Abstract
Objectives
Nivolumab has recently been shown in the phase III clinical trial CheckMate‐141 to have superior survival rates compared to the current standard of care chemotherapy for recurrent or metastatic platinum‐resistant head and neck squamous cell carcinoma (HNSCC). Nivolumab targets the immune inhibitory receptor programmed cell death 1 (PD‐1). Programmed cell death ligand 1 (PD‐L1) genomics have been poorly characterized in the context of HNSCC, including expression levels of PD‐L1 in individual tumors as well as related up or down‐regulated genes that might function as co‐targets.
Study Design
Data mining of The Cancer Genome Atlas (TCGA)
Methods
530 patients with HNSCC were pulled from the TCGA using cBioPortal. Primary tumor site data was available in 279 of the samples (52.6%), of which oral cavity was the most common site (61.6%) followed by larynx (25.8%). Other PD‐1‐sensitive tumors were analyzed to compare PD‐L1 expression in HNSCC relative to other tumors including bladder, renal cell carcinoma, melanoma, and lung carcinomas.
Results
A significant fraction of HNSCC tumors have genetic alterations in PD‐L1 (6.2%). HNSCC has the highest PD‐L1 expression of all of the tumor types examined, with a median 60‐fold increase. Several important genes were identified in this study including Caspase 7, ZFYVE9, and Plg‐R(KT) that have a strong relationship with alterations in PD‐L1.
Conclusion
In light of the role of PD‐1 and PD‐L1 as key immunotherapy targets in HNSCC, several potential co‐targets identified in this study warrant further investigation. Further, while the number of genetic alterations were small in head and neck carcinomas, alterations in PD‐L1 expression were highly significant.
Level of Evidence
NA
Keywords: Immunotherapy, head and neck cancer, programmed death ligand
INTRODUCTION
The Programmed Death 1 (PD‐1) immune checkpoint comprises the PD‐1 T‐cell co‐inhibitory receptor and the Programmed death ligand 1 and 2 and PD‐L2 ligands.1 The pathway mediates immune resistance in the local environment. Monoclonal antibodies blocking PD‐1 activate the immune system against several tumors, including kidney, lung, melanoma, and colon.2 PD‐L1 expression in tumor specimens can identify patients that are likely to have a response to PD‐1 blockade with monoclonal antibodies, and PD‐L1 expression tests are approved by the Food and Drug Administration to guide clinical decision making in advanced non–small‐cell lung cancer and melanoma.3 Tumor mutational burden, which creates new neoantigens for immune recognition, also influences tumor responsiveness to anti–PD‐1 therapy, as has the presence of DNA mismatch repair defects.4, 5
Despite the development of novel therapies, many patients with squamous cell carcinoma of the head and neck (HNSCC) have a poor prognosis. In addition to the traditional teaching of a strong association with tobacco and alcohol, human papilloma virus (HPV) infection has also recently been identified as an important oncogenic risk factor.6, 7 Cancer genomics of HNSCC has shown a high tumor mutational burden.4 Patients with metastatic, platinum refractory disease have poor outcomes when chemotherapy is used in the relapsed setting.8 PD‐1 inhibition with nivolumab can improve survival in these patients, and tumors with high PD‐1 expression respond better to PD‐1 inhibition than those with no PD‐1 expression.9 In non–small‐cell lung cancer and melanoma, patients are much more likely to benefit from PD‐1 inhibition when PD‐L1 is expressed.3
Herein we sought to determine gene expression patterns, co‐occurring mutations, and copy number variations in HNSCC tumors known to have alterations in PD‐L1, and to compare HNSCC PD‐L1 expression with expression in other cancers that are known to respond favorably to anti‐PD‐1/PD‐L1 therapy.
MATERIALS AND METHODS
Patient Population and Tumor Genetics
The results included in our study are based upon data generated by the head and neck cancer (HNSCC) data found within the Cancer Genome Atlas (TCGA) Research Network (http://cancergenome.nih.gov/). The data set included 530 patients with HNSCC, of which 279 patients had full tumor sequencing and protein expression analysis data available and 530 patients had somatic copy number alteration (SCNA) data available.10 The patient population was 72.8 percent male with the tumor stage breakdown as follows: 5.1% Stage I, 14.5% Stage II, 15.5% Stage III, 48.9% Stage IVA, 2.3% Stage IVB, 0.2% Stage IVC. Tumor Subsite data was available for 279 patients including 61.6% oral cavity, 25.8% larynx, 11.8% oropharynx, and 0.7% hypopharynx. PD‐L1 expression data in the head and neck cancer data set was compared to other cancers that have shown sensitivity to PD‐1 inhibition including bladder, papillary, clear cell and chromophobe renal cell carinomas, melanoma as well as squamous and adenocarcinoma of the lung with data available in the TCGA. The TCGA data was analyzed using the open‐source web program cBioPortal.11, 12 Figures were generated using Plotly (Montreal, Canada), as functionality built into cBioPortal.
