Dear Editor,
We have read with great interest the recent article by Tufton and coworkers reporting on the risk of metastasis in patients with paraganglioma (PGL) tumours associated with germline SDHA mutations (Tufton et al. 2017). Herein, we report a further case of a malignant PGL in a 46-year-old man with a succinate dehydrogenase complex flavoprotein subunit A (SDHA) germline mutation (c.91C > T, p.Arg31*). In the case we describe, following the initial surgical removal of a left-sided retroperitoneal PGL, twenty-three years elapsed before the development of a bony metastasis in the eighth left rib. This observation is similar to that of Tufton and coworkers who reported two patients, who developed metastatic disease in 16 and 37 years, respectively, following initial diagnosis (Tufton et al. 2017). During investigations for this case, we found that the rib metastasis was avid on 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) computed tomography (CT), but not avid on radiolabeled I131 metaiodobenzylguanidine (MIBG) imaging (Fig. 1). This observation of SDHA-related malignant PGL is in keeping with SDHB- and SDHD-associated PGL, in which reduced avidity on MIBG imaging is due to reduced tumoural expression of noradrenaline transporters (Timmers et al. 2007). This clinical report supports the recent literature that suggests a risk of malignancy in patients with SDHA mutated PGL (Bausch et al. 2017, Casey et al. 2017, Tufton et al. 2017). The prolonged time interval between diagnosis and development of metastases in our case suggests that SDHA-related tumours are slow growing but it does support long-term surveillance programmes for patients with germline SDHA mutations.
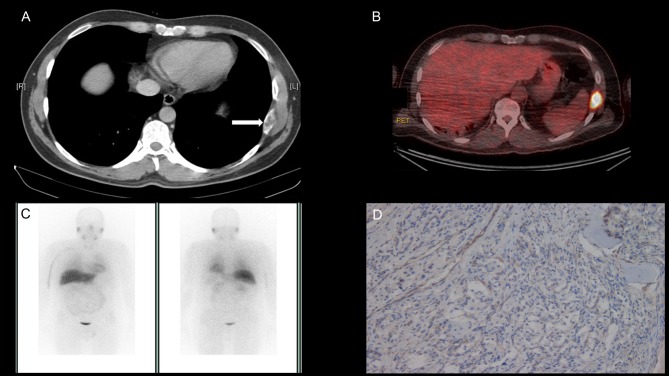
Figure 1.
(A) Demonstrates the metastatic deposit in the left 8th rib on CT as indicated by the arrow. (B) Shows the avid rib metastasis on 18F FDG PET CT and (C) shows no tumour avidity on I131 MIBG imaging. (D) Demonstrates loss of SDHB protein expression in the metastatic tumour deposit on SDHB immunostaining indicating SDH deficiency due to the detected germline mutation in SDHA (c.91C > T, p.Arg31).
Although mutations in SDHB, SDHC and SDHD gene subunits were first identified as predisposition genes for the development of phaeochromocytoma (PC) and PGL tumours over 15 years ago, SDHA was first associated with PGL only seven years ago (Burnichon et al. 2010) and, more recently, with wild-type gastrointestinal stromal tumours (WT GIST) (Evenepoel et al. 2015). It is now recognised that the SDHA gene is the most common SDHx germline mutation implicated in development of SDH-deficient WT GIST (Boikos et al. 2016). Recently, the European-American-Asian Pheochromocytoma-Paraganglioma Registry Study Group reported on 34 index cases with germline SDHA mutations and PC or PGL tumours. This group described a high prevalence of head and neck PGL in the SDHA cohort 15/34 (44%) and metastatic disease was reported in 4/34 (12%) (Bausch et al. 2017).
