ABSTRACT
Group B streptococcus (GBS) infection is a leading cause of death among newborns in developed countries. Data on the burden of GBS in Asian countries are lacking. This study aimed to understand (i) the rate of maternal rectovaginal GBS carriage, (ii) the rate of vertical transmission of GBS, as determined by culturing ear, umbilicus, and nasal swabs, and (iii) the distribution of GBS serotypes. This prospective observational study was conducted between September 2012 and November 2013 at Kumudini Women's Medical College Hospital, a secondary-level hospital in Mirzapur, Bangladesh. The study enrolled pregnant women who visited the outpatient clinic for antenatal care (ANC) and/or delivered a child in the inpatient department of Kumudini Women's Medical College Hospital and the babies born to those mothers. Among 1,151 enrolled pregnant women, 172 (15% [95% confidence interval [CI], 13 to 17%]) carried GBS; among 68 babies born to mothers with carriage, 26 (38% [95% CI, 27 to 51%]) had GBS on their body surfaces, indicating vertical transmission. Typing of the isolates (n = 172) identified all 10 GBS serotypes, most commonly types Ia (40% [69/172 isolates]), V (23% [40/172 isolates]), II (14% [24/172 isolates]), and III (12% [20/172 isolates]). This study shows that Bangladesh has all of the ingredients for invasive GBS disease, including colonization of mothers by invasive serotypes and vertical transmission to babies.
KEYWORDS: colonization, group B streptococcus, serotype, vertical transmission
INTRODUCTION
Achievement of Millennium Development Goal 4 was a significant public health triumph for Bangladesh. However, progress in reducing neonatal mortality rates in Bangladesh remains slow; in 2015, 62% of deaths at <5 years of age occurred in the neonatal period (an estimated 74,000 deaths) (1). About one-quarter of those deaths were due to infections with unknown etiology (2). Bangladesh and other low- and middle-income countries need to focus on improving neonatal survival rates, health, and development.
Group B streptococcus (GBS) is a leading cause of infections and deaths among newborns in developed countries and some low- and middle-income countries, especially in Africa (3–8). However, the data on neonatal GBS infections in Bangladesh and the South Asia region are scarce and broadly debated (9, 10). Recent publications from Bangladesh have suggested that invasive GBS disease in neonates is rare (11, 12). However, neither of those studies included newborns during the first hours of life, when they are most vulnerable to GBS sepsis.
To advance knowledge regarding the importance of neonatal GBS in Bangladesh, we hypothesized the following: (i) most Bangladeshi pregnant women do not carry GBS; (ii) if they do carry GBS, then the rate of transmission of GBS to their babies is low; (iii) if they do transmit GBS, then the serotypes are not invasive types. In this study, we aimed to test these hypotheses and to proceed to a conclusion through their sequential rejection, by examining (i) the maternal GBS carriage rate, (ii) the magnitude of GBS transmission from mother to baby, and (iii) the distribution of GBS serotypes.