Somatic Copy Number Alteration (SCNA) Analysis
Copy number alterations were calculated using Genomic Identification of Significant Target in Cancer 2.0 (GISTIC).13 This validated method identifies alterations that drive cancer growth by utilizing a probabilistic method for defining background rates of copy‐number alterations from focal cancer‐associated changes. The SCNA results were made available through the cBioPortal.
Protein Expression Analysis
Protein expression analysis was performed using a reverse phase protein array (RPPA) analysis. This is a high throughput platform that can analyze protein expression levels of a large number of biological samples using a dot‐blot platform and high‐quality antibodies. The RPPA results were made available through the cBioPortal.
Statistics
Statistical significance was calculated using imbedded algorithms within cBioPortal. A p‐value < 0.05 was used as a cutoff of significance level.
RESULTS
The genetic fingerprint of HNSCC within the TCGA dataset showed preservation of PD‐1 in the large majority of cases (93.8%). There were no PD‐L1 somatic mutations seen in 279 samples with whole genome sequencing. When assessing somatic copy number alterations, 25 of 530 samples amplified (4.7%) and 8 of 530 samples deleted (1.5%) PD‐L1.
We performed a comprehensive somatic copy number analysis of the alteration of 19,238 possible genes and found 112 co‐occurred or were mutually excluded with statistical significance when PD‐L1 was altered. Figure 1 is a volcano plot of these results. Supplemental Table 1 details all 112 genes and displays the percentages and p‐values for each.
Figure 1.
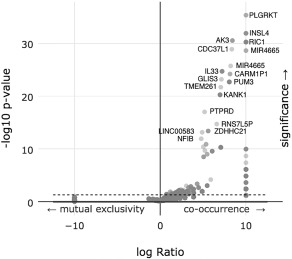
A volcano plot of an enrichment analysis of putative copy‐number alterations (Amplifications) from GISTIC with altered copy numbers of PD‐L1.
A similar analysis of deep deletions instead of amplification of 11,843 genes found 31 genes that co‐occurred or were mutually excluded with statistical significance. Figure 2 is a volcano plot of these results. Supplemental Table 2 details all 31 genes and displays the percentages and p‐values for each.
Figure 2.
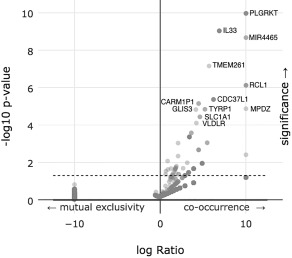
A volcano plot of an enrichment analysis of putative copy‐number alterations (Deep deletions) from GISTIC with altered copy numbers of PD‐L1.
Further, we performed a protein expression analysis using RPPA and found 5 proteins (IGFBP2, CASP7, CDKN2A, NF2, ITGA2) to have statistically significant changes when PD‐L1 was altered. Figure 3 is a volcano plot of these results. Supplemental Table 3 details all 5 genes and displays the percentages and p‐values for each. Of note, the signal to noise ratio was very high for insulin‐like growth factor binding protein 2 (over‐expressed, p‐value = 0.00069). In contradistinction caspase 7 was significantly under‐expressed (p‐value = 0.0127).
Figure 3.
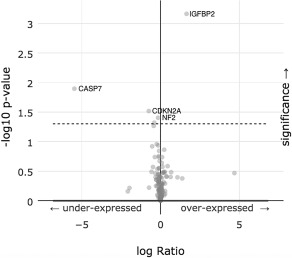
A volcano plot of an enrichment analysis of under‐expressed and over‐expressed proteins when PD‐L1 expression is altered.
While there were not any individual point mutations found within PD‐L1 in the dataset, we analyzed any associated mutations found in other genes when PD‐L1 was either amplified or deleted. There were 83 genes out of 12,682 analyzed that had a statistically significant relationship with PD‐L1. Figure 4 is a volcano plot of these results. Supplemental Table 4 details all 83 genes and displays the percentages and p‐values for each.
Figure 4.
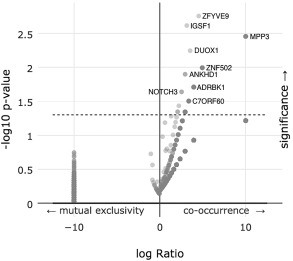
A volcano plot of an enrichment analysis of co‐occurring mutations when PD‐L1 expression is altered.
