Key Points
Intrathymic T-cell regeneration is facilitated by human proT-cells generated in vitro.
In vitro–generated human proT-cells home to the thymus, wherein they restore thymic structure.
Abstract
Hematopoietic stem cell transplantation (HSCT) is followed by a period of immune deficiency due to a paucity in T-cell reconstitution. Underlying causes are a severely dysfunctional thymus and an impaired production of thymus-seeding progenitors in the host. Here, we addressed whether in vitro–derived human progenitor T (proT)-cells could not only represent a source of thymus-seeding progenitors, but also able to influence the recovery of the thymic microenvironment. We examined whether co-transplantation of in vitro–derived human proT-cells with hematopoietic stem cells (HSCs) was able to facilitate HSC-derived T-lymphopoiesis posttransplant. A competitive transfer approach was used to define the optimal proT subset capable of reconstituting immunodeficient mice. Although the 2 subsets tested (proT1, CD34+CD7+CD5−; proT2, CD34+CD7+CD5+) showed thymus engrafting function, proT2-cells exhibited superior engrafting capacity. Based on this, when proT2-cells were coinjected with HSCs, a significantly improved and accelerated HSC-derived T-lymphopoiesis was observed. Furthermore, we uncovered a potential mechanism by which receptor activator of nuclear factor κb (RANK) ligand–expressing proT2-cells induce changes in both the function and architecture of the thymus microenvironment, which favors the recruitment of bone marrow-derived lymphoid progenitors. Our findings provide further support for the use of Notch-expanded progenitors in cell-based therapies to aid in the recovery of T-cells in patients undergoing HSCT.
Introduction
Hematopoietic stem cells (HSCs), which reside in the bone marrow (BM), are the ultimate source of all blood cell lineages. Although most hematopoietic lineages develop in the BM, T-cells are unique in that they must complete their development in the thymus. The thymus is composed of a 3-dimensional network of epithelial cells organized into distinct thymic epithelial cell (TEC) compartments with cortical TECs and medullary TECs located on the outer and inner thymus, respectively.1 These distinct stromal niches provide specialized environmental cues that support thymocytes undergoing different stages of maturation.2,3
Under steady state conditions, the thymus does not contain a self-renewing cell, and the production of T-cells throughout life must be maintained by the continued recruitment of blood-borne progenitors arriving from the BM.4,5 Human HSCs are found within the lineage negative (Lin−) CD34+CD38− compartment. Although HSCs do not directly seed the thymus,6 CD34+ cells are present in human thymus. CD7 is one of the earliest markers to appear during human T-cell ontogeny and analysis of human fetal and postnatal organs by Haddad et al7,8 showed that an early T-lineage progenitor in humans corresponds to a CD34+CD45RA+CD7+ population. A separate study by Hao et al9 has demonstrated that a rare population of Lin−CD34+CD7− thymocytes is detectable and thus may correspond to an earlier intrathymic progenitor. However, the exact nature of the thymus-seeding cell arriving from the BM remains unknown.10 Nevertheless, the presence of a Lin−CD34+CD38+CD10+CD45RA+CD7−/low common lymphoid progenitor population in human BM with T-cell potential has been described,11 as well as an umbilical cord blood-derived CD34+CD38−CD7+ common lymphoid progenitor population12 and a CD34+CD45RAhiCD7+ population from UCB and fetal BM possessing T-cell potential.7,8 Thus, candidate populations with T-cell potential have been reported from UCB and BM.
HSC transplantation (HSCT) is a mainstay for the treatment of hematologic malignancies, however, an extended delay in immune-reconstitution after transplantation leads to cases of morbidity and mortality and remains a clinical challenge.13 The increased susceptibility to opportunistic infections is specifically caused by poor T-cell recovery and the absence of de novo T-cell generation from HSC-derived progenitors found in the BM.14,15 Thus, the development of strategies to enhance T-lymphopoiesis remains an important task.16 One such strategy is the adoptive transfer of T-cell precursors to rapidly restore the T-cell compartment and T-cell–mediated immunity after HSCT. Zakrzewski et al17 demonstrated that infusion of in vitro–derived mouse CD4−CD8− double negative (DN) cells together with HSCs led increased T-cell reconstitution in both the thymus and periphery of mice that had undergone allogeneic HSCT. More importantly, in vitro–derived T-cells exhibited early graft-versus-tumor activity in HSCT recipients.
Previous work from our laboratory and others18,19 showed the generation of cells with a CD34+CD45RA+CD7++ thymus-colonizing phenotype derived from CD34+ UCB HSCs.18 These cells fulfilled the properties of a progenitor T (proT)-cell, as they were able to home to, settle, and differentiate in the thymus of recipient nonobese diabetic (NOD)/severe combined immunodeficiency (SCID)/γcnull (NSG) and RAG2−/−γc−/− mice. It was unclear, however, whether distinct subsets within the CD34+CD45RA+CD7++ population exhibited differential engraftment capacity in vivo. Additionally, it remained to be tested whether in vitro–derived proT-cells were capable of promoting human HSC-derived T-lymphopoiesis after HSCT.
In this study, we evaluated the efficiency of 2 distinct in vitro–generated proT subsets (CD5− proT1 and CD5+ proT2 CD34+CD45RA+CD7++) to engraft the thymus of NSG mice. Our findings reveal that both subsets colonize the thymus of immunodeficient mice. However, through competitive repopulation assays, we show that proT2 cells have an advantage in thymus reconstitution compared with the proT1 subset. Our findings also revealed that in vitro–generated proT2-cells are capable of enhancing thymus reconstitution from HSC-derived progenitors after transplantation into irradiated NSG mice. Recipients receiving both HSC and proT2-cells exhibited accelerated HSC-derived chimerism and higher thymus cellularity compared with HSC-only–injected mice. Finally, we addressed a potential mechanism by which proT2 cells were able to promote earlier T-cell reconstitution from BM-derived progenitors by showing that in vitro–generated proT-cells restored the thymic architecture in NSG mice.
Taken together, these findings support the use of OP9-DL cells for the generation of human proT-cells with the potential for adoptive cell transfer in the clinical setting during periods of immunodeficiency after HSCT.
Methods
UCB samples
Human UCB samples were obtained and HSC containing fractions were purified as previously described18 (details are provided in the supplemental Methods, available on the Blood Web site). Sorted human HSCs (Lin− CD34+ CD38−) were >99% pure, as determined by post-sort analysis. Cord blood samples were collected by following approved guidelines established by the Research Ethics Board of Sunnybrook Health Sciences Centre.
Mice
NOD.cg-PrkdcscidIL2rgtm/Wjl/Sz (NSG) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed and bred in a pathogen-free facility. All animal procedures were approved by the Sunnybrook Health Science Centre Animal Care Committee.