RESULTS
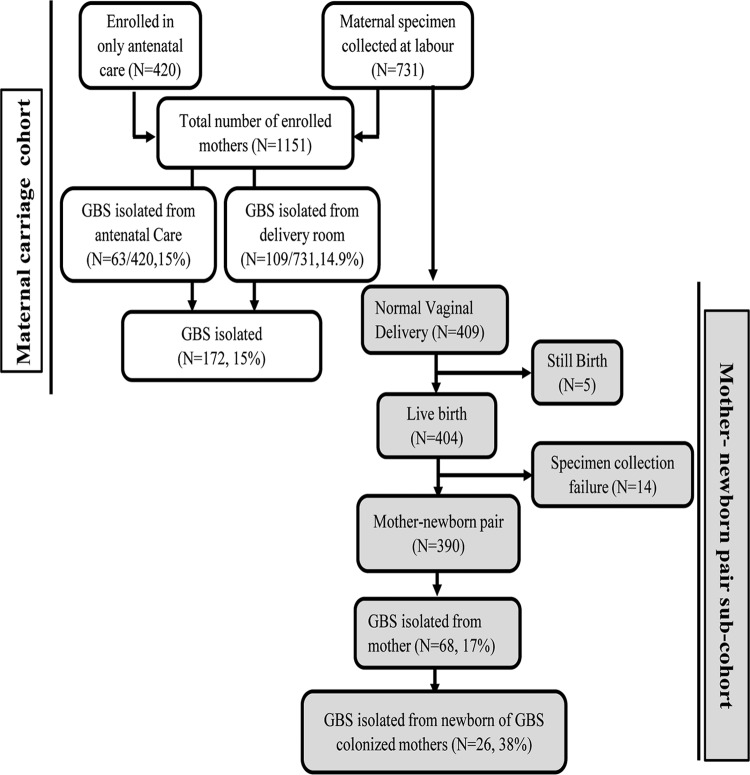
A total of 1,151 pregnant women (420 at the antenatal care [ANC] clinic and 731 at the delivery room) were enrolled in the maternal colonization study (Fig. 1 and Table 1). The overall GBS carriage rate among them was 15% (95% confidence interval [CI], 13% to 17%) (172/1,151 women); GBS was isolated from both vaginal and rectal swabs for 5% (57/1,151 women), from rectal swabs for 2% (26/1,151 women), and from vaginal swabs alone for 8% (89/1,151 women) (Table 2). The gestational ages (mean ± standard deviation) of the mothers enrolled at the ANC clinic and the delivery room were 34.6 ± 3.0 weeks (median, 34.0 weeks) and 38.5 ± 2.2 weeks (median, 39.0 weeks), respectively. GBS carriage rates were similar among the pregnant women enrolled at the ANC clinic (15.0% [63/420 women]) and the delivery room (14.9% [109/731 women]).
FIG 1.
Profile of GBS maternal carriage and transmission study, showing the total numbers of mothers enrolled in the study and of specimens collected and GBS isolates isolated from mothers and newborns.
TABLE 1.
Characteristics of enrolled mothers and newborn infants
| Variable | No. (%) |
P | |
|---|---|---|---|
| GBS carriage | No GBS carriage | ||
| Maternal age (n = 1,151) | 0.40 | ||
| <18 yr (n = 17 [1.5%]) | 4 (23.5) | 13 (76.5) | |
| 18–35 yr (n = 1,131 [98.2%]) | 167 (14.7) | 964 (85.3) | |
| >35 yr (n = 3 [0.3%]) | 1 (33.3) | 2 (66.6) | |
| Educational level (n = 1,151) | 0.75 | ||
| No schooling (n = 28 [2.5%]) | 4 (14.2) | 24 (85.8) | |
| Less than primary (n = 75 [6.5%]) | 14 (18.7) | 61 (81.3) | |
| Primary-secondary (n = 889 [77.2%]) | 133 (14.9) | 756 (85.1) | |
| More than secondary (n = 159 [13.8%]) | 21 (13.2) | 138 (86.8) | |
| Gestational age (n = 1,151) | 0.94 | ||
| >37 wk (n = 702 [61%]) | 103 (14.7) | 599 (85.3) | |
| 34–37 wk (n = 270 [23.5%]) | 42 (15.6) | 228 (84.4) | |
| <34 wk (n = 179 [15.5%]) | 27 (15) | 152 (85) | |
| How many times pregnant (n = 1,151) | 0.18 | ||
| 1 (n = 513 [44.5%]) | 87 (17) | 426 (83) | |
| 2 or 3 (n = 582 [50.5%]) | 76 (13) | 506 (87) | |
| >3 (n = 56 [5%]) | 9 (16) | 47 (84) | |
| Antibiotic during pregnancy (n = 1,151) | 0.76 | ||
| Yes (n = 13 [1%]) | 2 (15.4) | 11 (84.6) | |
| No (n = 1,125 [98%]) | 169 (15) | 956 (85) | |
| Unknown (n = 13 [1%]) | 1 (7.7) | 12 (92.3) | |
| Delivery method (n = 1,151) | 0.34 | ||
| Normal (n = 409 [35.5%]) | 68 (16.6) | 341 (83.4) | |
| Cesarean (n = 322 [28%]) | 41 (12.7) | 281 (87.3) | |
| Unknown (only ANC) (n = 420 [36.5%]) | 63 (15) | 357 (85) | |
| Birth weight (n = 390) | 0.13 | ||
| <2,500 g (n = 73 [18.7%]) | 2 (2.7) | 71 (97.3) | |
| >2,500 g (n = 317 [81.3%]) | 27 (8.5) | 290 (91.5) | |
| Newborn infant sex (n = 390) | 0.38 | ||
| Male (n = 212 [54.4%]) | 18 (8.5) | 194 (91.5) | |
| Female (n = 178 [45.6%]) | 11 (6.2) | 167 (93.8) | |
TABLE 2.