Here, we report the case of a 46-year-old man who first presented at age 23 years with headache, heat intolerance and abdominal pain. He was subsequently diagnosed with a left-sided retroperitoneal paragangliomama, which was surgically resected. The man had no family history of endocrine tumours and no additional relevant medical history. Following surgery, the patient was surveyed in primary care with annual urinary metanephrine testing. Twenty-three years later he was referred to our specialist neuroendocrine tumour service at Cambridge University Hospital NHS Foundation Trust, due to an elevated urinary normetanpehrine level (urinary normetanephrine 5870 nmol/24 h, reference range 0–4900; urinary metanephrine 756 nmol/24 h, reference range 0–2000), which was first observed 3 months earlier. On review, the patient denied symptoms suggestive of catecholamine excess and, importantly, denied any of the symptoms that he reported at the time of his initial presentation. Review of systems revealed that his only complaint was left side rib pain. Previous investigations in primary care included a plain chest radiograph which did not reveal any abnormality.
We performed plasma metanephrine testing and found an elevated normetanephrine level (2864 pmol/L, reference range <1000 pmol/L) in the context of normal plasma metanephrine (197 pmol/L, reference range <600 pmol/L) and methoxytyramine measurements (95.6 pmol/L, reference range <180 pmol/L). In light of the elevated normetanephrine level in urine and plasma, CT of the neck, thorax, abdomen and pelvis was performed and a metastatic deposit in the eighth left rib with no evidence of additional disease was identified (Fig. 1A). An 131I-MIBG scan was performed both for staging purposes and to evaluate suitability for 131I-MIBG therapy. Interestingly, the metastatic deposit was not avid (Fig. 1C). An 18F-FDG PET CT confirmed avidity (maximum standard uptake value of 19.1) in the left rib lesion (Fig. 1B) but no additional disease was identified. The patient was referred for a thoracotomy and surgical resection of the 8th rib after appropriate pre-operative optimisation with alpha-blockade. Subsequently, histological analysis confirmed the diagnosis of a metastatic paraganglioma with SDHB immunonegativity. SDHA immunohistochemistry was not performed (Fig. 1D).
Next generation sequencing of SDHA, SDHB, SDHC, SDHD, SDHAF2, MAX, TMEM127, VHL, RET and FH was performed on germline DNA from this patient after obtaining informed consent. The Illumina Trusight-One assay was used for sequencing and a mean coverage depth of >20-fold was achieved for 98% of the regions sequenced. Whole exon deletions, duplications and large rearrangements are not detected using this method and multiple ligation probe analysis was performed for VHL, SDHB, SDHC and SDHD. This testing identified a pathogenic truncating mutation in the SDHA gene (c.91C > T, p.Arg31*), which was confirmed by Sanger sequencing.
Post-operatively, repeat plasma metanephrine levels returned to normal (normetanephrine, 391 pmol/L; metanephrine, 180 pmol/L). This patient will remain under regular clinical follow-up for the development of synchronous or metachronous tumours. Given his germline SDHA mutation status and previous disease avidity on 18F-FDG PET CT, going forward, this will be the preferred surveillance imaging modality if there is no detectable disease on conventional cross-sectional imaging using CT/MRI, in the presence of elevated plasma metanpehrine or methoxytyramine levels.
In summary, the case we describe provides additional evidence for the risk of metastatic disease in SDHA mutated PGL. Moreover, our report highlights the utility of 18F-FDG PET CT in the detection of metastatic disease in patients with SDHA mutations, as has previously been demonstrated in cases of SDHB and SDHD related tumours (Timmers et al. 2007). Interestingly, and with relevance to optimal surveillance strategies for patients with germline SDHA mutations, the significant lag period (23 years) between initial presentation and the development of metastatic disease described in our case was also observed in two other cases of SDHA mutated malignant PGL (Tufton et al. 2017). The incidence of metastatic disease in SDHB related PC and PGL has been reported in two studies as 19% (Benn et al. 2006) and 16% (Srirangalingam et al. 2008) over a mean follow-up of 48 and 70 months, respectively. These data suggest that the development of metastatic disease may occur earlier in the disease course of SDHB-associated PGL/PC. Further study is required to define the risk of malignant disease in SDHA-related PGL/PC and the median interval for the development of malignancy. Recent literature suggests that life-long surveillance, as recently recommended by the European Society of Endocrinology (Plouin et al. 2016), is crucial for patients with SDHA gene mutations, in addition to those patients with mutations in other SDH subunits (SDHB/SDHC/SDHD). However, we acknowledge that prospective studies with extended follow-up periods of 15–20 years may be necessary to reveal the true incidence of metastasis in SDHx related PGL and to stratify individual surveillance protocols for patients based on the SDHx germline subunit mutation.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this article.
