Abstract
Objectives
Published data examining the efficacy of olfactory training (OT) has used standardized concentrations of odorants and the Sniffin’ Sticks testing method. Although well‐validated, these methods are costly and time‐intensive for the average otolaryngology practice. The purpose of our study was to evaluate the efficacy of using essential oils at random concentrations and the University of Pennsylvania Smell Test (UPSIT) for training and testing, and compare this with the existing data on OT.
Study Design
Randomized Clinical TrialMethods: Patients presenting to a tertiary care rhinology center with subjective loss of smell and olfactory loss measured by UPSIT were randomized to OT or control for 6 months. Only patients with loss of smell greater than one‐year duration, and loss associated with post‐infectious and idiopathic etiologies were included. Baseline UPSIT was compared to 6‐month UPSIT. An accepted 10% change or better was used to establish a significant improvement on UPSIT.
Results
43 patients were enrolled. Eight patients were lost to follow‐up, with a total of 35 completing the study. Age ranged from 39–71 with an average of 56. Of 19 patients in the OT group, 6 showed significant improvement (32%), while only two out of 16 patients (13%) in the control group improved. Increasing age and duration of loss were significantly correlated to lack of improvement.
Conclusion
Allowing patients to use random concentrations of essential oils to perform OT is as effective as published data using controlled concentrations of odorants for post‐infectious and idiopathic olfactory loss.
Level of Evidence
1b.
Keywords: olfactory loss, olfactory training, olfaction, smell loss, randomized controlled trial
INTRODUCTION
With estimates ranging from 2.7 million to 15 million Americans suffering from chronic olfactory problems and over a quarter of Americans over the age of 65 having documented olfactory loss, the need for a treatment paradigm is evident.1, 2 Although basic science research is rapidly expanding our knowledge of the olfactory system, the pathologic mechanisms that lead to the majority of cases of olfactory loss are still poorly understood. This is complicated by numerous etiologies noted in the literature as potentially causing or contributing to smell loss.3 Growing evidence from Europe suggests that a simple protocol known as olfactory training has the potential to improve olfactory function in a significant portion of those suffering from loss from a wide variety of etiologies.4, 5, 6, 7, 8 A recent systematic review and meta‐analysis confirmed that olfactory training as conducted in those studies is beneficial in patients with olfactory loss.9 However, while the training protocol of specific concentrations of odorants using the Sniffin’ Sticks method of delivery produces standardized data collection for research purposes, it may not be as feasible to use these same methods in a busy otolaryngology practice with patients in charge of purchasing their own supplies for the training protocol. A common practice in centers across the United States treating patients with olfactory dysfunction has been to instruct patients to obtain their own odorants and perform the protocol themselves, but this methodology remains unproven. Our objective was to elucidate if olfactory training with self‐purchased essential oils at uncontrolled concentrations would result in the same beneficial results as prior studies using standardized odorant concentrations.
METHODS
Study design was approved by the Institutional Review Board, and all patients enrolled in the study provided written informed consent. Patients were all seen in the setting of a tertiary care rhinology center, and all had subjective loss of smelling ability from either post‐infectious or idiopathic etiologies for at least one‐year duration. The University of Pennsylvania Smell Identification Test (UPSIT) was used to objectively confirm olfactory loss. If patients were noted to have hyposmia or anosmia on the UPSIT, they were eligible for inclusion in the study. Exclusion criteria were age less than 18, pregnancy, duration of smell loss less than one year, etiology of smell loss other than post‐infectious or idiopathic, prior sinus, skull base or brain surgery, and cognitive or psychiatric dysfunction. Patients were then randomized using a computerized random number generator to either the olfactory training arm or the control arm. The control arm received no intervention. The olfactory training (OT) arm was instructed to obtain essential oils of rose, lemon, eucalyptus and clove. Neither brand, concentration nor cost of the essential oils was specified, and patients were allowed to select whichever they preferred, as long as they were of those four scents. Patients in the OT arm were instructed to open each essential oil container, hold it under their nose, and breathe slowly and deeply for 15 seconds. They were then instructed to give themselves a 15 second break in between scents and rotate through all four scents. They were also asked to focus on what they remembered these odors smelling like before their loss of smell while performing this exercise, in order to boost concentration. They were told to perform this training protocol twice a day, every day, for 6 months. They were also asked to keep journal entries of each training session to boost adherence to the protocol and provide a way for us to objectively monitor compliance. Patients in this arm were also all contacted via phone or email at three months to monitor and encourage adherence to the protocol at the midpoint. Patients in both the control arm and the OT arm returned to the office at six months and were again tested using the UPSIT. A 10% or greater change in UPSIT score was considered significant (4 points).10 To detect a medium effect with a power of 0.95 and an alpha of 0.05, we recruited 43 participants. Percent improvement in scores between the control and OT arms were compared with Chi‐square testing and p < 0.05 was considered significant. As in the one other randomized controlled trial examining the efficacy of olfactory training, we set our primary endpoint as having greater than 20% of patients in a group achieve significant improvement (double the rate of spontaneous remission within 16 weeks).4, 8
RESULTS
A total of 43 patients were enrolled in the study. Twenty patients were randomized to the OT group and twenty‐three to the control group. Over the course of the six‐month trial period, eight patients were lost to follow up, leaving 35 for final analysis, with 19 in the OT group and 16 in the control group.