GBS colonization rate among pregnant women
| Rectal swab result | No. (%) of vaginal swabs |
||
|---|---|---|---|
| GBS positive | GBS negative | Total | |
| GBS positive | 57 (4.95) | 26 (2.25) | 83 (7.21) |
| GBS negative | 89 (7.73) | 979 (85.05) | 1,068 (92.79) |
| Total | 146 (12.7) | 1,005 (87.48) | 1,151 (100) |
Mother-newborn dyads (n = 409) for enrolled pregnant women who had normal vaginal deliveries at Kumudini Women's Medical College Hospital (KWMCH) were selected for the vertical transmission cohort. Among those dyads, 19 pairs were excluded due to stillbirth (n = 5) or wiping of the newborn prior to specimen collection (n = 14), leaving 390 mother-newborn pairs for estimation of the rate of vertical transmission. GBS was isolated from 17% of the mothers (68/390 mothers) and 7% of the babies (29/390 babies). The rate of vertical transmission of GBS was 38% (95% CI, 27 to 51%) (26/68 mothers). Among colonized newborns, the highest yield was from ear swabs (83% [24/29 babies]), followed by the nose (59% [17/29 babies]) and the umbilicus (48% [14/29 babies]) (Table 3). GBS could not be isolated from 3 mothers whose babies were positive for GBS.
TABLE 3.
Isolation of GBS from different body sites of newborns
| Colonization site | No. (%) of newborns GBS positive |
|---|---|
| Ear only | 9 (31.03) |
| Umbilicus only | 1 (3.5) |
| Nose only | 2 (6.9) |
| Ear and umbilicus | 2 (6.9) |
| Ear and nose | 4 (13.8) |
| Umbilicus and nose | 2 (6.9) |
| Ear, umbilicus, and nose | 9 (31.0) |
| Total | 29 (100) |
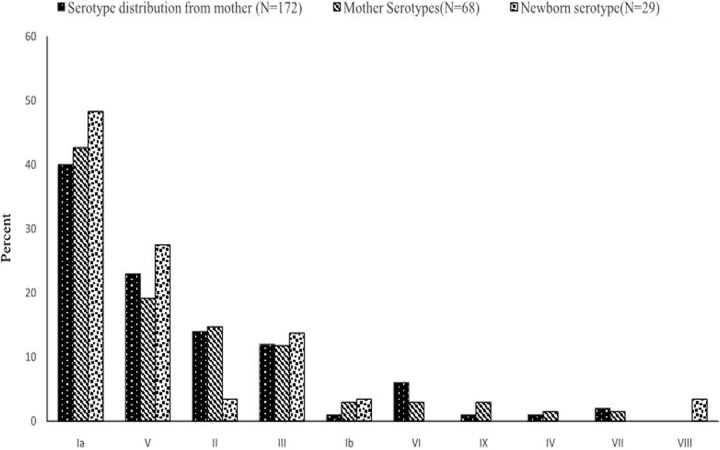
All isolates from mothers (n = 172, including the carriage group and the vertical transmission subgroup) and infants (n = 29) were available for capsular typing. Among the isolates from mothers, types Ia (40% [69/172 isolates]), V (23% [40/172 isolates]), II (14% [24/172 isolates]), and III (12% [20/172 isolates]) were predominant. The serotypes of isolates from the mother-baby dyads were invariably identical (Fig. 2). The three isolates from infants whose mothers' rectovaginal swabs did not yield any GBS were types Ia (n = 2) and III (n = 1).
FIG 2.
Distribution of GBS serotypes among all mothers and mother-newborn dyads.
DISCUSSION
The study showed that 15% of pregnant women in the Mirzapur subdistrict of Bangladesh carried GBS, which was notably higher than reports of maternal colonization rates of 8% in China (13) and 7.7% from our previous study in urban Bangladesh (14). A recent meta-analysis also reported a lower prevalence of rectovaginal GBS colonization in Southeast Asia (11.1%), although the prevalence varied widely in India (2% to 16%) (15–19). However, this rate of maternal carriage was lower than those in sub-Saharan Africa (21% to 22%) (15, 20, 21), North America and South America (19.7% to 24%) (15, 22), and Europe (19%) (15).
