Abstract
Objectives
To review evidence of hearing loss as a risk factor for dementia.
Data Sources: PubMed
Review methods: A systematic review was conducted using the PubMed database using the search terms (hearing loss OR presbycusis) AND (dementia OR cognitive decline). Initially, 488 articles were obtained. Only those studies evaluating an association between hearing loss and incident dementia or cognitive decline were included in the analysis. This resulted in 17 articles which were thoroughly evaluated with consideration for study design, method for determining hearing loss and cognitive status, relevant covariates and confounding factors, and key findings.
Results
All of the 17 articles meeting inclusion criteria indicate that hearing loss is associated with dementia or cognitive decline. The methods used among the studies for ascertaining hearing loss and dementia were notably varied. For hearing loss, peripheral auditory function was tested far more than central auditory function. For peripheral audition, pure tone audiometry was the most commonly reported method for defining hearing loss. Only a few studies measured central auditory function by using the Synthetic Sentence Identification with Ipsilateral Competing Message test (SSI‐ICM) and the Staggered Spondaic Word Test (SSW). Dementia was most often defined using the Mini Mental State Exam (MMSE). However, many studies used extensive batteries of tests to define cognitive status, often including a neuropsychologist. Confounding variables such as cardiovascular risk factors were measured in 17 studies and family history of dementia was only evaluated in 1 study. Overall, the methods used by studies to ascertain hearing loss, cognitive status and other variables are valid, making their evaluation appear reliable.
Conclusion
While each of the studies included in this study utilized slightly different methods for evaluating participants, each of them demonstrated that hearing loss is associated with higher incidence of dementia in older adults.
Level of Evidence
Level V, systematic review.
Keywords: agre‐related hearing loss, dementia, cognitive decline, Alzheimer's disease, presbycusis
INTRODUCTION
Dementia and hearing loss are both highly prevalent neurologic conditions in older adults, each having considerable impact on quality of life.1, 2 A growing body of literature suggests that these two conditions are interrelated and that hearing loss may be a risk factor for the development of dementia in older adults.3, 4 Though several epidemiological studies have demonstrated this association, the causal link of how hearing loss increases the risk of developing dementia is not well understood.
Several possible means have been identified. One line of thought is based on the impact of hearing loss on cortical processing. Hearing loss increases the cognitive load, diverting cognitive resources to auditory processing at the expense of other cognitive processes such as working memory.5, 6 Another hypothesis is that hearing loss leads to social isolation, which has been shown to contribute to dementia.7, 8 The third prominent explanation is that there is a common cause to both diseases and that hearing loss is the early manifestation of the underlying pathology.9, 10, 11 It is also possible that these proposed mechanisms are not mutually exclusive, and decline in one pathway consequentially affects the others.
Better understanding the etiology behind the connection between hearing loss and dementia could help lead to interventions that preserve cognitive function in hearing loss patients. In this way, hearing loss could serve as a potential modifiable risk factor. It is suggested that interventions delaying the onset of dementia by even one year would decrease the worldwide prevalence of dementia by 10%.12 Thus, there is compelling motivation to pinpoint the role hearing loss has on cognitive decline. The object of this study is to further investigate this connection. This will be performed through a systematic review of epidemiological studies published on the topic. Each study will be evaluated with consideration to design, method for determining hearing loss and dementia, relevant covariates and confounding factors, key findings, and conclusion.
METHODS
The MEDLINE database was used to search for all English and non‐English studies linking hearing loss to cognitive decline up through June 1, 2016 using the following search term: (hearing loss OR presbycusis) AND (dementia OR cognitive decline). The search details are as follows: (“hearing loss”[MeSH terms]) OR (“hearing”[all fields] AND “loss”[all fields]) OR (“hearing loss”[all fields]) OR (“presbycusis”[MeSH terms]) OR (“presbycusis”[all fields]) AND ((“dementia”[MeSH terms] OR “dementia”[all fields]) OR ((“cogn int conf adv cogn technol appl”[Journal] OR “cognitive”[all fields]) AND “decline”[all fields])). From this criteria, 488 articles were identified. Articles were excluded if they did not examine hearing loss as well as dementia or cognitive decline. This narrowed the collection to 131 relevant articles.
The remaining 131 articles were evaluated through their abstracts, and where necessary, full text. Articles were removed if they did not test the connection of hearing loss to incident dementia or cognitive impairment. All news articles, non‐academic research, case reports, editorials, commentary and reviews were removed. Three non‐English articles were excluded because they could not be translated or acquired. Of the 131 articles, 17 fit the inclusion criteria and evaluated the correlation of hearing loss with dementia (see Table 1).
Table 1.
