI] [PubMed] [Google Scholar]
- 48.Potter WZ, Scheinin M, Golden RN, Rudorfer MV, Cowdry RW, Calil HM, Ross RJ, Linnoila M. Selective antidepressants and cerebrospinal fluid. Lack of specificity on norepinephrine and serotonin metabolites. Archives of General Psychiatry 1985. 42 1171–1177. ( 10.1001/archpsyc.1985.01790350045009) [DOI] [PubMed] [Google Scholar]
- 49.Delay J, Laine B, Buisson JF. The action of isonicotinyl-hydrazide used in the treatment of depressive states. Annal Medical Psychology 1952. 110 689–692. ( 10.1111/j.2042-7158.1952.tb13203.x) [DOI] [PubMed] [Google Scholar]
- 50.Ban TA. An Oral History of Neuropsychopharmacology: The First Fifty Years, Peer Interviews, 2011. [Google Scholar]
- 51.Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. Journal of the American Academy of Child and Adolescent Psychiatry 2004. 43 491–499. ( 10.1097/00004583-200404000-00016) [DOI] [PubMed] [Google Scholar]
- 52.Lykouras L, Markianos M, Hatzimanolis J, Malliaras D, Stefanis C. Association of biogenic amine metabolites with symptomatology in delusional (psychotic) and nondelusional depressed patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry 1995. 19 877–887. ( 10.1016/0278-5846(95)00117-E) [DOI] [PubMed] [Google Scholar]
- 53.Tuckwell HC, Koziol JA. On the concentration of 5-hydroxyindoleacetic acid in schizophrenia: a meta-analysis. Psychiatry Research 1996. 59 239–244. ( 10.1016/0165-1781(95)02741-6) [DOI] [PubMed] [Google Scholar]
- 54.Pedersen AT, Batsakis JG, Vanselow NA, McLean JA. False-positive tests for urinary 5-hydroxyindoleacetic acid. Error in laboratory determinations caused by glyceryl guaiacolate. JAMA 1970. 211 1184–1186. ( 10.1001/jama.1970.03170070054016) [DOI] [PubMed] [Google Scholar]
- 55.McGregor RF, Phillips G, Romsdahl MM. Analysis of urinary 5-hydroxyindoleacetic acid by thin layer chromatography: elimination of interference by glyceryl guaiacolate. Clinica Chimica Acta 1972. 40 59–65. ( 10.1016/0009-8981(72)90251-3) [DOI] [PubMed] [Google Scholar]
- 56.Honet JC, Casey TV, Runyan JW., Jr False-positive urinary test for 5-hydroxyindoleacetic acid due to methocarbamol and mephenesin carbamate. New England Journal of Medicine 1959. 261 188–190. ( 10.1056/NEJM195907232610407) [DOI] [PubMed] [Google Scholar]
- 57.Feldman JM, Butler SS, Chapman BA. Interference with measurement of 3-methoxy-4-hydroxymandelic acid and 5-hydroxyindoleacetic acid by reducing metabolites. Clinical Chemistry 1974. 20 607–610. [PubMed] [Google Scholar]
- 58.Ross G, Weinstein IB, Kabakow B. The influence of phenothiazine and some of its derivatives on the determination of 5-hydroxyindoleacetic acid in urine. Clinical Chemistry 1958. 4 66–76. [PubMed] [Google Scholar]
- 59.Vallon JJ, Badinand A, Bichion C. Trial of elimination of interferences due to basic phenothiazines in the colorimetric determination of 5-hydroxyindolylacetic acid. Annales de Biologie Clinique 1971. 32 359–365. [PubMed] [Google Scholar]
- 60.Yamaguchi Y, Hayashi C. Simple determination of high urinary excretion of 5-hydroxyindole-3-acetic acid with ferric chloride. Clinical Chemistry 1978. 24 149–150. [PubMed] [Google Scholar]
- 61.Ahlberg CD. Interference in the fluorometric quantitation of urinary 5-hydroxyindoleacetic acid by aspirin. Biochemical Pharmacology 1971. 20 497–500. ( 10.1016/0006-2952(71)90091-8) [DOI] [PubMed] [Google Scholar]
- 62.Coward S, Boa FG, Sherwood RA. Sulfasalazine interference with HPLC assay of 5-hydroxyindole-3-acetic acid. Clinical Chemistry 1995. 41 765–766. [PubMed] [Google Scholar]