The higher prevalence of GBS carriage in this study, compared to our previous study, may be due to differences in the study populations; the prior study was conducted in an urban population, and the current study was conducted in a rural population. It is unclear why the urban population would have a lower colonization rate; however, similar differences between urban and rural populations were found in Zimbabwe (20).
GBS was isolated from the body surface of 7% of newborns. In three cases, the organism was isolated from newborns but not from their mothers. We assume that these cases represented vertical transmission, since collection of specimens immediately after birth, without any further handling and/or wiping of the newborns, makes the acquisition of GBS from other sources unlikely. We calculated a vertical transmission rate of 38% (26/68 women) among women who were colonized. This rate of transmission is lower than those reported in other countries (∼50%) with high prevalence rates of maternal GBS carriage and invasive disease in newborns (23, 24).
The present study reveals some similarities and some differences in the GBS serotype distribution in this population, compared to other reports. The leading serotypes in this population, i.e., types Ia, V, and II, are also among the top 5 global serotypes (9). However, type III, which has high invasive potential and is the global leader in causing invasive disease (9), ranked fourth among colonized mothers. In a separate study at a different location (Sylhet) in rural Bangladesh, serotype VII was most prevalent (25), whereas it ranked 6th in this population; serotype VII has also been reported in Thailand and Kuwait (22). The differences in serotype distributions between Mirzapur and Sylhet and in carriage rates between Dhaka and Mirzapur could be due to difference in the geographic regions and the demographic characteristics of the populations. These differences suggest that a more comprehensive understanding of GBS dynamics in Bangladesh is needed and perhaps could be developed through a well-coordinated multisite study.
This study has a number of limitations. It was based in a single hospital in rural Bangladesh and enrolled a relatively small number of pregnant women, we did not add rectal swabs from the babies for the vertical transmission subcohort, which might have led to higher rates of isolation of GBS from the babies' body surface, and we did not include active surveillance for invasive diseases and thus could not elucidate the invasive potential of the isolates in our population. Determination of invasive potential would be important to address the debate regarding invasive GBS disease in this population. The potential of particular serotypes to invade may vary based on the specific clone (sequence type). For example, serotype V, which was the second most common type in our series, had low invasive potential in African countries (4% to 6%) (8, 26) but high invasive potential in the United States (28%) and England (13%) (27, 28). Furthermore, the study sample size was not adequate to evaluate the proportion of newborns who went on to develop GBS disease.
Despite these limitations, this is the first study of its kind in Bangladesh to show maternal carriage, vertical transmission, and the serotype distribution of GBS. This study thus shows that Bangladesh has all of the ingredients for invasive GBS disease. At this point, we need to understand better the local factors leading to low levels of detection of invasive GBS disease in this population. It would be prudent to plan now for a larger multisite study with additional components, including follow-up monitoring for invasive GBS disease, assays for anti-GBS antibodies in mothers' blood and newborns' cord blood, and sequence typing of isolates, and a vaccine probe study, to better understand the burden of invasive GBS disease.
MATERIALS AND METHODS
Study duration.
This prospective, cross-sectional study was conducted in KWMCH at Mirzapur, Bangladesh, between September 2012 and November 2013.
Study site.
KWMCH is located in the Mirzapur subdistrict of Bangladesh, which has a mixed rural and periurban population totaling 407,781. The economy of Mirzapur depends mainly on agriculture (demographic surveillance system [DSS], unpublished data). The majority (n = 288,000) of the population of the Mirzapur subdistrict has been under our DSS since 2007. On average, 5,000 deliveries occur in the surveillance area each year, 58% of which take place in health facilities (DSS, unpublished data), in contrast to the national average facility birth rate of 37% (29).
KWMCH was established in 1938 as a private trust hospital, and it has 750 beds. This hospital is the main primary- and secondary-level health care provider for the population of the Mirzapur subdistrict and the nearby areas. Twenty-one percent of the deliveries that occur in the DSS area take place at this facility, whereas 37% occur at other government hospitals and private clinics (DSS, unpublished data).