Generation of proT-cells and OP9-DL1 cell coculture
OP9-DL1 cell20 cocultures were performed as previously described,18 and details are provided in the supplemental Methods.
Flow cytometry
Fluorophore-conjugated antibodies were commercially purchased (BD Biosciences or eBioscience), and used as previously described.18,21-23 Details are included in the supplemental Methods.
HSC/proT2-cell coinjection into immunodeficient mice
Lin− CD34+CD38−/lo HSCs from an HLA-A2+–typed UCB and HLA-A2− CD34+CD7++CD5+ proT2-cells sorted from day 10 to 12 of HSC/OP9DL1 coculture were resuspended in a mixture containing recombinant human interleukin 7 (rhIL-7) (2.5 μg) and an IL-7 antibody M25 (0.5 μg) (provided by Dr C. Surh, Scripps Research Institute, La Jolla, CA) and 0.2 × 105 (HSCs): 2 × 105 (proT2)-cells injected (30 μL/mouse) intrahepatically into irradiated (120 rads) 2- to 5-day-old neonates. As controls, NSG mice were injected with either HSCs or proT2-cells alone. Mice were boosted with an IL-7/M25 mixture every 5 days. Thymus, spleen, and BM were harvested at either 3, 6, 9, or 12 weeks after intrahepatic transplant.
ProT1/ProT2-cell coinjection
For competitive transfer experiments, CD34+CD38−/lo HSCs from an HLA-A2−-typed and HLA-A2+-typed CB was sorted and placed into separate (HLA-A2− vs HLA-A2+) and individual OP9-DL1 cell cocultures and grown for 10-12 days, as described above. Cocultures were sorted for CD34+CD7++CD5− proT1 and CD34+CD7++CD5− proT2 cells and 1 × 105 of each subset were coinjected as a mixture with IL-7/M25 into 2-5 day old neonates, as indicated above.
Quantitative real time reverse transcriptase polymerase chain reaction
Preparation of complementary DNA samples from thymuses, amplification conditions, and primers used are detailed in the supplemental Methods.
Histology
Preparation of thymuses sections and staining procedures are detailed in the supplemental Methods.
Statistics
The data and error bars are presented as mean ± standard error of the mean. To determine statistical significance, a two-tailed unpaired Student t test was used using Prism software. Statistical significance was determined as P < .05.
Results
Differential ability of proT1- and proT2-cells to engraft the thymus immunodeficient mice
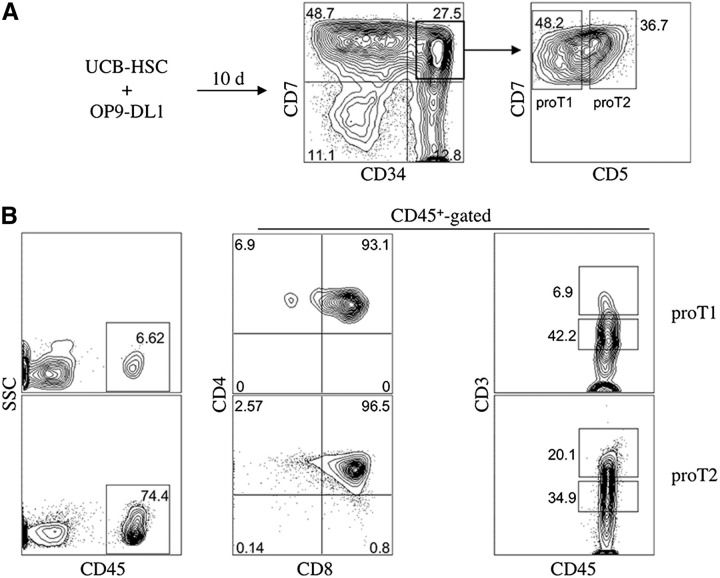
We have previously reported that 2 in vitro–derived subsets, CD34+CD7++CD5− (proT1) and CD34+CD7++CD5+ (proT2), generated from human HSC/OP9-DL1 cocultures display a differential capability to engraft mouse fetal thymus lobes in vitro.18 Whether they possessed a differential ability to home and reconstitute the thymus in vivo remains to be assessed. To address this, we injected NSG neonatal mice with either proT1- or proT2-cells derived from a day 10 coculture (Figure 1A) and analyzed them for thymus engraftment. Figure 1B shows a representative flow cytometric profile of human CD45-gated CD4, CD8, and CD3 cell surface expression in the thymus of mice injected with proT1- or proT2-cells. Although the proT1-injected mouse possessed a similar percentage of CD4+CD8+ double positive (DP) T-cells (93%) compared with proT2-cells (96%), they had lower levels of CD3 expression and only 6.9% of thymocytes expressed high levels of CD3 compared with 20% in the proT2-injected mice. Thus, the more advanced differentiation state of proT2-cells at the time of injection is highlighted by the more mature status of cells in the thymus of the proT2-injected mouse compared with the proT1-injected mouse. When decreasing numbers of each subset were injected into separate mice, proT1- and proT2-cells displayed a similar frequency of thymus-engraftment (Table 1). Engraftment was still observed for both subsets when as low as 1 × 104 cells/mouse were injected, however, a failure to engraft was seen with administration of 0.5 × 104 cells/mouse.
Figure 1.
Flow cytometric analysis of engraftment and differentiation of in vitro–derived CD34+ CD7++ CD5− (proT1)- and CD34+ CD7++ CD5+ (proT2)-cells in immunodeficient mice. (A) Human UCB CD34+CD38−/lo cells were differentiated on OP9-DL1 cells for 10 days and CD34+CD7++ CD5− (proT1)- and CD34+ CD7++ CD5+ (proT2)-cells were sorted by flow cytometry. (B) Neonatal NSG mice were injected intrahepatically with 2.0 × 105 cells of either subset (n ≥ 8 mice/group). Thymuses were harvested after 4 weeks and stained for CD45, CD4, CD8, and CD3. The percentage of human CD45+ cells present in the thymus of NSG mice transplanted with proT1-cells (average thymus cellularity 27.6 × 104 ± 6.6 × 104) or proT2 cells (average thymus cellularity 31.6 × 104 ± 4.0 × 104) are shown. CD4, CD8, and CD3 cell surface expressions are shown on CD45+-gated thymocytes. The results shown are representative of at least 3 independent experiments. SSC, side scatter.
Table 1.
Thymic engraftment analysis of in vitro derived proT subsets
| Cell number injected | Percent engraftment* | |
|---|---|---|
| proT1 | proT2 | |
| 0.75-1.0 × 105 | 50 (9/18)† | 62 (13/21) |
| 0.20-0.25 × 105 | 10 (1/10) | 33 (3/9) |
| 0.1 × 105 | 30 (3/10) | 12.5 (1/8) |
| 0.05 × 105 | 0 (0/5) | 0 (0/8) |
Engraftment defined as >1% human CD45+ cells as analyzed by flow cytometry after 4-week postinjection.