Table of the 17 identified publications that evaluate hearing loss as a risk factor for cognitive decline.
| Author | Year | Database Used | Number of Participants | CV Risk Factors | Hearing Aid use | Other Dementia risk factors | Hearing Loss Criteria | Dementia Criteria | Key Finding | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Amieva22Prospective Cohort | 2015 | Personnes Agees QUID study | 3,670 | NA | Measured and attenuates cognitive decline (β = 0.07, P = 0.08) | Depression, social isolation | Self‐Report | French version of Lawton Scale.MMSE. | Self‐reported hearing loss associated with cognitive decline at 25 year follow up (β = −0.04, P = 0.01). Hearing aids attenuate cognitive decline (β = 0.07, P = 0.08) | Self‐reported hearing loss associated with cognitive decline at 25 year follow up. Hearing aids attenuate cognitive decline. |
| Deal4Prospective Cohort | 2015 | Atherosclerosis Risk in Communities Neurocognitive Study | 253 | Diabetes, Hypertension, Smoking | Decreased likelihood of cognitive decline | Depression, | PTA (500, 1000, 2000, 4000 Hz; mild 25‐40, moderate 40‐70, severe >70)) | DSST, 2013 Delayed word recall test (DWRT). Incidental Learning Test, Logical Memory Test I and II, Word fluency Test, Animals Naming Test, Boston Naming Test, and Trail making A and B, digit span backwards test | Standard Deviations for moderate/severe HL = ‐.47 (P=0.02), no HL = ‐.29 (P=0.02) | Moderate association between moderate/severe HL and memory performance |
| Deal13Prospective Cohort | 2016 | Health, Aging, and Body Composition Study | 3,075 | Diabetes, HTN, smoking, cerebrovascular disease | Measured but did not statistically decrease cognitive decline with use. | NA | PTA (500, 1000, 2000, 4000 Hz; Mild 26‐40 dBHL, moderate/severe > 40 dBHL) | 3‐MS, record of prescribed dementia medication, Selective Reminding Test, Boxes Test, Digit Copying Test, Pattern Comparison Test, and Letter Comparison Test | Moderate/severe hearing loss associated with increased risk of incident dementia over 9 years (Hazard ratio: 1.55, 95% CI: 1.10, 2.19).For every 10 dBHL, HR = 1.14, 95% CI = 1.03‐1.25) | Hearing loss is associated with increased risk of developing dementia in older adults |
| Gates14Prospective Cohort | 1996 | Framingham Heart Study | 1662 | NA | NA | NA | PTA (500, 1000, 2000 Hz; >40dB), SSI‐ICM, SSW | MMSE (<24 considered dementia), neurologist and neuropsychologist evaluation. | The relative risk of developing dementia with hearing loss was 6 in subjects with very poor scores (<50%) in one ear on the SSI‐ICM (P=.2), Relative Risk 12.5 with poor scores in both ears (p=0.001) | Central auditory dysfunction precedes senile dementia and may be an early marker for senile dementia. |
| Gurgel15Prospective Cohort | 2014 | Cache County Study on Memory, Health, and Aging | 4,463 | Diabetes, Hypertension, Smoking, Hyperlipidemia | NA | APOE‐E allele | Clinician ascertainment during interview | 3MS screen, followed by neuropsychologist, and geropsychiatrist evaluation. | HR 1.27 p=0.026 (with 95% CI 1.03‐1.56);'‐0.26 points/year faster decline on 3MS‐R with HL | Elderly individuals with HL have an increased rate of developing dementia |
| Lin16Prospective Cohort | 2004 | Study of Osteoporotic Fractures (SOF) | 6,112 | Diabetes, Smoking, Cerebrovascular disease/stroke, | Did not improve cognition | NA | Pure tone audiometry (>40dB at 2 kHz considered hearing loss; no average taken) | 3MS | In those with hearing impairment, there was a trend toward greater odds of cognitive decline over time (OR51.38, 95% CI50.95–2.00). | Hearing impairment lead to greater odds of cognitive impairment |
| Lin3Cross‐Sectional | 2011 | Baltimore Longitudinal Study of Aging | 639 | Diabetes, Hypertension, Smoking | No cognitive improvement | NA | PTA (500, 1000, 2000, 4000 Hz; mild 25‐40, moderate 40‐70, severe >70)) | complete neurological and neuropsychological exam (if older than 65); BOMC (if younger than 65) | The risk of incident all‐cause dementia increased log‐linearly with the severity of baseline hearing loss (1.27 per 10 db loss, 95% CI: 1.06 ‐ 1.50); The risk of incident AD also increased with baseline hearing loss (1.20 per 10 dB of hearing loss, CI 0.94‐1.53) | Hearing loss is independently associated with incident all‐cause dementia |
| Lin54Prospective Cohort | 2011 | National Health and Nutritional Examination Survey | 605 | Diabetes, Hypertension, Smoking, Cerebrovascular Disease | Significantly associated with higher cognitive scores DSST, difference of 7.4, p=0.03). | NA | PTA (500, 1000, 2000, 4000 Hz; mild 25‐40, moderate 40‐70, severe >70) | DSST | Greater hearing loss was significantly associated with lower scores on the DSST after adjustment for demo‐graphic factors and medical history (DSST score difference of −1.5 [95% confidence interval: −2.9 to −0.23] per 10 dBof hearing loss). | Hearing loss is independently associated with lower scores on the DSST |
| Lin24Prospective Cohort | 2011 | Baltimore Longitudinal Study of Aging | 347 | Diabetes, Hypertension, Smoking | Not associated with any scores of cognition) | Depression, | PTA (500, 1000, 2000, 4000 Hz; >25 dB considered hearing loss) | MMSE, Free Recall, Stroop Mixed, Trail Making B, Free and Cued Selective Reminding Test (FCSRT), Trail Making A, Stroop Color and Words, Category and Letter Fluency, American Version of the Nelson Adult Reading Test (AMNART) | The reduction in cognitive performance associated with a 25 dB hearing loss was equivalent to the reduction associated with an age difference of 6.8 years. | Hearing loss is independently assocatiated with lower scores on tests of memory and executive function |