Normal (uncomplicated) vaginal deliveries at KWMCH are conducted for an average fee of $1.50, which includes costs for the bed, food, physician consultations, and nursing services. On average, the obstetric department of KWMCH conducts 2,500 deliveries per year (2012 to 2013), 47% by cesarean section. The country has no intrapartum antibiotic prophylaxis (IAP) policy, and IAP is not practiced at KWMCH.
Study eligibility criteria. (i) Rectovaginal carriage.
Separate collection of vaginal and rectal swab specimens and isolation of GBS from those specimens were performed to measure the carriage rate among the mothers. Pregnant women were included in the study at gestational ages of ≥30 weeks, if they were either attending the outpatient department for antenatal care (ANC) check-ups or coming directly for delivery without ANC, were not enrolled in another study, and, gave verbal informed consent. Pregnant women with antibiotic administration in the previous 2 weeks were excluded from the study.
(ii) Vertical transmission.
Vertical transmission was measured based on the isolation of GBS from infants immediately after birth. Newborns of enrolled mothers who had normal vaginal deliveries at KWMCH were included in this subcohort. Babies who were wiped at birth or bathed before the collection of swabs or whose mother was not swabbed were excluded from the study.
Specimen collection.
Separate lower vaginal and rectal swabs were collected from eligible women with rayon-tipped swabs. For assessment of vertical transmission, separate swabs were collected from the ear, nose, and umbilicus of each newborn of enrolled mothers, immediately after delivery.
Specimen processing.
All swabs were placed in Amies transport medium at 4°C and sent to the hospital microbiology laboratory within <1 h, and processing at the laboratory was completed within the next 2 h. Specimens were processed using procedures described elsewhere (30). In brief, swabs were inoculated onto 5% sheep blood agar and also into Todd-Hewitt broth (2 ml) supplemented with gentamicin (8 μg/ml) and nalidixic acid (15 μg/ml), and the samples were incubated at 37°C for 18 h. An aliquot of 10 μl of Todd-Hewitt broth was subsequently inoculated onto 5% sheep blood agar plates. The plates were observed 24 h after inoculation; if there was no suspected growth of GBS, then they were reincubated for an additional 24 h. Suspected GBS colonies were subjected to CAMP testing (31). The results for the CAMP-test-positive isolates were confirmed with the latex agglutination test (LAT) (Oxoid, Basingstoke, Hants, UK) (32). Confirmed GBS isolates were stored at −70°C in medium containing skim milk, tryptone, glucose, and glycerol until they were transported to the department of microbiology at Dhaka Shishu Hospital for serotyping.
Capsular typing of GBS isolates.
Serotyping of GBS isolates was performed by LAT using GBS type-specific antisera against Ia, Ib, and II to IX capsular antigens (Statens Serum Institute, SSI, Denmark), following the method described by Slotved et al. (33). GBS strains of types Ia (strain 2008232728), Ib (strain 2008232580), II (strain 2008232738), III (strain 2008232579), IV (strain 2011201884), V (strain 2008232731), VI (strain 2010230042), VII (strain 4832-06), and VIII (strain 5030-08) were used as serotype-specific controls (kindly provided by the Centers for Disease Control and Prevention, Atlanta, GA). All GBS isolates, including strains that were not typeable by LAT, were subjected to PCR following the method described by Poyart et al. (34). In brief, DNA was extracted using the QIAamp DNA minikit (Qiagen, Hilden, Germany), according to the manufacturer's instructions, and was subjected to PCR for the GBS dltS gene. Serotyping by LAT and PCR methods showed 100% concordance.
Data analysis.
Data were analyzed using STATA 13.1 (StataCorp LP, College Station, TX) to estimate rates (with 95% CIs) of maternal colonization and vertical transmission.
Ethical standards.
This study was reviewed and approved by the institutional review board of the Bangladesh Institute of Child Health (Dhaka, Bangladesh). Verbal informed consent was obtained from pregnant women for their participation and their newborn's participation in the study.
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of Tahmina Rahman and other research staff members based at Kumudini Women's Medical College Hospital and in the laboratories.
Centers for Disease Control and Prevention (prime donor USAID) provided financial support to implement the study (grant 5U01CI000628-03).
S.K.S. has received grants from GlaxoSmithKline (GSK), Pfizer, WHO, CDC, and the Bill and Melinda Gates Foundation for studies unrelated to the submitted work. G.L.D. is on the GSK-Save the Children R&D Advisory Board. All other authors declare no conflicts of interest.