- 63.Zuetenhorst JM, Korse CM, Bonfrer JM, Peter E, Lamers CB, Taal BG. Daily cyclic changes in the urinary excretion of 5-hydroxyindoleacetic acid in patients with carcinoid tumors. Clinical Chemistry 2004. 50 1634–1639. ( 10.1373/clinchem.2004.032151) [DOI] [PubMed] [Google Scholar]
- 64.Bhagat CI, Dick M. Naproxen interferes positively with 5-hydroxyindoleacetate assay. Clinical Chemistry 1982. 28 1240. [PubMed] [Google Scholar]
- 65.Walker PL, Pettit BR, Sandler M. Interference by naproxen in the urinary 5-hydroxyindoleacetic acid assay is due to a metabolite, desmethylnaproxen. Annals of Clinical Biochemistry 1987. 24 177–181. ( 10.1177/000456328702400209) [DOI] [PubMed] [Google Scholar]
- 66.Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. American Journal of Clinical Pathology 2000. 113 75–86. ( 10.1309/QAW1-X5XW-BVRQ-5LKQ) [DOI] [PubMed] [Google Scholar]
- 67.Walsh FX, Langlais PJ, Bird ED. Liquid-chromatographic identification of acetaminophen in cerebrospinal fluid with use of electrochemical detection. Clinical Chemistry 1982. 28 382–383. [PubMed] [Google Scholar]
- 68.Davidson FD. Paracetamol-associated interference in an HPLC-ECD assay for urinary free metadrenalines and catecholamines. Annals of Clinical Biochemistry 2004. 41 316–320. ( 10.1258/0004563041201626) [DOI] [PubMed] [Google Scholar]
- 69.Drouard-Troalen L, Assicott MJ, Bonnay MM. Paracetamol interference with vanilmandelic and homovanillic acid quantification by the Bio-Rad HPLC kit. Annales de Biologie Clinique 2002. 60 626–627. [PubMed] [Google Scholar]
- 70.Pfafflin A, Mussig K, Schleicher E. Interference of paracetamol (acetaminophen) with a commercially available high-performance liquid chromatography analysis of serotonin leading to falsely low serotonin levels. Annals of Clinical Biochemistry 2009. 46 146–148. ( 10.1258/acb.2008.008116) [DOI] [PubMed] [Google Scholar]
- 71.Daya S, Anoopkumar-Dukie S. Acetaminophen inhibits liver trytophan-2,3-dioxygenase activity with a concomitant rise in brain serotonin levels and a reduction in urinary 5-hydroxyindole acetic acid. Life Sciences 2000. 67 235–240. ( 10.1016/S0024-3205(00)00629-9) [DOI] [PubMed] [Google Scholar]
- 72.van Berkel A, Lenders JW, Timmers HJ. Diagnosis of endocrine disease: biochemical diagnosis of phaeochromocytoma and paraganglioma. European Journal of Endocrinology 2014. 170 R109–R119. ( 10.1530/EJE-13-0882) [DOI] [PubMed] [Google Scholar]
- 73.Eisenhofer G. Screening for pheochromocytomas and paragangliomas. Current Hypertension Reports 2012. 14 130–137. ( 10.1007/s11906-012-0246-y) [DOI] [PubMed] [Google Scholar]
- 74.Monsaingeon M, Perel Y, Simonnet G, Corcuff JB. Comparative values of catecholamines and metabolites for the diagnosis of neuroblastoma. European Journal of Pediatrics 2003. 162 397–402. ( 10.1007/s00431-003-1175-1) [DOI] [PubMed] [Google Scholar]
- 75.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. Journal of Pharmacology and Experimental Therapeutics 2003. 305 800–811. ( 10.1124/jpet.103.049270) [DOI] [PubMed] [Google Scholar]
- 76.Eisenhofer G, Keiser H, Friberg P, Mezey E, Huynh TT, Hiremagalur B, Ellingson T, Duddempudi S, Eijsbouts A, Lenders JW. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. Journal of Clinical Endocrinology and Metabolism 1998. 83 2175–2185. ( 10.1210/jcem.83.6.4870) [DOI] [PubMed] [Google Scholar]
- 77.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacological Reviews 2004. 56 331–349. ( 10.1124/pr.56.3.1) [DOI] [PubMed] [Google Scholar]