| Lin3Prospective Cohort | 2013 | Health, Aging, and Body Composition Study | 1,984 | Diabetes, Hypertension, Smoking, Cerebrovascular disease/stroke | No cognitive improvement | Depression | PTA (250, 500, 1000, 2000, 4000, 6000, 8000 Hz; >25dB considered hearing loss) | 3MS (<80 considered dementia, or decline in score of 5 points), DSST | hazard ratio, 1.24; 95%CI, 1.05‐1.48. Hearing impairment > 25 dB/2kHz: (mild 25‐40: 1.19 with 95% CI 0.99‐1.44), (moderate 40‐70: 1.36 with 95% CI 1.08‐1.70), (severe > 70: N/A) | Hearing loss is independently associated with accelerated cognitive decline and incident cognitive impairment in community‐dwelling older adults |
| Quaranta25Cross‐Sectional | 2014 | Great Age Study; Italy | 488 | NA | NA | NA | PTA (Threxhold in Hz not specified; >35 dB, present or absent);SSI‐ICM | MMSE;Neurological Exam, Clinical Dementia Rating Scale, Unified Parkinson Disease Rating Scale part III, Epworth Sleepiness Scale‐Italian Version, Eating Assessment Tool, Frontal Assessment Battery, Digit Modalities Test‐Oral Version, Trail Making Test, Controlled Oral Word Association, Clock‐Drawing Test, Boston Naming Test‐Short form, Token Test, Rey's Verbal Learning Test | OR 4.2, p=0.05 AD associated with CAPD; OR 1.8, p=0.31 AD with hearing thresholds; OR 1.6, p=0.31 MIC with hearing impairment; OR 2.4 p=0.03 Cognitive Impairment (dementia and MCI) and CAPD; OR 1.6, p=0.03cognitive impairment (Dementia and MCI) with hearing impairment (CAPD and Peripheral HL); OR 1.5, p=0.08 Cognitive Impairment and hearing thresholds | The use of hearing tests and early diagnosis and treatment of ARHL may potentially slow cognitive impairment |
| Tay26Cross‐Sectional | 2006 | Blue Mountains Eye Study, Australia | 3,509 | Diabetes, Hypertension, Smoking, Hyperlipidemia, Cerebrovascular disease/stroke | NA | NA | PTA (250, 500, 1000, 2000, 4000, 6000, 8000 Hz; mild 20‐40, mod/severe >40) | MMSE (<24 considered dementia) | Persons with moderate to severe hearing loss had a lower mean MMSE score than those without hearing loss (28.1 v. 28.7, p<0.001) | Age‐related correlation between sensory and cognitive function in a normal ageing sample. |
| Teipel27Cross‐Sectional | 2015Cross sectional | Claims data of the Allgemeine Ortskrankenkasse, the largest public health insurance company in Germany | 1,338,462 | Diabetes, Hypertension, Hyperlipidemia, Cerebrovascular disease/stroke | NA | NA | ICD‐10 codes | ICD‐10 codes | Regional (based on 2 digit postal code) dementia prevalence increased by 0.23% when HL prevalence increased by 1 SD | Hearing impairment is a risk factor for dementia |
| Tomioka23Prospective Cohort | 2015 | The Fujiwara‐Kyo Study, Nara, Japan | 4,427 | Diabetes, Hyperlipidemia, BMI, smoking, HTN, cerebrovascular disease | Not associated with any scores of cognition | Depression | Self‐report | Tokyo Metropolitan Institute of Gerontology Index of Competence (TMIG‐IC).MMSE (<24 considered dementia) | Baseline HL associated with decline in intellectual activity (OR = 1.39, 95% CI = 1.02‐1.76) at 5 year follow up | Hearing loss is associated with measurable cognitive dysfunction within 5 years. |
| Uhlmann27Case‐Control | 1989 | Adult Medicine Clinics at Harborview Medical Center and University Hospital in Seattle, WA | 200 | NA | Did not change odds of dementia, but did not directly test its effect on cognition | Depression, Family History (OR 3.3, 95% CI 1.7‐6.4) | PTA (500, 1000, 2000, 3000 Hz; Mild 20‐29, moderate 30‐39, mod/severe >40) | MMSE (<24 considered dementia) | Mild HL (1.5 with 95% CI 0.4‐5.4), Moderate HL (2.2 with 95% CI 0.6‐7.8), Severe HL (4.1 with 95% CI 1.1 to 15.8) | Hearing impairment contributes to cognitive dysfunction in older adults |
| Wallhagen18Prospective Cohort | 2008 | Alameda County Study (California) | 2,002 | Diabetes, Hypertension, Smoking, Crrebrovascular disease/stroke | NA | NA | Self‐report | (Self report on the hearing impairment scale using the following categories: dif‐ficulty remembering things, forgetting where one put something, trouble finding the right word, and difficulty paying attention). | Baseline hearing impairment increases risk of cognitive decline 5 years later (OR = 1.22, 95% CI = 1.14 to 1.29).Each point increase in hearing impairment scale makes increases likelihood of developing dementia by 22% 5 years later. | A relatively strong relationship between baseline hearing impairment and subsequent poorer cognitive function was found in both existing and new cases of cognitive impairment |
| Wen21Prospective Cohort | 2016 | Taiwan's National Health Insurance Research Database | 6,546 | Diabetes, history of head injury, diabetes, vascular diseases | Not measured | Depression | ICD‐9 codes | ICD‐9 codes | Hearing loss associated with increased risk of dementia (OR = 1.577) | Hearing loss associated with increased risk of dementia |
Each article included was thoroughly evaluated with thoughtful consideration given to the many design elements discussed below.