Numbers in parentheses represent the number of mice engrafted over the total number of mice injected.
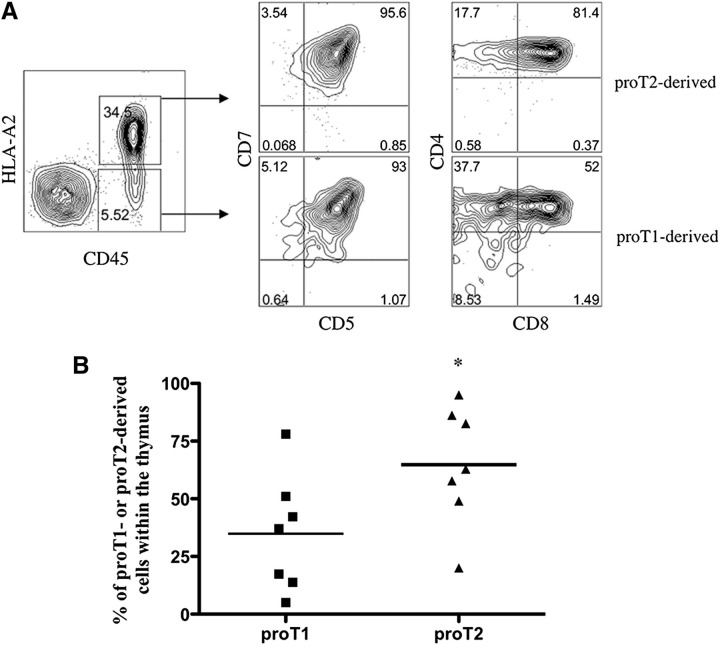
To more clearly determine whether one subset or another exhibited better thymus engraftment capacity, proT1- and proT2-cells were placed in a competitive repopulation assay, and the cell of origin traced based on differences in the expression of the major histocompatibility complex (MHC) class I molecule HLA-A2. In vitro–generated HLA-A2− proT1- and HLA-A2+ proT2-cells were injected in a 1:1 ratio (1 × 105 of each subset) into neonatal mice and analyzed 17 days after injection. Figure 2A shows the presence of proT1- and proT2-derived cells in the thymus, as indicated by CD45+HLA-A2− (5.5%) and CD45+HLA-A2+ (34.5%) expression, respectively. When examined, we observed that 93% of proT1-cells acquired CD5 on the cell surface. Furthermore, the thymus of mice injected with proT1-cells also possessed 38% CD4+CD8− immature single positive (SP) T-cells and >50% of cells had progressed to the DP stage. In contrast, >80% of cells found within the thymus of the proT2-injected mouse contained CD4+CD8+ T-cells. More importantly, when the ratio of proT1- vs proT2-cells was analyzed in the thymus, proT2-cells were present at a significantly higher ratio (P = .049; n = 7) compared with proT1-cells (Figure 2B).
Figure 2.
Analysis for the presence of proT1-derived and proT2-derived cells in the thymus of competitively reconstituted immunodeficient mice. (A) 1:1 mixture of sorted HLA-A2− proT1-cells (1 × 105) and sorted HLA-A2+ (1 × 105) proT2-cells were injected into nonirradiated NSG neonatal mice and the thymuses harvested and analyzed after 17 days (n = 7 mice/group). Shown is flow cytometric analysis of human CD45 and HLA-A2 cell surface expression and CD5, CD7, CD8, and CD4 expression on CD45+HLA-A2−– and CD45+HLA-A2+–gated cells for proT1- or proT2-derived cells, respectively. (B) Percentage of HLA-A2− or HLA-A2+ cells over total human CD45+ cells for individual mice shown. The results shown are representative of at least 3 independent experiments. *P < .05.
In vitro–derived proT2-cells differentiate into functionally mature T-cells in vivo
Both present and previous work from our laboratory have demonstrated that in vitro–generated proT-cells reconstitute the thymus of immunodeficient mice. However, whether proT-cells obtained from HSC/OP9-DL1 cocultures could develop into functional human T-cells in a physiological setting remained to be explored. A representative flow cytometric analysis of the thymus of an NSG mouse at 8-weeks postinjection of proT2-cells is shown in supplemental Figure 1A. Human CD45 expression was observed and these cells were CD4+CD8− and CD4−CD8+ SP T-cells, with few DN and DP thymocytes. These SP T-cells coexpressed T-cell receptor (TCR)αβ and CD3, and appeared to be mature naïve T-cells, as indicated by CD27 and CCR7 coexpression (not shown).
We tested whether these phenotypically mature thymocytes were responsive to stimulation by anti-CD3/CD28 stimulation. Prior to activation, thymocytes obtained from proT2-injected mice were loaded with carboxyfluorescein diacetate succinimidyl ester (CFSE) to assess their proliferative ability. Shown in supplemental Figure 1B, stimulated thymocytes exhibited an increase in side scatter and forward scatter (not shown) compared with nonstimulated cells. We also observed that ∼70% of cells found within the stimulated wells displayed an upregulation of the activation marker CD25 and loss of CFSE, indicating proliferation. This was in contrast to the lack of CD25 upregulation and maintenance of high CFSE levels remaining in nonstimulated human thymocytes. In addition, stimulated thymocytes downregulated CD27 and upregulated CD38 on the cell surface indicating functional responsiveness. Supplementary Figure 1C shows that human T-cells were also detected at low levels (1% CD45) in the spleen of mice at 8-weeks postinjection of proT2-cells, and similar to the thymocytes, these cells were also capable of proliferation and activation marker upregulation (86% CD25+CFSE−) after TCR/CD28 stimulation.
In vitro–derived proT2-cells promote the entry of HSC-derived progenitors into the thymus of irradiated mice after HSCT
A major concern after HSCT is the slow recovery of T-cells in the thymus leading to an increase in opportunistic infections and mortality in patients.14 Different strategies aimed at enhancing T-cell reconstitution are being developed and tested in clinical trials.24-27 Previous work by Zakrzewski et al27 demonstrated that DN proT-cells generated from mouse hematopoietic progenitors cocultured on OP9-DL1 cells were able to enhance T-lymphopoiesis at coinjection after HSCT. However, it was unknown whether human proT-cells coinjected with HSCs during transplantation were capable of facilitating T-cell reconstitution from HSC-derived progenitors.