Funding
The authors thank the following funding agencies: NIHR (E R M), European Research Council Advanced Researcher Award (E R M), the British Heart Foundation (E R M) and Health Research Board Ireland (R C).
References
- Bausch B, Schiavi F, Ni Y, Welander J, Patocs A, Ngeow J, Wellner U, Malinoc A, Taschin E, Barbon G, et al. 2017. Clinical characterization of the pheochromocytoma and paraganglioma susceptibility genes SDHA, TMEM127, MAX, and SDHAF2 for gene-informed prevention. JAMA Oncology [in press]. ( 10.1001/jamaoncol.2017.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, Croxson M, Dahia PL, Elston M, Gimm O, et al. 2006. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. Journal of Clinical Endocrinology and Metabolism 91 827–836. ( 10.1210/jc.2005-1862) [DOI] [PubMed] [Google Scholar]
- Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon FC, Georger S, Trent JC, von Mehren M, Wright JA, Schiffman JD, et al. 2016. Molecular subtypes of KIT/PDGFRA Wild-type gastrointestinal stromal tumors: a report from the National Institutes Of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncology 2 922–928. ( 10.1001/jamaoncol.2016.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnichon N, Brière JJ, Libé R, Vescovo L, Rivière J, Tissier F, Jouanno E, Jeunemaitre X, Bénit P, Tzagoloff A, et al. 2010. SDHA is a tumor suppressor gene causing paraganglioma. Human Molecular Genetics 19 3011–3020. ( 10.1093/hmg/ddq206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey RT, Ascher DB, Rattenberry E, Izatt L, Andrews KA, Simpson HL, Challis B, Park S-M, Bulusu VR, Lalloo F, et al. 2017. SDHA related tumorigenesis: a new case series and literature review for variant interpretation and pathogenicity. Molecular Genetics and Genomic Medicine 5 237–250. ( 10.1002/mgg3.279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenepoel L, Papathomas TG, Krol N, Korpershoek E, de Krijger R, Persu A, Dinjens WN. 2015. Toward an improved definition of the genetic and tumor spectrum associated with SDH germ-line mutations. Genetics in Medicine 17 610–620. ( 10.1038/gim.2014.162) [DOI] [PubMed] [Google Scholar]
- Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C, Steichen O. & Guideline Working Group 2016. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. European Journal of Endocrinology 174 G1–G10. ( 10.1530/EJE-16-0033) [DOI] [PubMed] [Google Scholar]
- Srirangalingam U, Walker L, Khoo B, MacDonald F, Gardner D, Wilkin TJ, Skelly RH, George E, Spooner D, Monson JP, et al. 2008. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clinical Endocrinology 69 587–596. ( 10.1111/j.1365-2265.2008.03274.x) [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. 2007. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. Journal of Clinical Oncology 25 2262–2269. ( 10.1200/JCO.2006.09.6297) [DOI] [PubMed] [Google Scholar]
- Tufton N, Ghelani R, Srirangalingam U, Kumar VKA, Drake W, Iacovazzo D, Skordilis K, Berney DM, Al-Mrayat M, Khoo B, et al. 2017. SDHA mutated paragangliomas may be at high risk of metastasis. Endocrine-Related Cancer 24 L43–L49. ( 10.1530/ERC-17-0030) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a