REFERENCES
- 1.United Nations Children's Fund. 2015. Levels and trends in child mortality report 2015. United Nations Children's Fund, New York, NY. [Google Scholar]
- 2.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 3.Zea-Vera A, Ochoa TJ. 2015. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr 61:1–13. doi: 10.1093/tropej/fmu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, Robinson MJ, Collinson A, Heath PT. 2011. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed 96:F9–F14. doi: 10.1136/adc.2009.178798. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID, Hale EC, Shankaran S, Kennedy K, Carlo WA, Watterberg KL, Bell EF, Walsh MC, Schibler K, Laptook AR, Shane AL, Schrag SJ, Das A, Higgins RD. 2011. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics 127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigaúque B, Roca A, Mandomando I, Morais L, Quintó L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardají A, Nhalungo D, Soriano-Gabarró M, Flannery B, Menendez C, Levine MM, Alonso PL. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 7.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MPE, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JAG. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 8.Gray KJ, Bennett SL, French N, Phiri AJ, Graham SM. 2007. Invasive group B streptococcal infection in infants, Malawi. Emerg Infect Dis 13:223–229. doi: 10.3201/eid1302.060680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, Heath PT. 2012. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 379:547–556. doi: 10.1016/S0140-6736(11)61651-6. [DOI] [PubMed] [Google Scholar]
- 10.Dagnew AF, Cunnington MC, Dube Q, Edwards MS, French N, Heyderman RS, Madhi SA, Slobod K, Clemens SAC. 2012. Variation in reported neonatal group B streptococcal disease incidence in developing countries. Clin Infect Dis 55:91–102. doi: 10.1093/cid/cis395. [DOI] [PubMed] [Google Scholar]
- 11.Rivera L, Sáez-Llorens X, Feris-Iglesias J, Ip M, Saha S, Adrian PV, Madhi SA, Boudville IC, Cunnington MC, Casellas JM, Slobod KS. 2015. Incidence and serotype distribution of invasive group B streptococcal disease in young infants: a multi-country observational study. BMC Pediatr 15:143. doi: 10.1186/s12887-015-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darmstadt GL, Saha SK, Choi Y, El Arifeen S, Ahmed NU, Bari S, Rahman SM, Mannan I, Crook D, Fatima K, Winch PJ, Seraji HR, Begum N, Rahman R, Islam M, Rahman A, Black RE, Santosham M, Sacks E, Baqui AH. 2009. Population-based incidence and etiology of community-acquired neonatal bacteremia in Mirzapur, Bangladesh: an observational study. J Infect Dis 200:906–915. doi: 10.1086/605473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu B, Li D, Cui Y, Sui W, Huang L, Lu X. 2014. Epidemiology of group B streptococcus isolated from pregnant women in Beijing, China. Clin Microbiol Infect 20:O370–O373. doi: 10.1111/1469-0691.12416. [DOI] [PubMed] [Google Scholar]
- 14.Chan G, Modak J, Mahmud A, Baqui A, Black R, Saha S. 2013. Maternal and neonatal colonization in Bangladesh: prevalences, etiologies and risk factors. J Perinatol 33:971–976. doi: 10.1038/jp.2013.99. [DOI] [PubMed] [Google Scholar]
- 15.Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA. 2016. Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis 16:1076–1084. doi: 10.1016/S1473-3099(16)30055-X. [DOI] [PubMed] [Google Scholar]
- 16.Narava S, Rajaram G, Ramadevi A, Prakash G, Mackenzie S. 2014. Prevention of perinatal group B streptococcal infections: a review with an Indian perspective. Indian J Med Microbiol 32:6–12. doi: 10.4103/0255-0857.124286. [DOI] [PubMed] [Google Scholar]
- 17.Dechen TC, Sumit K, Ranabir P. 2010. Correlates of vaginal colonization with group B streptococci among pregnant women. J Global Infect Dis 2:236–241. doi: 10.4103/0974-777X.68536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharmila V, Joseph NM, Babu TA, Chaturvedula L, Sistla S. 2011. Genital tract group B streptococcal colonization in pregnant women: a South Indian perspective. J Infect Dev Ctries 5:592–595. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary M, Rench MA, Baker CJ, Singh P, Hans C, Edwards MS. 2016. Group B streptococcal colonization among pregnant women in Delhi, India. Pediatr Infect Dis J doi: 10.1097/inf.0000000000001514. [DOI] [PubMed] [Google Scholar]