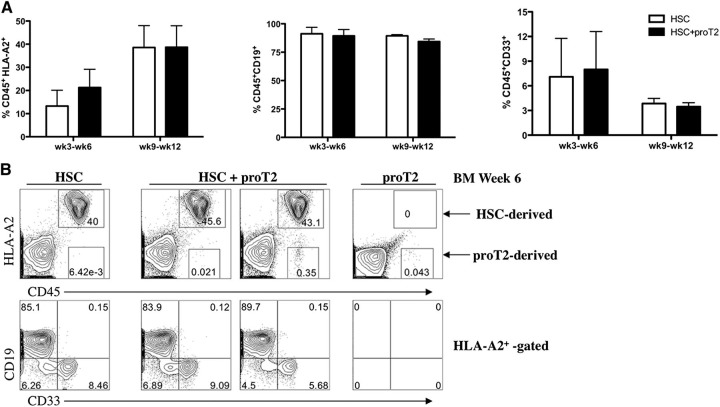
To address this question, we generated HLA-A2− proT2 cells by HSC/OP9-DL1 coculture, and coinjected them with HLA-A2+CD34+CD38–/lo UCB-HSCs into irradiated newborn NSG mice. The engraftment of HSCs in these mice was confirmed by examining the BM (Figure 3A) and spleen (not shown) for the presence of human CD45+ HLA-A2+ cells at 3 to 12 weeks after injection (Figure 3A and not shown). The percentage of HSC-derived cells in the BM did not differ from the coinjected mice at any of the time-points analyzed. Furthermore, these human cells correspond to CD19+ B cells and CD33+ myeloid cells, with the exception of the week 3 time-point when myeloid cells were absent (data not shown).
Figure 3.
Analysis of engraftment and differentiation of HSCs and proT2-cells in the BM of immunodeficient mice. (A) Percentage of human CD45+HLA-A2+ (left), CD19 (middle), and CD33 (right) HSC-derived engraftment at 3 to 6 weeks, and 9 to 12 weeks after intrahepatic injection of neonatal NSG mice receiving either HSCs (white) or HSCs with proT2-cells (black). (B) Flow cytometric analysis of CD45 vs HLA-A2, and CD19 and CD33 on CD45+HLA-A2+–gated cells in the BM at 6 weeks postintrahepatic injection. Table 2 shows the number of mice analyzed. The results shown are representative of at least 3 independent experiments.
Figure 3B shows the flow cytometric analysis of human CD45+HLA-A2+ cells (40%) in the BM of 1 mouse injected with HSCs with 85% of these corresponding to CD19+ B cells and a lower frequency corresponding to CD33+ myeloid cells. A similar profile was observed in mice coinjected with HSCs, along with proT2-cells (middle panels), confirming that proT2-cells do not interfere with HSC engraftment in the BM and also validating the presence of HSC-derived cells in these mice. The coinjected mice displayed very low levels of human engraftment originating from proT2-cells (<0.5%), as in vitro generated proT2-cells are inefficient at engrafting the BM when injected into neonatal immunodeficient mice. A representative flow cytometric analysis of a mouse injected with only proT2-cells is also shown (right panel). A similar reconstitution profile was observed in the spleen of these mice (data not shown).
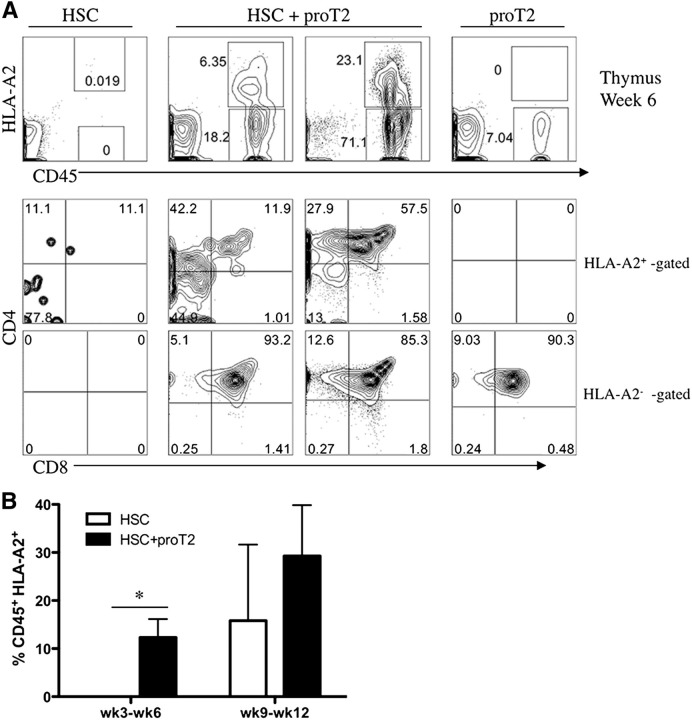
In contrast to the profile observed in the BM of the HSC-only injected mouse, Figure 4A shows the near absence of HSC-derived cells in the thymus of these mice. The absence of T-cells at 3- to 6-weeks post-HSC–injection in neonatal mice was not surprising as T-cell reconstitution is not usually observed in these recipients as early as 6 weeks. In striking contrast, at examination of the thymuses of HSC/proT2-coinjected mice, the mice clearly possessed a CD45+HLA-A2+ population, thus indicating thymus reconstitution from an incoming HSC-derived progenitor. As expected, these mice contained proT2-derived cells (18% and 71%) in the thymus, which were CD45+HLA-A2−. The middle panel of Figure 4A highlights the more immature status of HSC/BM-derived progenitors (HLA-A2+-gated) compared with proT2-derived cells (HLA-A2−-gated) indicated by the larger proportion of DN cells in the HSC-derived compartment, as compared with the near absence of this population in the proT2-generated compartment. Nonetheless, DP cells were emerging from HSC-derived cells in the thymus of coinjected mice. As previously shown,18 flow cytometric analysis of the thymus of proT2-only injected mice revealed the presence of proT-derived cells and no HLA-A2+ cells. Figure 4B highlights the significant difference in the percentage of HSC-derived T-lineage cells present in the thymus when coinjected, as compared with HSC-only–injected mice. Of note, a significant facilitation of HSC/BM-derived engraftment is seen at early time-points (3-6 weeks), whereas only a trend to enhanced engraftment is seen at later time-points (9-12 weeks).
Figure 4.
Analysis of engraftment and differentiation of HSCs and proT2-cells in the thymus of immunodeficient mice. (A) Flow cytometric analysis of CD45 vs HLA-A2, and CD4 and CD8 on CD45+HLA-A2+–gated (HSC-derived) and CD45+HLA-A2− –gated (proT2-derived) cells in the thymus at 6 weeks after intrahepatic injection of NSG mice receiving either HSCs, HSCs with proT2-cells, or proT2-cells. (B) Percentage of human CD45+HLA-A2+ HSC-derived engraftment at 3 to 6 weeks and 9 to 12 weeks after intrahepatic injection of neonatal mice receiving either HSCs (white) or HSCs with proT2 cells (black). Table 2 shows the number of mice analyzed. The results shown are representative of at least 3 independent experiments. *P < .05.