RESULTS
Twelve of the 17 studies (70.6%) included in this analysis were prospective cohort studies measuring hearing loss as a risk factor and some form of dementia as an outcome.3, 4, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Four of the 17 studies (23.5%) were cross‐sectional.23, 24, 25, 26 Twelve of the studies followed participants living in the United States. Five took place in other localities: Italy (1/17),24 Australia (1/17),25 Germany (1/17),26 Taiwan (1/17),20 and Japan (1/17).22 The number of participants ranged anywhere from 200 to 1,338,462. All of the prospective studies measured hearing and cognition at baseline except for one, which measured it at the end of the period observed.
Dementia Status Ascertainment
To determine cognitive function and level of decline, 64.7% (11/17) of the studies used either the Mini‐Mental State Exam (MMSE),3, 14, 21, 22, 24, 25, 27 or more recent variations of the MMSE entitled the Modified Mini Mental State Exam (3MS or 3MS‐R).13, 15, 16, 17 Three studies used the Digital Symbol Substitution Test (DSST) to assess cognition,4, 17, 19 one of which also employed the 3MS.17 Other instruments to measure cognition included a system of self‐report (1/17),18 complete neurological exams (2/17),14, 23 or extensive batteries of cognitive tests (5/17)3, 4, 13, 24 (see Table 1). Only one study (1/17) evaluated family history of dementia.27
Hearing Loss Ascertainment
Among the studies examined, there was variation regarding criteria for determining baseline hearing acuity. The most consistently reported measure for determining hearing loss was pure tone audiometry at 58.8% (11/17).3, 4, 13, 14, 16, 17, 19, 23, 24, 25, 27 Seven of the 11 studies using audiometry (7/11) obtained a pure tone average (PTA) using the thresholds 500, 1,000, 2,000, and 4,000 Hz,3, 4, 13, 17, 19, 23, 25 two of which measured thresholds beyond this range.17, 25 One study did not use thresholds higher than 3,000 Hz;27 another did not measure above 2,000 Hz.14 One study obtained a single measure at 2,000 Hz and did not obtain an average.16 Even among these 11 studies using audiometry, the classification for the severity of hearing loss varied slightly. Three studies (3/17) used the common system classifying mild as >25 dB to 40 dB, moderate as 40 dB to 70 dB, and severe as >70 dB.4, 19, 23 One study stratified hearing loss as mild (20–29 dB), moderate (30‐39 dB), and severe (>40 dB).27 Another study stratified as mild (20–40 dB), moderate/severe (>40 dB).25 Another study stratified as mild (26–40 dB HL) and moderate/severe (>40 dB HL).13 Five studies did not stratify at all and simply measured hearing loss as present or absent for anything above 25 dB (2/17),3, 17 35 dB (1/17),24 or 40 dB (2/17),14, 16 respectively. In addition to using PTA, two studies employed the Synthetic Sentence Identification with Ipsilateral Competing Message test (SSI‐ICM),14, 24 one of which also used the Staggered Spondaic Word (SSW) test.14 The six studies not using audiometry used medical codes such as ICD‐9 and ICD‐10 (2/17),20, 26 or more subjective measures such as clinician ascertainment of hearing loss during an interview (1/17)15 and self‐report of hearing loss (3/17).18, 21, 22 No studies reported specifically on word recognition or speech discrimination scores as a metric of hearing loss. Hearing aids were controlled for 58.8% (10/17),3, 4, 13, 16, 17, 19, 21, 23 only one of which did not directly test the effects of hearing aids on cognitive function.13 Six found no reduction in cognitive decline with hearing aid use.13, 16, 17, 23 Only three studies determined that hearing aids decreased cognitive decline.4, 19, 21
Covariates
Many studies controlled for potentially confounding variables such as diabetes, hypertension, smoking status, high cholesterol, and history of stroke or cerebrovascular disease.4, 13, 15, 16, 17, 18, 19, 20, 22, 23, 25, 26 Some studies controlled for less commonly tested variables such as depression (7/17)4, 17, 20, 21, 22, 27 and a genetic test indicating the presence of the APOE‐e allele which has been identified as being associated with Alzheimer's Disease (AD) (1/17).15
Evaluating Hearing Loss as a Risk Factor for Dementia
All of the studies (17/17) indicated that hearing loss is independently associated with higher incidence of dementia. The majority of the studies (12/17) quantified the relationship using standard deviations, hazard ratio, relative risk, or odds ratio.4, 13, 14, 15, 16, 17, 18, 20, 21, 22, 24, 26, 27 Four studies (4/17) found a dose‐response relationship between the severity of hearing loss and an increased risk of cognitive decline.13, 18, 19, 23 One study found that for every 10 dB HL at baseline, there was a 1.27 increased risk for all‐cause dementia and 1.20 increased risk for developing AD.23 Another study found the dose response curve to be a 1.5 point score decrease in the DSST cognitive test for every 10 dB HL.19 A third study found that for every 1 point increase in the hearing impairment scale, the likelihood of developing dementia five years later increased by 22%.18 Another study found the dose response to be an increased hazard ratio of 1.14 for every 10 dB HL.13 Two of the studies (2/17) also employed other measurement systems; for example, a 25 dB hearing loss is the equivalent cognitive performance of an individual 6.8 years older (1/17),3 or moderate to severe hearing loss resulted in worse scores on the MMSE (1/17).25 One study (1/17) quantifying the relationship with a hazard ratio also found a 0.26 points per year faster decline on 3MS‐R when HL was present (see Table 1).15
DISCUSSION
All of the studies evaluated in this review found hearing loss to be associated with dementia or cognitive decline. This substantially supports the hypothesis that hearing loss is a risk factor for dementia. Because of the considerable variability among the studies regarding how dementia, hearing loss, and potential confounding variables were determined, careful scrutiny and comparison of these methods is crucial to fully appreciating the validity of this observed association.