- 20.Mavenyengwa RT, Afset JE, Schei B, Berg S, Caspersen T, Bergseng H, Moyo SR. 2010. Group B Streptococcus colonization during pregnancy and maternal-fetal transmission in Zimbabwe. Acta Obstet Gynecol Scand 89:250–255. doi: 10.3109/00016340903398029. [DOI] [PubMed] [Google Scholar]
- 21.Sinha A, Russell LB, Tomczyk S, Verani JR, Schrag SJ, Berkley JA, Mohammed M, Sigauque B, Kim S-Y. 2016. Disease burden of group B streptococcus among infants in sub-Saharan Africa: a systematic literature review and meta-analysis. Pediatr Infect Dis J 35:933–942. doi: 10.1097/INF.0000000000001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, Mohle-Boetani J, Gershman K, Schaffner W, Petit S, Zansky SM, Morin CA, Spina NL, Wymore K, Harrison LH, Shutt KA, Bareta J, Bulens SN, Zell ER, Schuchat A, Schrag SJ. 2009. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 360:2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 23.Beal S, Dancer S. 2006. Antenatal prevention of neonatal group B streptococcal infection. Rev Gynaecol Perinatal Pract 6:218–225. doi: 10.1016/j.rigapp.2006.05.006. [DOI] [Google Scholar]
- 24.Berardi A, Rossi C, Creti R, China M, Gherardi G, Venturelli C, Rumpianesi F, Ferrari F. 2013. Group B streptococcal colonization in 160 mother-baby pairs: a prospective cohort study. J Pediatr 163:1099–1104.e1091. doi: 10.1016/j.jpeds.2013.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Islam MS, Saha SK, Islam M, Modak JK, Shah R, Talukder RR, El Arifeen S, Baqui AH, Darmstadt GL, Mullany LC. 2016. Prevalence, serotype distribution, and mortality risk associated with group B streptococcus colonization of newborns in rural Bangladesh. Pediatr Infect Dis J 35:1309–1312. doi: 10.1097/INF.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 26.Madhi SA, Radebe K, Crewe-Brown H, Frasch CE, Arakere G, Mokhachane M, Kimura A. 2003. High burden of invasive Streptococcus agalactiae disease in South African infants. Ann Trop Paediatr 23:15–23. doi: 10.1179/000349803125002814. [DOI] [PubMed] [Google Scholar]
- 27.Weisner AM, Johnson AP, Lamagni TL, Arnold E, Warner M, Heath PT, Efstratiou A. 2004. Characterization of group B streptococci recovered from infants with invasive disease in England and Wales. Clin Infect Dis 38:1203–1208. doi: 10.1086/382881. [DOI] [PubMed] [Google Scholar]
- 28.Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 29.National Institute of Population Research and Training, Mitra and Associates, ICF International. 2016. Bangladesh demographic and health survey 2014. ICF International, Rockville, MD. [Google Scholar]
- 30.Verani JR, McGee L, Schrag SJ. 2010. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. Centers for Disease Control and Prevention, Atlanta, GA. [PubMed] [Google Scholar]
- 31.Wilkinson HW. 1977. CAMP-disk test for presumptive identification of group B streptococci. J Clin Microbiol 6:42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerrero C, Martinez J, Menasalvas A, Blazquez R, Rodriguez T, Segovia M. 2004. Use of direct latex agglutination testing of selective broth in the detection of group B streptococcal carriage in pregnant women. Eur J Clin Microbiol Infect Dis 23:61–62. doi: 10.1007/s10096-003-1052-x. [DOI] [PubMed] [Google Scholar]
- 33.Slotved H-C, Kaltoft M, Skovsted I, Kerrn M, Espersen F. 2004. Simple, rapid latex agglutination test for serotyping of pneumococci (Pneumotest-Latex). J Clin Microbiol 42:2518–2522. doi: 10.1128/JCM.42.6.2518-2522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poyart C, Tazi A, Réglier-Poupet H, Billoët A, Tavares N, Raymond J, Trieu-Cuot P. 2007. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J Clin Microbiol 45:1985–1988. doi: 10.1128/JCM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]