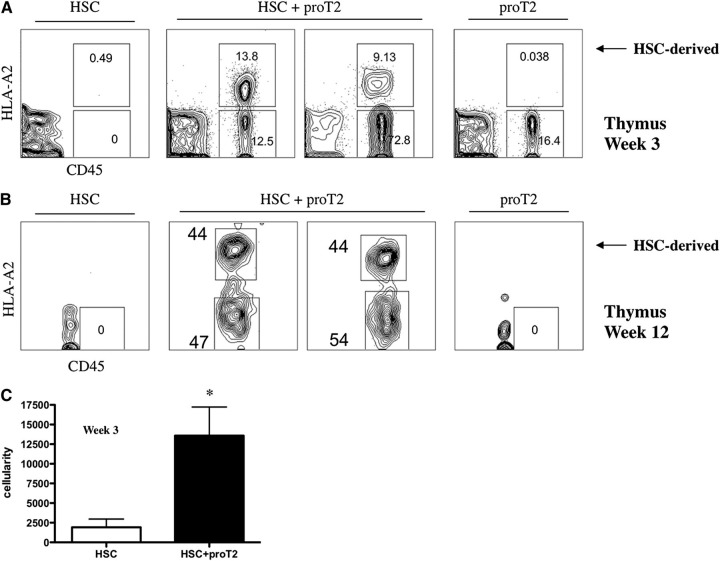
We sought to further examine T-lymphopoiesis originating from HSC-derived progenitors when coinjected with proT2-cells at earlier time-points, after only 3 weeks, a period when T-lymphopoiesis after HSCT is not typically observed. Figure 5A demonstrates the absence of HSC-derived CD45+HLA-A2+ cells in the thymus of mice receiving HSCs alone. In contrast, we observed the presence of HSC-derived human thymocytes in mice that received in vitro–generated proT2-cells and HSCs at this early time-point. Further facilitation of HSC-derived T-lymphopoiesis was still evident when mice were analyzed after 12 weeks (Figure 5B). Additionally, mice receiving both HSCs with proT2-cells displayed a significant difference in thymus cellularity when compared with mice receiving HSCs alone (Figure 5C). Table 2 illustrates the overt improvement in T-cell reconstitution from HSC-derived progenitors when combined with proT2 coinjection. The frequency of thymus-engraftment (based on CD45+HLA-A2+ expression) from a BM-derived progenitor increased significantly (P < .05) when coinjected mice are analyzed at weeks 3 to 6 and 9 to 12.
Figure 5.
Analysis of early and late engraftment, and differentiation of HSCs and proT2-cells in the thymus of immunodeficient mice. Flow cytometric analysis of CD45 vs HLA-A2 in the thymus at (A) 12 weeks and at (B) 3 weeks, postintrahepatic injection of neonatal NSG mice receiving HSCs, HSCs with proT2-cells, or proT2-cells (n = 5 and n = 12 mice/group, respectively). Human CD45− cells represent cells of host mouse origin. (C) Thymus cellularity in mice receiving HSCs or HSCs with proT2-cells 3 weeks after injection. The results shown are representative of at least 3 independent experiments. Cellularity was calculated based on human CD45+ cells obtained by flow cytometry. *P < .05.
Table 2.
Analysis for the engraftment* of HSC-derived cells in NSG mice
| Cells injected | ||||
|---|---|---|---|---|
| HSC† | HSC + proT2‡ | |||
| Weeks after injection | Percent in BM§ | Percent in thymus§ | Percent in BM§ | Percent in thymus§ |
| 3-6 | 89 (8/9) | 11 (1/9) | 100 (8/8) | 63 (5/8)|| |
| 9-12 | 100 (13/13) | 31 (4/13) | 100 (13/13) | 70 (9/13)|| |
Engraftment of HSC-derived cells defined by the presence of >1% human CD45+ HLA-A2+ in the thymus or BM of host NSG mice.
HSC were sorted from UCB as Lin− CD34+ CD38−/low cells, 2 × 104 cells injected/mouse.
The proT2-cells were generated from HSC/OP9-DL1 cultures after 10-12 days, and they were sorted as CD34+ CD7++ CD5+ cells, 2 × 105 cells injected/mouse.
Numbers in parentheses represent the number of mice engrafted of the total number injected.
P < .05 for the presence of HLA-A2+ cells in the thymus of HSC+ proT2-injected mice in comparison with HSC alone.
In vitro–derived proT2-cells alter the thymic stromal architecture in NSG mice
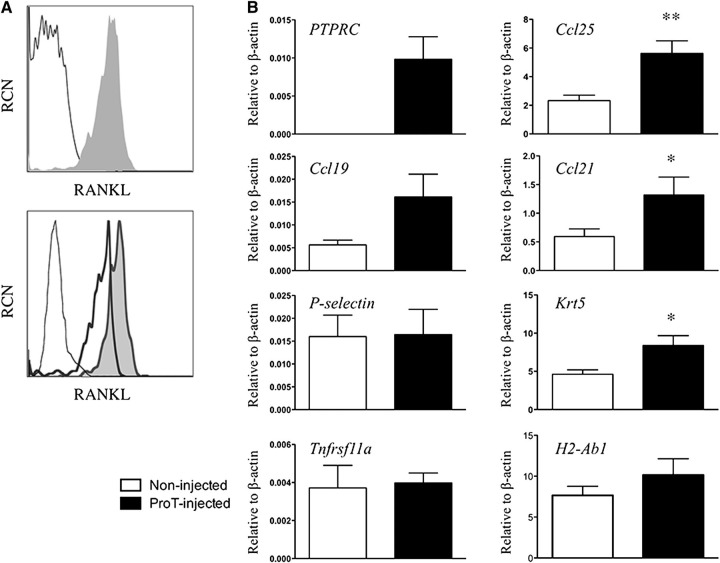
Given the enhanced thymic recruitment of BM-derived progenitors at co-transplantation with in vitro–derived proT-cells, we sought to determine a plausible mechanism by which proT-cells could be facilitating this occurrence. The critical interplay between thymocytes and TECs in the generation of a fully formed thymic microenvironment is well accepted.28,29 Thus, we sought to assess whether proT-cells could be acting on stromal elements within the NSG thymus, positively affecting thymic function. As shown in Figure 6A, proT-cells from a day 12 coculture expressed RANK ligand (RANKL), a tumor necrosis factor receptor superfamily ligand that interacts with RANK on TECs to influence their functional maturation.28 However, RANKL expression was detected at low levels on the starting HSCs used to generate the proT-cells (top plot). Of note, while both proT-cell populations expressed high levels of RANKL, the proT2 subset expressed 2.5× higher levels than the proT1-cells (bottom plot).
Figure 6.