Survival was not significantly affected in patients with or without genetic alterations in PD‐L1 (p‐value = 0.647). The median survival of the PD‐L1 altered group was 47.9 months, and was 56.9 months for the PL‐1 unaltered group. A survival curve is given in Figure 5. Of note, there were 33 patients included in the PL‐1 altered group, of which 16 were deceased and 17 were censored.
Figure 5.
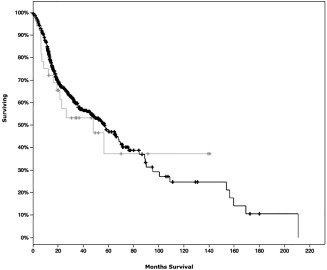
Survival curves of patients without alterations in PD‐L1 (black) compared to patients with genetic alterations in PD‐L1 (gray).
Expression of PD‐L1 may be independent of copy number variations or somatic mutations. Figure 6 illustrates several different tumor types and expression levels of PD‐L1. Interestingly, HNSCC has the highest PD‐L1 expression of all of the examined tumor types. The median expression of PD‐L1 is a 60‐fold increase from baseline.
Figure 6.
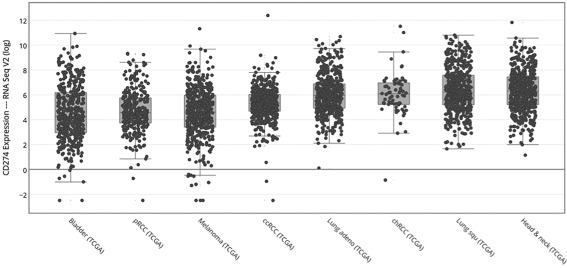
PD‐LI1 expression in selected anti‐PD‐L1 sensitive cancers. CD274 is synonymous for PD‐L1. The y‐axis is plotted as a log base of 2. pRCC = papillary renal cell carcinoma, ccRCC = clear cell renal cell carcinoma, chRCC = chromophobe renal cell carcinoma.
DISCUSSION
The PD‐1–blocking antibodies pembrolizumab and nivolumab are promising therapies for patients with advanced metastatic melanoma and non–small‐cell lung cancer.14, 15 Nivolumab was recently approved for the treatment of a renal cell carcinoma, and has now shown antitumor efficacy in HNSCC.8, 16 Despite promising results, some cancers appear to be refractory to anti‐PD‐1 therapy. Factors known to be associated with anti‐PD‐1 response include tumor PD‐L1 expression, high tumor mutational burden, and the presence of CD8 T cells at the invasive tumor margin.17 Determining further mechanisms of PD‐1 resistance and new therapeutic targets remains critical.
The incidence of PD‐L1 expression in HNSCC appears most similar to non‐small cell lung cancer. Median PD‐L1 expression in HNSCC exceeds that of other anti‐PD‐1 sensitive cancers.
Plg‐R(KT) was found to be have the strongest association to PD‐L1 in both deletion and amplification copy number variations. Plg‐R(KT) is a plasminogen receptor found to be a control point regulating cell surface proteolysis. Plg‐R(KT) is broadly expressed in migratory cells (e.g. leukocytes) and in breast cancer, leukemic and neuronal cells.18 The gene plays an important role in macrophage recruitment in the inflammatory process, and its absence significantly impairs macrophage recruitment in mice.19 Plg‐R(KT) activates plasminogen, which acts to inhibit cellular apoptosis.20 Therapeutic knockdown of Plg‐R(KT) could promote cancer cell apoptosis.
The most significant gene associated with mutations in PD‐L1 altered cancers was zinc finger FYVE domain containing 9 (ZFYVE9). ZFYVE9 recruits unphosphorylated SMAD2/SMAD3 to the TGF‐beta receptor. Once phosphorylated, SMAD2/SMAD3 dissociates from ZFYVE9 and translocates into the nucleus. TGF‐beta signaling has several known functions in both tumor growth and suppression, but may help tumor cells proliferate and escape immune response.21 This pathway's relationship to PD‐L1 merits further investigation. It may be a candidate target for multimodality therapy to prevent PD‐1 resistance or to provide synergistic efficacy with PD‐1 inhibition.