- 78.Woo HI, Yang JS, Oh HJ, Cho YY, Kim JH, Park HD, Lee SY. A simple and rapid analytical method based on solid-phase extraction and liquid chromatography-tandem mass spectrometry for the simultaneous determination of free catecholamines and metanephrines in urine and its application to routine clinical analysis. Clinical Biochemistry 2016. 49 573–579. ( 10.1016/j.clinbiochem.2016.01.010) [DOI] [PubMed] [Google Scholar]
- 79.Grouzmann E, Lamine F. Determination of catecholamines in plasma and urine. Best Practice and Research Clinical Endocrinology and Metabolism 2013. 27 713–723. ( 10.1016/j.beem.2013.06.004) [DOI] [PubMed] [Google Scholar]
- 80.Battelli MG. Dosage colorimétrique de la substance active des capsules surrénales. Comptes Rendus des Séances de la Société de Biologie 1902. 54 571. [Google Scholar]
- 81.Von Euler US, Hamberg U. Colorimetric estimation of noradrenalin in the presence of adrenalin. Science 1949. 110 561 ( 10.1126/science.110.2865.561) [DOI] [PubMed] [Google Scholar]
- 82.Engelman K, Portnoy B, Lovenberg W. A sensitive and specific double-isotope derivative method for the determination of catecholamines in biological specimens. American Journal of the Medical Sciences 1968. 255 259–268. ( 10.1097/00000441-196804000-00007) [DOI] [PubMed] [Google Scholar]
- 83.Hjemdahl P, Daleskog M, Kahan T. Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: comparison with a radioenzymatic method. Life Sciences 1979. 25 131–138. ( 10.1016/0024-3205(79)90384-9) [DOI] [PubMed] [Google Scholar]
- 84.Chan EC, Ho PC. High-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometric method for the analysis of catecholamines and metanephrines in human urine. Rapid Communications in Mass Spectrometry 2000. 14 1959–1964. () [DOI] [PubMed] [Google Scholar]
- 85.Pisano JJ. A simple analysis for normetanephrine and metanephrine in urine. Clinica Chimica Acta 1960. 5 406–414. ( 10.1016/0009-8981(60)90146-7) [DOI] [PubMed] [Google Scholar]
- 86.Gupta RN, Price D, Keane PM. Modified Pisano method for estimating urinary metanephrines. Clinical Chemistry 1973. 19 611–614. [PubMed] [Google Scholar]
- 87.Crawford GA, Gallery ED, Gyory AZ. Removal of interference by antihypertensive drugs in the spectrophotometric assay of metanephrines. Clinica Chimica Acta 1987. 169 117–119. ( 10.1016/0009-8981(87)90400-1) [DOI] [PubMed] [Google Scholar]
- 88.Stroes JW, Putters J, van Rijn HJ. An improved spectrophotometric procedure for the determination of urinary metanephrines. Journal of Clinical Chemistry and Clinical Biochemistry 1987. 25 483–486. [DOI] [PubMed] [Google Scholar]
- 89.Corcuff JB, Gatta B, Ducassou D, Simonnet G. Antihypertensive drug urapidil metabolites interfere with metanephrines assays. Clinical Endocrinology 2001. 55 280 ( 10.1046/j.1365-2265.2001.1225b.x) [DOI] [PubMed] [Google Scholar]
- 90.de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP. Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors. Journal of Clinical Endocrinology and Metabolism 2009. 94 2841–2849. ( 10.1210/jc.2009-0303) [DOI] [PubMed] [Google Scholar]
- 91.Marrington R, Johnston J, Knowles S, Webster C. Measurement of urinary metadrenaline and normetadrenaline by liquid chromatography tandem mass spectrometry for the diagnosis of phaeochromocytoma. Annals of Clinical Biochemistry 2010. 47 467–475. ( 10.1258/acb.2010.010060) [DOI] [PubMed] [Google Scholar]
- 92.Peitzsch M, Pelzel D, Glockner S, Prejbisz A, Fassnacht M, Beuschlein F, Januszewicz A, Siegert G, Eisenhofer G. Simultaneous liquid chromatography tandem mass spectrometric determination of urinary free metanephrines and catecholamines, with comparisons of free and deconjugated metabolites. Clinica Chimica Acta 2013. 418 50–58. ( 10.1016/j.cca.2012.12.031) [DOI] [PubMed] [Google Scholar]
