ABSTRACT
In early Lyme disease (LD), serologic testing is insensitive and seroreactivity may reflect active or past infection. In this study, we evaluated a novel assay for the direct detection of three species of Borrelia spirochetes in whole blood. The T2 magnetic resonance (T2MR) assay platform was used to amplify Borrelia DNA released from intact spirochetes and to detect amplicon. Analytical sensitivity was determined from blood spiked with known concentrations of spirochetes, and the assay's limit of detection was found to be in the single-cell-per-milliliter range: 5 cells/ml for B. afzelii and 8 cells/ml for Borrelia burgdorferi and Borrelia garinii. Clinical samples (n = 66) from confirmed or suspected early LD patients were also analyzed. B. burgdorferi was detected using T2MR in 2/2 (100%) of blood samples from patients with confirmed early LD, based on the presence of erythema migrans and documentation of seroconversion or a positive real-time blood PCR. T2MR detected B. burgdorferi in blood samples from 17/54 (31%) of patients with probable LD, based on the presence of erythema migrans without documented seroconversion or of documented seroconversion in patients with a compatible clinical syndrome but without erythema migrans. Out of 21 clinical samples tested by real-time PCR, only 1 was positive and 13 were negative with agreement with T2MR. An additional 7 samples that were negative by real-time PCR were positive with T2MR. Therefore, T2MR enables a low limit of detection (LoD) for Borrelia spp. in whole blood samples and is able to detect B. burgdorferi in clinical samples.
KEYWORDS: Lyme disease, diagnostics
INTRODUCTION
Lyme disease (LD) is a tick-borne infection caused by pathogenic spirochetes of the Borrelia burgdorferi sensu lato genospecies. B. burgdorferi sensu stricto has been identified as the main causative agent in North America, accounting for 20,000 to 30,000 confirmed cases annually reported to the Centers for Disease Control (CDC) in the United States (http://www.cdc.gov/lyme/stats/chartstables/casesbyyear.html). However, estimates indicate that there may be closer to 300,000 new cases yearly (1–3). In addition to B. burgdorferi sensu stricto, LD in Europe is also caused by B. afzelii and B. garinii, with an estimated 60,000 to 90,000 cases per year (4). Other Borrelia species can cause LD, including, but not limited to, B. bavariensis, B. bisettii, and B. lusitainiae, although these species represent a minority of cases (5–7) Recently, B. mayonii was discovered to be a distinct species from B. burgdorferi causing LD in the upper Midwest of the United States and may represent an emerging causative agent of LD in the United States (8). Early LD is variably characterized by the presence of an erythema migrans (EM) rash and flu-like symptoms, including fever, fatigue, and myalgia (9, 10). If left untreated, later-stage manifestations, such as arthritis, carditis, or neuroborreliosis, may develop (10), underscoring the necessity for early detection and treatment.
Early LD is diagnosed in the clinic by recognition of the EM rash, other associated symptoms, and a compatible exposure history (11, 12). However, the diagnosis can be difficult, as 5 to 30% of patients do not present with an EM rash (10, 12) and early-stage symptoms may be completely lacking or nonspecific (13). Laboratory confirmation of clinical diagnosis is performed using serology. In the United States, the CDC currently recommends a two-tiered approach in which a positive or equivocal enzyme immunoassay (EIA) is followed by a Western immunoblot (WB) (14). However, the interpretation of serologic tests can be subjective, and high variability in test results has been reported between laboratories (15, 16). Because multiple Borrelia species cause LD in Europe, geographic regions use different species for antigen preparations, which can complicate the diagnostic approach for patients that have recently traveled (17). In addition, the antibodies elicited by LD-related spirochetes have been found to persist long after infection and treatment, preventing the deconvolution of a present versus past infection (18). Commercial U.S. laboratories performed approximately 3.4 million LD diagnostic tests in 2014, most of them serologic, and yet only 5.8% of two-tiered serologic tests in regions where LD is endemic were positive (1). One potential explanation for this low positivity rate is that serologic testing is performed too early in the disease progression in many cases. Since IgM and IgG antibody titers take time to reach detectable levels, 1 to 2 weeks and 4 to 6 weeks, respectively, there exists a window in which diagnosis of early LD is not possible using the two-tiered method (14).
While directly detecting Borrelia spirochetes would theoretically enable diagnosis of LD within this window, no direct detection methods are currently recommended by the CDC or approved by the FDA. Culture of clinical samples, most commonly performed using skin biopsy specimens from EM lesions, can require several weeks for positive results due to the low growth rate of Borrelia spp. (9), thus limiting its clinical utility. Moreover, few clinical laboratories offer this service, which would be impractical to perform on a large scale. PCR has been used to detect spirochetes in skin biopsy specimens, cerebrospinal fluid (CSF), synovial fluid, and blood (9, 10). PCR, however, is not widely used for LD detection due to its low sensitivity. While estimates of sensitivity vary between the various nonstandardized PCR methods, the averages are 69% for skin biopsy of EM lesions, 38% for CSF samples in neuroborreliosis, 78% for synovial fluid in Lyme arthritis, and 14% for blood in early LD (9). Interrogation of small sample volumes can lead to reduced sensitivity if the concentration of spirochetes in the sample volume is low. Variations of PCR methodologies have demonstrated improved sensitivity, including the use of nested PCR (19, 20). New detection systems, such as electrospray ionization mass spectrometry, have also been shown to improve sensitivity compared with standard PCR (21). However, false-positive results can be a problem for PCR, in particular nested PCR, as the amplified product can contaminate successive reactions.