Gene expression analyses from thymuses obtained from in vitro–derived proT-injected mice. (A) Flow cytometric analysis of RANK ligand (RANKL) expression on purified CD34+CD38− cells (not shaded) and day 11 in vitro–derived CD34+CD7+-gated proT-cells (shaded) (top); and in vitro–derived proT1- (not shaded, thick line) and proT2-cells (shaded) (bottom). Unstained cells are included as a control (not shaded, thin dashed line). (B) Quantitative real time reverse transcriptase polymerase chain reaction analysis for the expression of human PTPRC (CD45), and mouse Ccl25, Ccl19, Ccl21, Selp (P-selectin), Krt5 (Cytokeratin-5), Tnfrsf11a (RANK), and H2-Ab1 (MHC class II) from mouse thymus extracts of NSG mice injected with proT-cells or control noninjected mice after 3 weeks. Transcript levels for all genes were normalized to mouse β-actin. These results are the average of 3 independent experiments, with the exception of Selp (n = 2), with error bars corresponding to standard error of the mean. Asterisks represent statistical significance as determined by Student t tests. The results shown are representative of 3 independent experiments. *P < .05; **P < .005.
Next, we examined whether the expression of critical TEC-derived chemokines, such as CC19, CCL21, and CCL25, known to recruit BM and blood-borne progenitors,6,30,31 was affected by the presence of proT-cells. First, we confirmed the engraftment of proT-cells, as indicated by the presence of human CD45 (PTPRC) transcripts within the thymuses of pro-T–injected mice vs noninjected mice (Figure 6B). With the examination of Ccl25 and Ccl21 transcript levels, our analyses revealed a marked upregulation of both chemokines in thymuses obtained from proT-injected mice as compared with noninjected mice after 3 weeks. Finally, consistent with the role of RANKL-expressing cells in directly influencing TEC maturation, the expression of Cytokeratin-5 (Krt5) was increased in the thymuses of proT-injected mice compared with noninjected littermates. Of note, thymic expression of P-selectin (Selp), RANK (Tnfrsf11a), and MHC class II (H2-Ab1) were not affected in proT-cell–injected mice (Figure 6B). The expression levels for RANK in either of the NSG mouse thymuses were about half of that observed from wild-type (WT) thymus (data not shown), whereas RANK expression was not detectable in CD45+ thymocytes, as previously reported.32 Taken together, in vitro–derived proT-cells appear to induce TEC maturation and the expression of key chemokines involved in progenitor recruitment to the thymus.
In light of these findings, we sought to evaluate whether injection of proT2-cells were also facilitating the recruitment of BM-derived progenitors through structural alteration of the thymic microenvironment. To this end, immunohistologic analyses were performed on the thymuses from control (noninjected) mice or proT-injected mice. Shown in Figure 7, the thymus of an adult WT mouse, control, or proT-injected (CD34+CD7++) NSG mouse were sectioned and stained with anti-Cytokeratin 5 (K5) and anti-Cytokeratin 8 (K8) expressed on thymic medullary and cortical epithelial cells, respectively. As expected, the thymus of the WT mouse possessed a cortex, medulla, and a defined corticomedullary boundary shown by anti-Cytokeratin staining. In contrast, the control NSG mouse exhibited highly disorganized thymic architecture lacking a cortex and medulla. Rather, a dense network of disorganized epithelial cells was observed. Strikingly, when human in vitro–generated proT2-cells are injected into NSG mice, a reorganization of the cortex and medulla (with a clear boundary between the 2 stromal niches) was observed. These findings suggest that a critical effect of proT-mediated enhanced thymic reconstitution may be due to the RANKL-induced differentiation and reorganization of the thymic architecture that likely leads to more effective recruitment of BM-derived T-lymphocyte progenitors.
Figure 7.
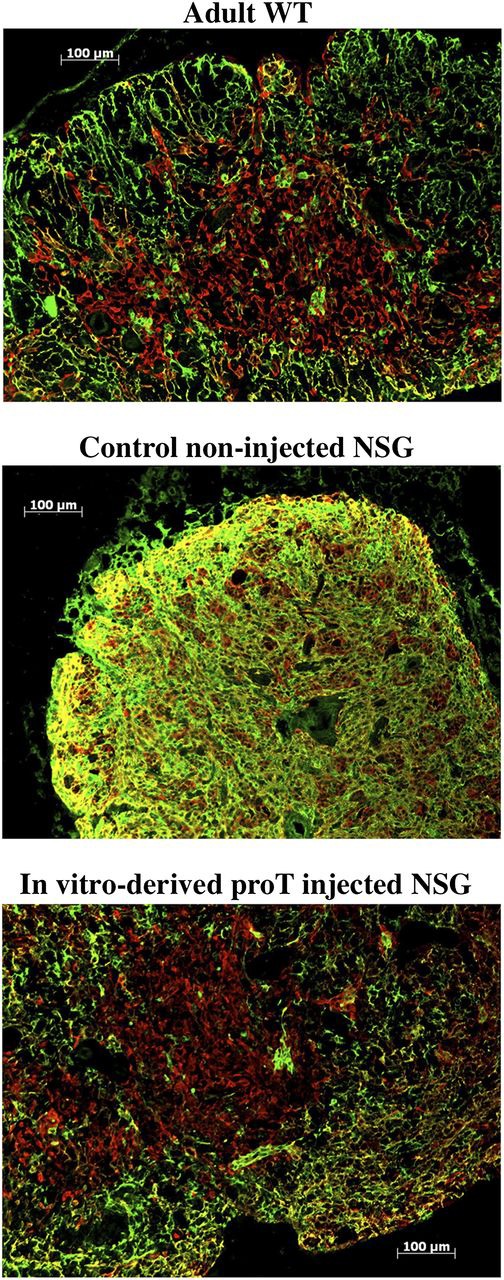
Immunohistologic analysis of thymus. Sections of the thymus from an adult WT mouse (top); control noninjected NSG mouse (middle); and in vitro–derived proT-injected NSG mouse (bottom) 6 weeks after intrahepatic injection into neonates (n = 6). Thymus tissues were stained with anti-Cytokeratin 8 (green; cortical) and anti-Cytokeratin 5 (red; medullary). The results shown are representative of 3 independent experiments.
Discussion
The identification of different progenitors possessing T-cell potential from CB and BM is well established.9,10,33,34 Previously, we characterized a CD34+CD7++ T-progenitor subset generated from UCB-HSC cocultures with OP9-DL1 cells.18 Additionally, this population was capable of homing to and engrafting the thymus of immunodeficient mice. The CD34+CD7++ can be fractionated into CD5− and CD5+ subsets, both capable of reconstituting host thymus lobes in vitro.18 However, we noted that CD34+CD7++CD5+ cells (proT2) possessed a threefold higher engrafting capacity in vitro, as compared with the CD5− counterpart (proT1). Our present findings indicate that while both subsets displayed thymus-settling ability in vivo, proT2-cells possessed increased thymus engraftment function when placed in competition with proT1-cells. Although it is possible that both subsets are similarly capable of homing, the proT2 subset may have a proliferative advantage leading to a higher frequency of proT2-derived cells in the thymus of NSG mice. Nonetheless, given their more differentiated state and expression of homing molecules,18 it is simply more likely that proT2-cells are more effective at migration and/or entering the host thymus.