Most of the studies (8/17) utilized the Mini Mental State Exam (MMSE), or a variation of it, the Modified Mini Mental State Exam (3MS). In the MMSE, the patient is instructed to perform a series of basic tasks. The patient's performance is quantified numerically and is assigned a score from 0 to 30. A score below 24 usually indicates dementia. However, some flaws have been found in the sensitivity of the MMSE, which led to the development of the 3MS which is similar but slightly longer and extends the scoring system to a range of 0 to 100.28 Both the 3MS and MSSE are valid for detecting and tracking dementia but have difficulty identifying lower levels of cognitive impairment.28, 29 Three articles (3/17) utilized the Digit Symbol Substitution Test (DSST) to assess dementia.4, 17, 19 It is a sensitive executive function test which has patients code a series of numbers with symbols.30 It is thought that the faster the patient matches the symbols with the correct numbers, the better their cognition is. This particular test does not rely heavily on auditory function, as the patient does not receive continual instruction from the clinician to complete it. However, other cognitive function tests which rely on a patient using auditory processing—for example, responding to spoken questions—are significantly impacted by an auditory impairment.31, 32, 33 If patients have hearing loss, they may perform worse on certain cognitive tests, though they may not have any cognitive dysfunction. This could make the correlation of hearing loss with dementia appear higher than it really is. Tests relying on auditory instruction from the clinician include the MMSE and the 3MS, discussed previously. However, several studies (7/17) used a variety of methods for evaluating cognition that did not rely heavily on auditory function, including a variety of tests that rely almost entirely on the patient's ability to use a pen, paper and cognitive faculties.4, 13, 17, 18, 19, 23, 24
Hearing loss was also measured a variety of ways. Some studies used more subjective measures. Gurgel et al. and Wallhagen et al. used clinician assessment of HL and patient self‐report of HL, respectively, and found a strong correlation between HL and incidence of dementia years later.15, 18 Although less quantitative, studies have shown subjective measures to be a reliable method for ascertaining HL, but may reveal a slightly higher prevalence of hearing loss than objective measures.34, 35, 36 Though accurate, from this method it is not possible to determine how HL severity corresponds to dementia risk.
Pure tone audiometry was the most common method for determining hearing loss (11/17).3, 4, 13, 14, 16, 17, 19, 23, 24, 25, 27 Pure tone averages (PTA) can quantify the severity of hearing loss (HL) and categorize HL as mild (25‐40 dB HL), moderate (40‐70 dB HL), and severe (>70 dB HL). Stratification of HL severity provides information helpful in elucidating the relationship of HL to dementia. Three studies showed that as hearing loss severity increases, the odds ratio (OR) and hazard ratio (HR) for developing dementia increases accordingly.17, 23, 27 Uhlmann et al. noted the most drastic relationship, showing mild HL corresponded with an OR of 1.5, moderate HL with an OR of 2.2, and severe HL with an OR of 4.1.27 One limitation of the studies evaluated was that none reported on word recognition scores (WRS) or speech discrimination scores that are obtained in routine audiometry as predictors for dementia. The WRS has been recognized as an important metric in reporting hearing outcomes. Indeed, it may be the most important metric of hearing acuity because it has predictive value on how well an individual will respond to aural rehabilitation (i.e., hearing aids). This should be considered a covariate in future studies.37
In discussing accuracy of hearing loss ascertainment, it should be noted there are several studies that indicate that pure tone audiometry fails to adequately assess central auditory processing.38, 39, 40 This would indicate that while PTA is an accurate measure of hearing loss, it may not lend itself to proving or disproving different hypotheses of hearing loss and dementia.