Age ranged from 39 to 71 with a mean age of 56. Duration of smell loss ranged from 12 months to 38 months, with a mean of 24 months. There was no significant difference between control and intervention groups regarding age, gender, duration of loss, severity of loss or etiology (idiopathic versus post‐infectious).
In the OT group, 6/19 (32%) showed a 10% or greater improvement on the UPSIT, while in the control group only 2/16 (13%) had this result. (Fig. 1) The raw numeric difference between the groups was not statistically significant, but the OT group did reach the primary endpoint of >20% with significant improvement, whereas the control group did not. This is similar to results from the one other RCT published evaluating olfactory training.8 Both increasing age and increased duration of smell loss correlated with lack of improvement in UPSIT. (Figs. 2 and 3)
Figure 1.
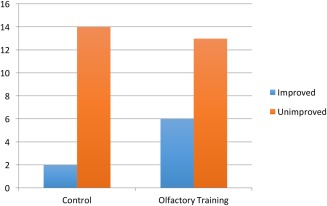
Significant improvement (10% change or greater) in UPSIT
Figure 2.
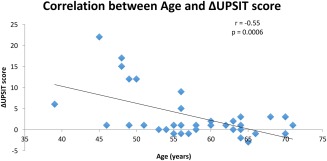
Correlation between Age and Change in UPSIT Score
Figure 3.
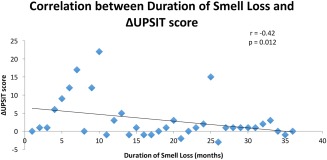
Correlation between Duration of Smell Loss and Change in UPSIT Score
Compliance was excellent, at 98% in all patients who remained in the study and were not lost to follow‐up.
Adverse events were tracked. Two patients in the control arm experienced self‐limited nose bleeds during the time of the study. One patient in the OT arm experienced phantosmia for two days (a smell described as “rotten meat”), and another patient in the OT arm experienced nasal irritation, but in neither circumstance did it preclude them from completing the training protocol.
DISCUSSION
Published prospective studies utilizing olfactory training with standardized concentrations of pure odorants have demonstrated varying rates of improvement, with percentage of patients improving from training ranging from 30% to 68%, and with the duration of smell loss and etiology of smell loss also extremely varied in these studies.4, 5, 6, 7 However, in the only other randomized controlled trial using OT, when including those patients with duration of smell loss greater than 12 months, the percentage of patients experiencing improvement with OT was 26%, compared to 15% in the control group.8 Our results of 32% of patients showing significant improvement in our OT group compared with 13% in our control group are very similar. Although it sets the bar higher for finding a positive result, we specifically chose to exclude patients with olfactory loss less than 12 months, because it is difficult to ascertain how many of those patients are still experiencing spontaneous recovery. In addition, this is also why we placed our primary endpoint to be at least 20% of patients in a group achieving significant improvement, as we wished to avoid any possible confounding from spontaneous remission. It is not possible, based on our study design, to know whether increasing purity and concentration of an odorant may give the training protocol incrementally greater efficacy, but random concentration nonetheless appears equally efficacious.
As we continue to learn more about olfactory training, nuances are beginning to appear suggesting adding more scents to the training protocol and extending the time period can be beneficial in increasing efficacy.11, 12 Certainly as we move forward, we will incorporate these new findings into recommended clinical protocols involving essential oils.
We used the UPSIT as our testing methodology and many other studies have used Sniffin’ Sticks. There have been studies performed comparing and validating that clinical change on one is comparable to the other, so we felt this to be a valid comparison.13, 14, 15
Limitations of the study include small number of participants and lack of a placebo in our control group. Although our numbers were small, they were actually similar to numbers in other prospective studies, and although smaller than in the other RCT that was a multi‐center study, the fact that we were still able to demonstrate similar results lends credibility to our data.