Although the ability to migrate and occupy the thymus provide a key initiating step to the establishment of the T-cell compartment, it was significant to investigate whether in vitro–generated proT-cells, after completing their maturation in a host thymus, have the ability to restore functional T-cell competence. To this end, we observed that human thymocytes and peripheral cells (splenocytes) displayed hallmarks of being functionally competent T-cells by their ability to proliferate and upregulate activation markers with TCR stimulation. This result is consistent with Shultz et al35 that previously reported T-cell cytotoxic ability in NSG mice reconstituted with CD34+ HSCs. One constraint regarding the injection of in vitro–derived proT-cells is that although maturation based on phenotypic differentiation is readily observed in the thymus of NSG mice, maintenance in the periphery in the absence of other human cells is inefficient, resulting in low numbers of human T-cells in the spleen. Although rhIL-7/M25 is periodically provided, human MHC:Ag contact and additional cell types of human origin are likely required to support the maintenance and survival of peripheral T-cells in these mice. Indeed, Shultz generated HLA class I transgenic NSG mice that support human T-cell selection in an HLA-restricted manner.36 T-cells generated in these mice expressed cytotoxic molecules, such as Granzyme A and Granzyme B, and more importantly they exhibited an antiviral response to Epstein-Barr virus.36
Similar to our previous findings,18 Reimann et al37 recently demonstrated that UCB CD34+ cells cultured on immobilized Δ-like 4 generated T-cell progenitors were able to engraft NSG mice. Here, we extend these results by demonstrating that coinjection of a defined subset of in vitro–generated proT-cells dramatically enhances and accelerates HSC-derived thymopoiesis after HSCT. Additionally, we provide a plausible mechanism for the facilitated entry of HSC/BM-derived cells into the thymus of host mice. By using a low number of HSCs, a lack of T-cell generation at multiple time-points in irradiated neonatal mice receiving CD34+CD38−/lo cells was typically observed. This was not due to lack of HSC engraftment, as all mice displayed significant levels of human chimerism in the BM and spleen, consisting mostly of CD19+ B cells and some CD33+ myeloid cells. In contrast, when proT2-cells are coinjected with HSCs, thymus reconstitution is readily attained.
Based on the low number of cells used for engraftment, sometimes proT2-cells failed to engraft; however, it is important to note that T-lymphopoiesis originating from HSC/BM-derived progenitors only occurred in coinjected mice when there was evidence that proT2-cells also engrafted the thymus. This observation underscores the importance of proT-cells for HSC-derived T-lymphopoiesis in these mice. Our findings also revealed the appearance of HSC-derived thymocytes in mice as early as 3 weeks after coinjection. This phenomenon may or may not be due to HSCs directly “piggy-backing” with proT2-cells, as these cells home directly to the thymus after transplantation. This hypothesis may be tested by examining the thymuses of coinjected mice as early as 24 hours after injection. Although the appearance of HSC-derived cells in mice as early as 3 weeks does not allow us to discern whether HSCs are “piggy-backing” or not, it does indicate that proT2-cells are capable of facilitating HSC-derived T-cell reconstitution at a much earlier time as compared with mice receiving HSCs alone.
A plausible explanation for the observed facilitated entry is through the restoration of thymic architecture and function, due to inductive lympho-stromal interactions. The importance of thymocytes in the maintenance of the thymic microenvironment has been underscored by numerous studies showing that mice possessing an early block in T-cell development display abnormalities of thymic organization, with the degree of disorganization correlating with how early the block in T-cell development occurs.38,39 In this study, we demonstrate that the highly disorganized thymic structure of NSG mice was alleviated with injection of in vitro–derived proT-cells, inducing the organization of cortical and medullary environments important for T-lymphopoiesis. Although DN thymocytes influence cortex formation, studies have demonstrated that DP and SP thymocytes induce the medulla formation and maturation of medullary TECs.39,40 Specifically, positively selected thymocytes have been shown to regulate the development of the medulla through TNF superfamily receptor/ligand interactions, such as RANK/RANKL.32 Indeed, the high expression levels of RANKL on in vitro–derived proT-cells and the known cross-reactivity of human RANKL with mouse RANK,41 strongly support our hypothesis that the changes in epithelial cell organization that we observed are due to lympho-stromal interaction between in vitro–derived progenitors and TECs. Lending further support to our findings is the increased expression of CCL21 and CCL25 by thymus stromal cells in mice injected with proT-cells. However, the presence of proT-cells did not alter the expression of P-selectin, which is known to be important for entry to the thymus.42 Taken together, it is plausible that RANKL-expressing in vitro–generated proT2-cells (that directly settle within the thymus) alter the stromal microenvironment found in immunodeficient mice, thereby promoting the creation of new chemokine rich niches for immigrating HSC-derived progenitors. This in turn favors the migration or settling of these cells arriving from the BM to newly-enabled stromal signals and niches.
In summary, we have characterized 2 subsets of in vitro–derived progenitor T-cells for their ability to colonize the thymus of immunodeficient recipients, and differentiate into functionally mature T-cells. We noted a superior engraftment capacity by proT2-cells, and showed that this subset is able to promote T-lymphopoiesis from HSC-derived progenitors after HSCT. One caveat to these experiments is the use of immunodeficient neonatal mice, which provide a permissive environment for xenogeneic engraftment. Of higher relevance would be the use of adult mice for these studies. We have tested to determine whether in vitro–derived progenitors are capable of thymus settling in adult mice, and we observed a low frequency of mice with thymus engraftment (data not shown). The precise reasons for the difference in engraftment between the neonatal and the adult is unknown, however, the increase in the neonatal vasculature compared with adults,43 severe thymus involution and continued atrophy in adult NSG mice may be factors influencing human proT-cell engraftment in neonatal vs adult mice.
The use of in vitro–generated progenitors for clinical use is underway; Delaney et al 26 cultured CD34+ cells from UCB on immobilized Δ-like 1 (delta1ext-IgG) that were infused with an unmanipulated UCB unit into patients undergoing HSCT.26 Patients receiving 1 expanded cord and 1 unmanipulated cord displayed greater myeloid recovery compared with patients receiving 2 unmanipulated cords, in addition to shorter time to neutrophil recovery. Thus, the use of Notch-ligands as an approach to generate large numbers of proT-cells17-19,37 have the potential to become clinically relevant, as these could be used to accelerate T-cell reconstitution after HSCT.