Only two studies evaluated central auditory processing. They utilized the Synthetic Sentence Identification with Ipsilateral Competing Message test (SSI‐ICM) and the Staggered Spondaic Word Test (SSW).14, 24 Due to the fact that central auditory processing, peripheral auditory, and cognition function are all likely interrelated, studies are most powerful when all three elements are evaluated.41 Gates et al. showed that central auditory processing disorder (CAPD) was related significantly to performance decline on the MMSE.14 The relative risk for dementia in patients scoring poorly on the SSI‐ICM exam in one ear was 6 (p = 0.2), and was 12.5 (p = 0.001) for patients scoring poorly in both ears. Quaranta et al. also found that CAPD increased the odds ratio for developing Alzheimer's disease dementia (OR of 4.2 [p = 0.03]).
Cardiovascular risk factors, such as hypertension, diabetes, hyperlipidemia, and smoking, have been shown to increase one's risk of developing dementia.42, 43 Controlling for cardiovascular risk factors is necessary for accurately measuring the relationship of hearing loss to dementia. The majority of the studies (13/17) controlled for at least one cardiovascular risk factor. Whitmer et al. found in a group of 8,845 individuals that the hazard ratio (HR) for developing dementia if only one CV risk factor was present was 1.27.43 If four risk factors were present the HR rose to 2.37 (95% CI 1.10 to 5.10). Therefore, the presence of CV risk factors, could confound the association between hearing loss and dementia.
In 61.5% (9/17) of the included articles, the impact of hearing aid use on cognition was evaluated.3, 4, 16, 17, 19, 21, 23 Most of these studies (6/17) found no correlation between hearing aid use and level of cognitive function. The two studies that did find an association (2/17) found that hearing aid use diminished the likelihood of cognitive decline.4, 19, 21 However, among the studies, hearing aid use was usually determined by a yes or no question. Such simplistic measures leave significant gaps regarding compliance and proper management of hearing aids, thereby making their assessment of hearing aid impact on cognition less conclusive. Other articles not included in our systematic review indicate that hearing aids may not improve cognition.44, 45 Conversely, other studies have indicated that improving hearing through the use of hearing aids or cochlear implantation has positive effects on cognition.46, 47, 48, 49 Additional prospective studies with reliable methods for determining hearing aid use and compliance would be necessary to fully ascertain hearing aid impact on cognitive decline.
Family history of dementia was only controlled for in one of the studies. Uhlmann et al. found the odds ratio (OR) of having dementia in the presence of a family history of dementia was 3.3 (confidence interval 1.7‐6.4). However, having a family history of dementia doesn't necessarily preclude hearing loss from serving as a risk factor for dementia, as one proposed mechanism behind the HL‐dementia relationship is that both diseases are results of a shared underlying pathology. Therefore, it is unclear what impact controlling for family history would have on the conclusion of the remaining 16 articles. Evaluating family history in the context of studying hearing loss as a risk factor for dementia could provide valuable insight into the etiology behind the connection.
Based on the studies reviewed in this article, there is an overwhelming consensus that hearing loss is a risk factor for incident dementia. However, due to inherent limitations in epidemiological studies, these articles do not adequately describe exactly why hearing loss is a risk factor. There are multiple hypotheses on how hearing loss is associated with dementia: the cognitive load hypothesis, psychosocial hypothesis, and the common‐cause or shared neurobiological pathology hypothesis. In an extensive review on the cognitive load hypothesis, Pichora‐Fuller explains that as hearing diminishes, mental resources are diverted toward auditory perception. It is thought that as cognitive activities are continually neglected at the expense of hearing, dementia will ensue.50
An alternative hypothesis regarding the relationship of HL and cognitive decline involves various psychosocial factors that likely influence cognitive ability and function. Individuals with HL have difficulty communicating and maintaining interpersonal relationships, often resulting in social isolation.51 Mick et al. found in a nationally representative sample of 860 females between the ages of 60‐69 that hearing loss is associated with increased odds of social isolation with an odds ratio (OR) of 3.49 per 25‐dB HL (95% confidence interval).46 Fratiglioni et al. demonstrated that individuals who were single, living alone, and had few relatives/friends were at an increased risk for dementia.52 Thus, it is possible that as hearing loss sets in, social activity diminishes leading to dementia. Seven of the included studies (7/17) measured and controlled for depression.4, 17, 20, 21, 22, 23, 27 Since depression is a known risk factor for the development of dementia, controlling for this covariate strengthens the association that hearing loss is an independent risk factor to dementia in older adults.
Another notable hypothesis is the common‐cause hypothesis. In a comprehensive review of the potential causal links of hearing loss leading to dementia, Wayne et al. explain the possibility that a single factor associated with old age leads to both neurologic processes.10 Gates et al. suggested that central auditory dysfunction and executive dysfunction may arise from the same neurodegenerative process.11 If there is a single cause for both neurologic processes, it is possible that hearing loss is merely an early manifestation of dementia during its pre‐clinical phase.9
One of the notable limitations of this review article is that several non‐English articles were not able to be translated and evaluated. It is unclear how the inclusion of these potentially informative articles would impact the discussion of this study. In addition to non‐English articles not reviewed, there may have been other studies that investigated hearing loss and dementia but found no connection. It is possible that such studies were not published on account of their negative findings. The articles that were not published could potentially alter the conclusion of the current study. However, we believe that the 17 articles evaluated in this study are a strong and accurate representation of the topic. Another limitation of this review is the difficulty of comparing results due to the variety of tests used to assess HL and dementia. This is likely due to a lack of standardized assessment for both hearing loss and dementia. Additionally, there are limitations regarding how data is obtained, potentially obscuring the relationship of hearing loss as a cause of dementia. As mentioned previously, if the patient is administered a cognitive test that requires hearing, then the patient may not perform to their full cognitive potential. This potential flaw could obscure the connection, making it appear that hearing loss is associated with cognitive decline in cases where this may not be true. Furthermore, longitudinal epidemiologic studies are very time‐intensive and usually rely on data already gathered for a different purpose; therefore, the methods of assessment may not be ideal to the researcher. It is notable, however, that in spite of the different assessments utilized in this body of research, the conclusion for nearly all articles was the same: that hearing loss is associated with and is a risk factor for incident dementia.