- 93.Chan EC, Wee PY, Ho PC. Evaluation of degradation of urinary catecholamines and metanephrines and deconjugation of their sulfoconjugates using stability-indicating reversed-phase ion-pair HPLC with electrochemical detection. Journal of Pharmaceutical and Biomedical Analysis 2000. 22 515–526. ( 10.1016/S0731-7085(99)00308-8) [DOI] [PubMed] [Google Scholar]
- 94.Willemsen JJ, Ross HA, Lenders JW, Sweep FC. Stability of urinary fractionated metanephrines and catecholamines during collection, shipment, and storage of samples. Clinical Chemistry 2007. 53 268–272. ( 10.1373/clinchem.2006.075218) [DOI] [PubMed] [Google Scholar]
- 95.de Jong WH, Post WJ, Kerstens MN, de Vries EG, Kema IP. Elevated urinary free and deconjugated catecholamines after consumption of a catecholamine-rich diet. Journal of Clinical Endocrinology and Metabolism 2010. 95 2851–2855. ( 10.1210/jc.2009-2589) [DOI] [PubMed] [Google Scholar]
- 96.Eagle K. Toxicological effects of red wine, orange juice, and other dietary SULT1A inhibitors via excess catecholamines. Food and Chemical Toxicology 2012. 50 2243–2249. ( 10.1016/j.fct.2012.03.004) [DOI] [PubMed] [Google Scholar]
- 97.Madhavaram H, Woollard GA. Interference from Indian diet on the internal standard in a commercial method for the measurement of urinary metanephrines by high-performance liquid chromatography with electrochemical detection. Annals of Clinical Biochemistry 2014. 51 400–405. ( 10.1177/0004563213494079) [DOI] [PubMed] [Google Scholar]
- 98.Nishimura FT, Kimura Y, Abe S, Fukunaga T, Minami J, Tanii H, Saijoh K. Effects of functional polymorphisms related to catecholaminergic systems on changes in blood catecholamine and cardiovascular measures after alcohol ingestion in the Japanese population. Alcoholism Clinical and Experimental Research 2008. 32 1937–1946. ( 10.1111/j.1530-0277.2008.00778.x) [DOI] [PubMed] [Google Scholar]
- 99.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. Journal of Clinical Endocrinology and Metabolism 2003. 88 2656–2666. ( 10.1210/jc.2002-030005) [DOI] [PubMed] [Google Scholar]
- 100.Munion GL, Seaton JF, Harrison TS. HPLC for urinary catecholamines and metanephrines with alpha-methyldopa. Journal of Surgical Research 1983. 35 507–514. ( 10.1016/0022-4804(83)90040-9) [DOI] [PubMed] [Google Scholar]
- 101.Spilker B, Watson BS, Woods JW. Drug interference with measurement of metanephrines in urine. Annals of Clinical and Laboratory Sciences 1983. 13 16–19. [PubMed] [Google Scholar]
- 102.Savory DJ. Interference by Sotalol with the Pisano method for urinary metanephrines. Annals of Clinical Biochemistry 1984. 21 446 ( 10.1177/000456328402100519) [DOI] [PubMed] [Google Scholar]
- 103.Jauzac P, Sylla C, Valdiguie P, Couste-Blazy L. Elimination of interference by acebutolol and its metabolites in determinations of urinary metanephrines. Clinical Chemistry 1982. 28 1243–1244. [PubMed] [Google Scholar]
- 104.Madiedo G, Smurawa B. Interference by triamterene with assay for metanephrine. Clinical Chemistry 1980. 26 782. [PubMed] [Google Scholar]
- 105.Johnson LR, Reese M, Nelson DH. Interference in Pisano’s urinary metanephrine assay after use of x-ray contrast media. Clinical Chemistry 1972. 18 209–211. [PubMed] [Google Scholar]
- 106.van Laarhoven HW, Willemsen JJ, Ross HA, Beex LV, Lenders JW, Sweep FC. Pitfall in HPLC assay for urinary metanephrines: an unusual type of interference caused by methenamine intake. Clinical Chemistry 2004. 50 1097–1099. ( 10.1373/clinchem.2004.032912) [DOI] [PubMed] [Google Scholar]