Acknowledgments
The authors thank Gisele Knowles and Courtney McIntosh for their expertise in cell sorting; Sean Oh, Roxanne Holmes, John Xu, and Sanam Taheri for their technical support; and Dr Rose Kung and staff of the Women and Babies program at Sunnybrook Health Sciences Centre (Toronto, ON, Canada) for their ongoing support by providing umbilical cord blood.
This work was supported by grants to J.C.Z.-P. from The Krembil Foundation and the Canadian Institutes of Health Research (CIHR MOP-119538 and HOP-83070).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.A. performed and designed research, analyzed data, and wrote the paper; J.S., M. Mohatshami, M. Malm, and R.N.L.M.-M. performed experiments; E.H. provided crucial materials; P.M.B. and P.S. provided intellectual insight; M.R.v.d.B. contributed to the experimental approach; and J.C.Z.-P. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for G.A. is McEwen Centre for Regenerative Medicine, University Health Network, Toronto, ON, Canada; for M. Malm is Vaccine Research Center, Tampere, Finland; for R.N.L.M.-M. is Wellstat Therapeutics Inc., Gaithersburg, MD; and for P.S. is Centre Esther Koplowitz, Hospital Clinic de Barcelona, Barcelona, Spain.
Correspondence: Juan Carlos Zúñiga-Pflücker, Sunnybrook Research Institute, 2075 Bayview Ave, Room A3-31, Toronto, ON M4N 3M5, Canada; e-mail: jczp@sri.utoronto.ca.
References
- 1.Holländer G, Gill J, Zuklys S, Iwanami N, Liu C, Takahama Y. Cellular and molecular events during early thymus development. Immunol Rev. 2006;209:28–46. doi: 10.1111/j.0105-2896.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4(4):278–289. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- 3.Petrie HT, Zúñiga-Pflücker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 4.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 5.Goldschneider I. Cyclical mobilization and gated importation of thymocyte progenitors in the adult mouse: evidence for a thymus-bone marrow feedback loop. Immunol Rev. 2006;209:58–75. doi: 10.1111/j.0105-2896.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178(4):2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 7.Haddad R, Guardiola P, Izac B, et al. Molecular characterization of early human T/NK and B-lymphoid progenitor cells in umbilical cord blood. Blood. 2004;104(13):3918–3926. doi: 10.1182/blood-2004-05-1845. [DOI] [PubMed] [Google Scholar]
- 8.Haddad R, Guimiot F, Six E, et al. Dynamics of thymus-colonizing cells during human development. Immunity. 2006;24(2):217–230. doi: 10.1016/j.immuni.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Hao Q-L, George AA, Zhu J, et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7- lympho-myeloid thymic progenitors. Blood. 2008;111(3):1318–1326. doi: 10.1182/blood-2007-08-106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awong G, Zuniga-Pflucker JC. Thymus-bound: the many features of T cell progenitors. Front Biosci (Schol Ed) 2011;3:961–969. doi: 10.2741/200. [DOI] [PubMed] [Google Scholar]
- 11.Galy A, Travis M, Cen D, Chen B, Human T. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3(4):459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 12.Hao QL, Zhu J, Price MA, Payne KJ, Barsky LW, Crooks GM. Identification of a novel, human multilymphoid progenitor in cord blood. Blood. 2001;97(12):3683–3690. doi: 10.1182/blood.v97.12.3683. [DOI] [PubMed] [Google Scholar]
- 13.Storek J, Geddes M, Khan F, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30(4):425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 14.Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am J Hematol. 1997;54(2):131–138. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Mackall CL, Fleisher TA, Brown MR, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89(10):3700–3707. [PubMed] [Google Scholar]
- 16.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4(11):856–867. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 17.Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12(9):1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 18.Awong G, Herer E, Surh CD, Dick JE, La Motte-Mohs RN, Zúñiga-Pflücker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114(5):972–982. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- 19.Meek B, Cloosen S, Borsotti C, et al. In vitro-differentiated T/natural killer-cell progenitors derived from human CD34+ cells mature in the thymus. Blood. 2010;115(2):261–264. doi: 10.1182/blood-2009-05-223990. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17(6):749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 21.Awong G, Herer E, La Motte-Mohs RN, Zúñiga-Pflücker JC. Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature. BMC Immunol. 2011;12:22. doi: 10.1186/1471-2172-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awong G, La Motte-Mohs RN, Zúñiga-Pflücker JC. In vitro human T cell development directed by notch-ligand interactions. Methods Mol Biol. 2008;430:135–142. doi: 10.1007/978-1-59745-182-6_9. [DOI] [PubMed] [Google Scholar]
- 23.La Motte-Mohs RN, Herer E, Zúñiga-Pflücker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105(4):1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 24.Wils EJ, Cornelissen JJ. Thymopoiesis following allogeneic stem cell transplantation: new possibilities for improvement. Blood Rev. 2005;19(2):89–98. doi: 10.1016/j.blre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Alpdogan O, Hubbard VM, Smith OM, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107(6):2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg GL, Zakrzewski JL, Perales MA, van den Brink MR. Clinical strategies to enhance T cell reconstitution. Semin Immunol. 2007;19(5):289–296. doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitta T, Ohigashi I, Nakagawa Y, Takahama Y. Cytokine crosstalk for thymic medulla formation. Curr Opin Immunol. 2011;23(2):190–197. doi: 10.1016/j.coi.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 29.van Ewijk W, Holländer G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127(8):1583–1591. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 30.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202(1):21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Ueno T, Kuse S, et al. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood. 2005;105(1):31–39. doi: 10.1182/blood-2004-04-1369. [DOI] [PubMed] [Google Scholar]
- 32.Hikosaka Y, Nitta T, Ohigashi I, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29(3):438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Six EM, Bonhomme D, Monteiro M, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204(13):3085–3093. doi: 10.1084/jem.20071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11(7):585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 35.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 36.Shultz LD, Saito Y, Najima Y, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci USA. 2010;107(29):13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reimann C, Six E, Dal-Cortivo L, et al. Human T-lymphoid progenitors generated in a feeder-cell-free Delta-like-4 culture system promote T-cell reconstitution in NOD/SCID/γc(-/-) mice. Stem Cells. 2012;30(8):1771–1780. doi: 10.1002/stem.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holländer GA, Wang B, Nichogiannopoulou A, et al. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373(6512):350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- 39.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol. 1991;21(7):1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 40.Surh CD, Ernst B, Sprent J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J Exp Med. 1992;176(2):611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bossen C, Ingold K, Tardivel A, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281(20):13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 42.Rossi FM, Corbel SY, Merzaban JS, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6(6):626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 43.Cuddihy AR, Suterwala BT, Ge S, et al. Rapid thymic reconstitution following bone marrow transplantation in neonatal mice is VEGF-dependent. Biol Blood Marrow Transplant. 2012;18(5):683–689. doi: 10.1016/j.bbmt.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]