Although cross‐sectional studies provide valuable insight into the correlation of hearing loss and dementia, they cannot provide causality, making it difficult to observe the etiology behind the connection. More prospective studies should be done on this subject in order to identify causation rather than just correlation. Careful consideration for confounding variables such as cardiovascular risk factors help the investigator determine which variables, including HL, are influencing the development of dementia. Furthermore, identifying the exact reason hearing loss is a risk factor for dementia would require longitudinal studies to account for other variables, including social isolation and depression. Wayne et al. suggested that an effective way to identify why hearing loss precedes dementia is to intervene in various proposed causal pathways and observe their impact on cognition.10 Studies performed this way could reveal valuable insight as to why hearing loss is a risk factor for dementia. The more we know about dementia and its causes, the closer we are to a cure.
CONCLUSION
Multiple epidemiological studies have shown that hearing loss is an independent risk factor for the development of dementia. Future studies controlling for potentially confounding variables and mechanistic studies will be necessary to further elucidate this association.1
Figure 1.
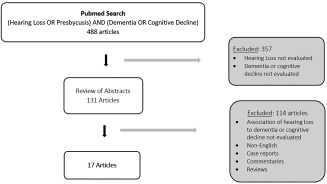
Schematic of PubMed Database Search Resulting in the Identification of 17 Publications that Evaluate Hearing Loss as a Risk Factor for the Development of Dementia or Cognitive Decline.
Support/Funding: No sources of support or funding were received for this work.
COI: None of the authors has a conflict of interest.
BIBLIOGRAPHY
- 1. Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist 2003;43:661–668. [DOI] [PubMed] [Google Scholar]
- 2. Bowling A, Rowe G, Adams S et al. Quality of life in dementia: a systematically conducted narrative review of dementia‐specific measurement scales. Aging Ment Health 2015;19:13–31. [DOI] [PubMed] [Google Scholar]
- 3. Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol 2011;68:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deal JA, Sharrett AR, Albert MS et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol 2015;181:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell J, Sharma A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci 2013;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erb J, Obleser J. Upregulation of cognitive control networks in older adults' speech comprehension. Front Syst Neurosci 2013;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shankar A, Hamer M, McMunn A, Steptoe A. Social isolation and loneliness: relationships with cognitive function during 4 years of follow‐up in the English Longitudinal Study of Ageing. Psychosom Med 2013;75:161–170. [DOI] [PubMed] [Google Scholar]
- 8. Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg 2014;150:378–384. [DOI] [PubMed] [Google Scholar]
- 9. Martini A, Castiglione A, Bovo R, Vallesi A, Gabelli C. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol 2014;19(Suppl 1):2–5. [DOI] [PubMed] [Google Scholar]
- 10. Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age‐related hearing loss and cognitive decline. Ageing Res Rev 2015;23:154–166. [DOI] [PubMed] [Google Scholar]
- 11. Gates GA, Gibbons LE, McCurry SM, Crane PK, Feeney MP, Larson EB. Executive dysfunction and presbycusis in older persons with and without memory loss and dementia. Cogn Behav Neurol 2010;23:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peracino A. Hearing loss and dementia in the aging population. Audiol Neurootol 2014;19(Suppl 1):6–9. [DOI] [PubMed] [Google Scholar]
- 13. Deal JA, Betz J, Yaffe K et al. Hearing impairment and incident dementia and cognitive decline in older adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gates GA, Cobb JL, Linn RT, Rees T, Wolf PA, D'Agostino RB. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg 1996;122:161–167. [DOI] [PubMed] [Google Scholar]
- 15. Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: a prospective, population‐based study. Otol Neurotol 2014;35:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin MY, Gutierrez PR, Stone KL et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004;52:1996–2002. [DOI] [PubMed] [Google Scholar]
- 17. Lin FR, Yaffe K, Xia J et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med 2013;173:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallhagen MI, Strawbridge WJ, Shema SJ. The relationship between hearing impairment and cognitive function: a 5‐year longitudinal study. Res Gerontol Nurs 2008;1:80–86. [DOI] [PubMed] [Google Scholar]
- 19. Lin FR, Thorpe R, Gordon‐Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci 2011;66:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wen YH, Wu SS, Lin CH et al. A Bayesian approach to identifying new risk factors for dementia: a nationwide population‐based study. Medicine 2016;95:e3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amieva H, Ouvrard C, Giulioli C, Meillon C, Rullier L, Dartigues JF. Self‐reported hearing loss, hearing aids, and cognitive decline in elderly adults: a 25‐year study. J Am Geriatr Soc 2015;63:2099–2104. [DOI] [PubMed] [Google Scholar]