Lack of placebo is the major limitation of both this study and most of the other studies evaluating this intervention. The two major reasons why we did not have a placebo arm in this study was the likelihood of patients’ friends and family members easily detecting odorless liquids and thus immediately giving away the placebo, as well as the fact that we wanted the patients to purchase essential oils at random concentrations instead of providing them with a “real” and “sham” OT kit. Lack of a placebo also leaves the control arm with no intervention and decreased motivation for testing and follow up, which could certainly have affected the results of this study. One could consider using the UPSIT instead of a full battery testing system such as the Sniffin’ Sticks system as a relative limitation, however as this study sought to simply replicate efficacy of olfactory training that has already been proven using standardized concentrations, the difference in exact testing methodology does not seem as important, as both are accepted methods.
One additional potential confounder is setting our bar of clinical improvement at 10% change, which shows improvement throughout the UPSIT except at the low end of the anosmic range (i.e., a movement from 10 to 15 is a 10% change, but still leaves the patient within the anosmic range). We went back to our initial data collection to evaluate how many patients we were including in our “improved” group that may have fallen into this category. None of our patients that had significantly improved were found in this category, therefore our results remained significant even when evaluating for this potential confounding factor.
A more pure comparison would have been to compare non‐standard odorant concentrations with standardized odorant concentrations in our own patient population instead of comparing our patients with data previously published. We were limited by patient population and cost and acknowledge this as a limitation of the study.
CONCLUSION
With millions of patients suffering from olfactory dysfunction, and little to no evidence that the multiple pills and sprays sold as “cures” provide benefit, olfactory training may give us an opportunity to help these patients. Although still not a cure for many patients, it does offer improvement for some. We now have additional information that variability in the concentration and delivery system of the odorants does not appear to detract from the outcome. Allowing patients to use random concentrations of essential oils to perform OT is as effective as published data using controlled concentrations of odorants for post‐infectious and idiopathic olfactory loss.
Conflicts of Interest: None
Financial Disclosures: Zara M. Patel: Consultant for Medtronic and Patara Pharma; Speakers Bureau for Intersect ENT
Sarah K. Wise: Scientific Advisory Board for Greer Pharmaceuticals, Research Support from Genentech
John M. DelGaudio: None
BIBLIOGRAPHY
- 1. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA 2002;288:2307–2312. [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope 2015;125:1102–1106. [DOI] [PubMed] [Google Scholar]
- 3. Henkin RI, Levy LM, Fordyce A. Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at The Taste and Smell Clinic in Washington, DC. Am J Otolaryngol 2013;34:477–489. [DOI] [PubMed] [Google Scholar]
- 4. Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009;119:496–499. [DOI] [PubMed] [Google Scholar]
- 5. Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post‐traumatic and postinfectious olfactory dysfunction. Laryngoscope 2013;123:E85–E90. [DOI] [PubMed] [Google Scholar]
- 6. Haehner A, Tosch C, Wolz M, et al. Olfactory training in patients with Parkinson's disease. PloS one 2013;8:e61680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geissler K, Reimann H, Gudziol H, Bitter T, Guntinas‐Lichius O. Olfactory training for patients with olfactory loss after upper respiratory tract infections. Eur Arch Otorhinolaryngol 2014;271:1557–1562. [DOI] [PubMed] [Google Scholar]
- 8. Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope 2014;124:826–831. [DOI] [PubMed] [Google Scholar]
- 9. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta‐analysis. Int Forum Allergy Rhinol 2016;6:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rotenberg BW, Saunders S, Duggal N. Olfactory outcomes after endoscopic transsphenoidal pituitary surgery. Laryngoscope 2011;121:1611–1613. [DOI] [PubMed] [Google Scholar]
- 11. Konstantinidis I, Tsakiropoulou E, Constantinidis J. Long term effects of olfactory training in patients with post‐infectious olfactory loss. Rhinology 2016;54:170–175. [DOI] [PubMed] [Google Scholar]
- 12. Altundag A, Cayonu M, Kayabasoglu G, et al. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope 2015;125:1763–1766. [DOI] [PubMed] [Google Scholar]
- 13. Stuck BA, Beule A, Damm M, et al. [Position paper “Chemosensory testing for expert opinion in smell disorders”]. Laryngo‐ rhino‐ otologie 2014;93:327–329. [DOI] [PubMed] [Google Scholar]
- 14. Gu D, Li P. [Comparison of application of several psychophysical olfactory test methods in clinic]. J Clin Otorhinolaryngol Head Neck Surg 2014;28:715–717. [PubMed] [Google Scholar]
- 15. Lawton M, Hu MT, Baig F, et al. Equating scores of the University of Pennsylvania Smell Identification Test and Sniffin' Sticks test in patients with Parkinson's disease. Parkinsonism Relat Disord 2016. DOI: 10.1016/j.parkreldis.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