Caspase 7 was found to have the strongest negative association with PD‐L1 expression. The caspase family of proteins play an intricate role in cell apoptosis and are frequently down‐regulated in cancer.22
The TCGA dataset gives an accurate portrayal of tumor cell expression of PD‐L1 in HNSCC. Notably absent are PD‐L1 expression data from tumor immune infiltrates. In colorectal cancer, high tumor neoantigen load correlated positively with lymphocytic infiltration and disease‐specific survival.23 Given the known somatic mutational burden in HNSCC,24 and the significant improvement in survival seen in HNSCC tumors in which PD‐L1 expression was greater than 1% (Checkmate 141), it is likely that looking purely at tumor gene expression within the TCGA can characterize the unique measures HNSCC tumors use to evade the host immune system and inform on genetic targets that may be useful in the treatment of HNSCC. An evaluation and further characterization of the tumor microenvironment within HNSCC and several other cancers could similarly produce new targets designed to activate the immune system against cancer.
Taken together, genes most associated with PD‐L1 expression in HNSCC are intricately involved in immune pathways, and the complex dysregulation of the immune response may make HNSCC vulnerable to other immunotherapy targets. Perhaps associated genetic abnormalities in PD‐L1 positive tumors can contribute to a “gene‐expression signature” that can identify HNSCC tumors most likely to respond to anti‐PD1 therapy similar to risk signatures that help guide clinical therapy in breast cancer.25
CONCLUSION
The PD‐L1 ligand is a powerful mechanism for cancer cell evasion of immune detection. Our algorithm, which analyzes PD‐LI expression in the context of associated genetic mutations, can predict actionable gene targets and may be informative for tumor prognosis. PD‐L1 expression in tumor specimens may identify patients that are likely to have a response to PD‐1 blockade with monoclonal antibodies, with a 60‐fold increase in PD‐L1 expression in our studied tumor samples. PD‐L1 expression tests are already approved by the Food and Drug Administration to guide clinical decision‐making in advanced non–small‐cell lung cancer and melanoma. We introduce this study as further evidence for a prospective study in HNSCC using a PD‐L1 expression‐based protocol to select patients that would be favorable candidates to receive PD‐L1 therapy.
Supporting information
Supporting Information
Conflicts of Interest: None.
Financial Disclosure: None
BIBLIOGRAPHY
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le DT, Uram JN, Wang H, et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus‐positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–269. [DOI] [PubMed] [Google Scholar]
- 8. Vermorken JB. Medical treatment in head and neck cancer. Ann Oncol 2005;16(Suppl 2):ii258–ii64. [DOI] [PubMed] [Google Scholar]
- 9. Gillison ML, Blumenschein G, Fayette J, et al. Nivolumab (nivo) vs investigator's choice (IC) for recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): CheckMate‐141. AACR annual abstracts. April 19, 2016. Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=b01a929a-449c-4fd7-89e5-7dc10238925f&cKey=7bbfb029-c64c-4ed8-97e9-515de57892d3&mKey=%7b1D10D749-4B6A-4AB3-BCD4-F80FB1922267%7d. Accessed November 1, 2016
- 10. The Cancer Genome Atlas Network . Comprehensive genomic characterization of head and neck squamous cell carinomas. Nature 2015;517:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cerami E, Gao J, Dogrusoz U, et al. The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal Sci Signal 2013;6:l1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy‐number alteration in human cancers. Genome Biol 2011;12(4):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 15. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 16. Ansell SM, Lesokhin AM, Borrello I, et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tumeh PC, Harview CL, Yearley JH, et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andronicos NM, Chen EI, Baik N, et al. Proteomics‐based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg‐RKT, a major regulator of cell surface plasminogen activation. Blood 2010;115:1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miles LA, Lighvani S, Baik N, et al. The plasminogen receptor, Plg‐R(KT), and macrophage function. J Biomed Biotechnol 2012;2012:250464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell JW, Baik N, Castellino FJ, Miles LA. Plasminogen inhibits TNFα‐induced apoptosis in monocytes. Blood 2006;107:4383–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gratchev A. TGF‐β signalling in tumour associated macrophages. Immunobiology 2016;222:75–81. [DOI] [PubMed] [Google Scholar]
- 22. Slee EA, Adrain C, Martin SJ. Executioner caspase‐3, ‐6, and ‐7 perform distinct, non‐redundant roles during the demolition phase of apoptosis. J Biol Chem 2001;276:7320–7326. [DOI] [PubMed] [Google Scholar]
- 23. Giannakia M, Mu XJ, Shukla SA et al. Genomic correlates of immune‐cell infiltrates in colorectal carcinoma. Cell Rep 2016;17:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alexandrov LB, Nik‐Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;502:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21‐gene expression assay in breast cancer. N Engl J Med 2015;373:2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