- 22. Tomioka K, Okamoto N, Morikawa M, Kurumatani N. Self‐reported hearing loss predicts 5‐year decline in higher‐level functional capacity in high‐functioning elderly adults: The Fujiwara‐Kyo Study. J Am Geriatr Soc 2015;63:2260–2268. [DOI] [PubMed] [Google Scholar]
- 23. Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 2011;25:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quaranta N, Coppola F, Casulli M et al. The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol Neurootol 2014;19(Suppl 1):10–14. [DOI] [PubMed] [Google Scholar]
- 25. Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology 2006;52:386–394. [DOI] [PubMed] [Google Scholar]
- 26. Teipel S, Fritze T, Ovari A et al. Regional pattern of dementia and prevalence of hearing impairment in Germany. J Am Geriatr Soc 2015;63:1527–1533. [DOI] [PubMed] [Google Scholar]
- 27. Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA 1989;261:1916–1919. [PubMed] [Google Scholar]
- 28. Tombaugh TN. Test‐retest reliable coefficients and 5‐year change scores for the MMSE and 3MS. Arch Clin Neuropsychol 2005;20:485–503. [DOI] [PubMed] [Google Scholar]
- 29. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini‐Mental State Exam (3MS) compared. J Clin Epidemiol 1997;50:377–383. [DOI] [PubMed] [Google Scholar]
- 30. Stephens R, Kaufman A. The role of long‐term memory in digit‐symbol test performance in young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2009;16:219–240. [DOI] [PubMed] [Google Scholar]
- 31. Wong LL, Yu JK, Chan SS, Tong MC. Screening of cognitive function and hearing impairment in older adults: a preliminary study. Biomed Res Int 2014;2014:867852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kravitz E, Schmeidler J, Beeri MS. Cognitive decline and dementia in the oldest‐old. Rambam Maimonides Med J 2012;3:e0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dupuis K, Pichora‐Fuller MK, Chasteen AL, Marchuk V, Singh G, Smith SL. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2015;22:413–437. [DOI] [PubMed] [Google Scholar]
- 34. Pacala JT, Yueh B. Hearing deficits in the older patient: “I didn't notice anything ” JAMA 2012;307:1185–1194. [DOI] [PubMed] [Google Scholar]
- 35. Moore DR, Edmondson‐Jones M, Dawes P et al. Relation between speech‐in‐noise threshold, hearing loss and cognition from 40‐69 years of age. PLoS One 2014;9:e107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu HY, Chin JJ, Tong HM. Screening for hearing impairment in a cohort of elderly patients attending a hospital geriatric medicine service. Singapore Med J 2004;45:79 – 84. [PubMed] [Google Scholar]
- 37. Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 2012;147:803–807. [DOI] [PubMed] [Google Scholar]
- 38. Golding M, Taylor A, Cupples L, Mitchell P. Odds of demonstrating auditory processing abnormality in the average older adult: the Blue Mountains Hearing Study. Ear Hear 2006;27:129–138. [DOI] [PubMed] [Google Scholar]
- 39. Strouse AL, Hall JW, 3rd, Burger MC. Central auditory processing in Alzheimer's disease. Ear Hear 1995;16:230–238. [DOI] [PubMed] [Google Scholar]
- 40. Cooper JC, Jr , Gates GA. Central auditory processing disorders in the elderly: the effects of pure tone average and maximum word recognition. Ear Hear 1992;13:278–280. [DOI] [PubMed] [Google Scholar]
- 41. Humes LE, Dubno JR, Gordon‐Salant S et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol 2012;23:635–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alonso A, Mosley TH, Jr , Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 2009;80:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 44. Allen NH, Burns A, Newton V et al. The effects of improving hearing in dementia. Age Ageing 2003;32:189–193. [DOI] [PubMed] [Google Scholar]
- 45. van Hooren SA, Anteunis LJ, Valentijn SA et al. Does cognitive function in older adults with hearing impairment improve by hearing aid use? Int J Audiol 2005;44:265–271. [DOI] [PubMed] [Google Scholar]
- 46. Dawes P, Emsley R, Cruickshanks KJ et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One 2015;10:e0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mosnier I, Bebear JP, Marx M et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg 2015;141:442–450. [DOI] [PubMed] [Google Scholar]
- 48. Boi R, Racca L, Cavallero A et al. Hearing loss and depressive symptoms in elderly patients. Geriatr Gerontol Int 2012;12:440–445. [DOI] [PubMed] [Google Scholar]
- 49. Acar B, Yurekli MF, Babademez MA, Karabulut H, Karasen RM. Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr 2011;52:250–252. [DOI] [PubMed] [Google Scholar]
- 50. Pichora‐Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am 1995;97:593–608. [DOI] [PubMed] [Google Scholar]
- 51. Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Lang Hear Res 1982;25:593–599. [DOI] [PubMed] [Google Scholar]
- 52. Fratiglioni L, Paillard‐Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 2004;3:343–353. [DOI] [PubMed] [Google Scholar]