- 107.Gardet V, Gatta B, Simonnet G, Tabarin A, Chene G, Ducassou D, Corcuff JB. Lessons from an unpleasant surprise: a biochemical strategy for the diagnosis of pheochromocytoma. Journal of Hypertension 2001. 19 1029–1035. ( 10.1097/00004872-200106000-00006) [DOI] [PubMed] [Google Scholar]
- 108.Barco S, Alpigiani MG, Ghiggeri GM, Talio M, Maffia A, Tripodi G, Cangemi G. Amoxicillin-associated interference in an HPLC-EC assay for urinary fractionated metanephrines: potential pitfall in pheochromocytoma biochemical diagnosis. Clinical Biochemistry 2014. 47 119–121. ( 10.1016/j.clinbiochem.2014.07.009) [DOI] [PubMed] [Google Scholar]
- 109.Dunand M, Donzelli M, Rickli A, Hysek CM, Liechti ME, Grouzmann E. Analytical interference of 4-hydroxy-3-methoxymethamphetamine with the measurement of plasma free normetanephrine by ultra-high pressure liquid chromatography-tandem mass spectrometry. Clinical Biochemistry 2014. 47 1121–1123. ( 10.1016/j.clinbiochem.2014.04.003) [DOI] [PubMed] [Google Scholar]
- 110.Peitzsch M, Adaway JE, Eisenhofer G. Interference from 3-O-methyldopa with ultra-high performance LC-MS/MS measurements of plasma metanephrines: chromatographic separation remains important. Clinical Chemistry 2015. 61 993–996. ( 10.1373/clinchem.2015.239962) [DOI] [PubMed] [Google Scholar]
- 111.Levreri I, Caruso U, Deiana F, Buoncompagni A, De Bernardi B, Marchese N, Melioli G. The secretion of ibuprofen metabolites interferes with the capillary chromatography of urinary homovanillic acid and 4-hydroxy-3-methoxymandelic acid in neuroblastoma diagnosis. Clinical Chemistry and Laboratory Medicine 2005. 43 173–177. ( 10.1515/CCLM.2005.029) [DOI] [PubMed] [Google Scholar]
- 112.Watanabe K, Miura I, Kanno-Nozaki K, Horikoshi S, Mashiko H, Niwa S, Yabe H. Associations between five-factor model of the positive and negative syndrome scale and plasma levels of monoamine metabolite in patients with schizophrenia. Psychiatry Research 2015. 230 419–423. ( 10.1016/j.psychres.2015.09.030) [DOI] [PubMed] [Google Scholar]
- 113.Dobson R, Burgess MI, Banks M, Pritchard DM, Vora J, Valle JW, Wong C, Chadwick C, George K, Keevil B, et al. The association of a panel of biomarkers with the presence and severity of carcinoid heart disease: a cross-sectional study. PLoS ONE 2013. 8 e73679 ( 10.1371/journal.pone.0073679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tohmola N, Itkonen O, Turpeinen U, Joenvaara S, Renkonen R, Hamalainen E. Preanalytical validation and reference values for a mass spectrometric assay of serum vanillylmandelic acid for screening of catecholamine secreting neuroendocrine tumors. Clinica Chimica Acta 2015. 446 206–212. ( 10.1016/j.cca.2015.03.041) [DOI] [PubMed] [Google Scholar]
- 115.Eisenhofer G, Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clinical Chemistry 2014. 60 1486–1499. ( 10.1373/clinchem.2014.224832) [DOI] [PubMed] [Google Scholar]
- 116.Osinga TE, Kema IP, Kerstens MN, de Jong WH, van Faassen M, Dullaart RP, Links TP, van der Horst-Schrivers AN. No influence of antihypertensive agents on plasma free metanephrines. Clinical Biochemistry 2016. 49 1368–1371. ( 10.1016/j.clinbiochem.2016.06.004) [DOI] [PubMed] [Google Scholar]
- 117.Eisenhofer G, Darr R, Pamporaki C, Peitzsch M, Bornstein S, Lenders JW. Supine or sitting? Economic and other considerations for use of plasma metanephrines for diagnosis of phaeochromocytoma. Clinical Endocrinology 2015. 82 463–464. ( 10.1111/cen.12602) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a