T2 magnetic resonance (T2MR) has been used for the rapid detection of Candida spp. in whole blood collected from patients with yeast fungemia (22, 23). Unlike for standard PCR methods, target DNA is amplified directly in whole blood without the use of DNA extraction and purification techniques. Superparamagnetic particles with conjugated probe sequences bind the target DNA, and the presence of the target DNA is identified using a T2MR reader. Target DNA is detected with high specificity even in the presence of a large background of human DNA in blood samples. T2MR has demonstrated a limit of detection (LoD) of 1 to 3 CFU/ml for Candida spp. in whole blood. In this study, we applied the T2MR method to the detection of Borrelia spirochetes in whole blood as a framework for the detection of early-stage LD.
RESULTS
Borrelia detection assay.
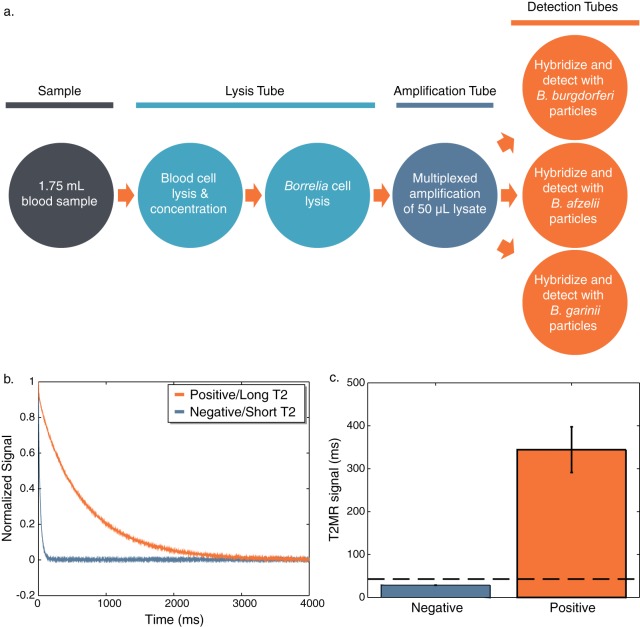
Figure 1a outlines the method developed to detect Borrelia cells in whole blood, which is similar to a previous method for the detection of Candida spp. (22). Blood cells in a 1.75-ml whole-blood sample were lysed with a detergent-based lysis buffer prior to concentration of Borrelia cells by centrifugation. After the pellet was washed, Borrelia cells were disrupted by bead beating and a single multiplex amplification reaction was used to amplify a conserved gene for each species. The amplicon was then divided into three separate detection tubes, and a single-particle suspension directed against one of the three species was added to each tube. Figure 1b shows the relaxation curves for a negative blood sample and a positive blood sample spiked with 20 cells/ml of B. burgdorferi as detected with particles directed against B. burgdorferi. An exponential fit of relaxation curves such as these provides the T2 signal [normalized signal = e−(t/T2)] (24). Figure 1c shows the T2 signal for a series of 8 negatives and 8 positives. A cutoff of 45 ms, which is >5 standard deviations above the baseline signal, is indicated by a dashed line. T2MR analysis is qualitative, not quantitative, and samples with T2 signals above this cutoff are considered positive.
FIG 1.
T2MR Borrelia detection assay and analysis of results. (a) Samples are minimally processed as depicted. The amplicon is added to three separate detection tubes and hybridized to particles directed against a particular species. (b) Relaxation curves are obtained from the T2MR reader, and the curves are fit with an exponential decay to find the T2MR signal. (c) A set of eight negative and eight positives spiked with 20 cells/ml of B. burgdorferi were detected with B. burgdorferi particles (error bars = 1 SD). The cutoff for the determination of a positive sample (45 ms) is indicated by a dashed line.
Spiked-sample analysis.
Analytic sensitivity was determined by spiking K2EDTA-anticoagulated whole blood with cultured Borrelia cells at concentrations of 5 to 100 cells/ml. We defined the limit of detection (LoD) using a conservative positivity rate method; the lowest concentration that had a ≥95% positivity rate for ≥20 replicates across multiple batches and days was determined to be the LoD. T2MR signals for the spiked samples were ∼100 times greater than for the negative samples. The LoD for both B. burgdorferi and B. garinii was 8 cells/ml, while for B. afzelii the LoD was 5 cells/ml (Table 1). Negative samples were run with every assay to identify if contamination occurred. Over a 2-month period with 46 experiments run by 3 operators, 0/204 negatives were false positives (+2.3% upper 95% confidence interval by modified Wald method [25]). No incidence of cross-reactivity was observed for any of the species (see Table S1 in the supplemental material), and freezing was not shown to negatively affect the samples (Table S2).
TABLE 1.
Analytical sensitivity of whole blood spiked with Borreliaa
| Target | Concn (cells/ml) | No. positive/total no. of samples | Positivity rate (%) | Avg T2MR signal (ms) | SD T2MR signal (ms) | CV (%) |
|---|---|---|---|---|---|---|
| B. burgdorferi | 10 | 116/120 | 97 | 339 | 116 | 34 |
| 8 | 75/78 | 97 | 374 | 159 | 43 | |
| B. afzelii | 10 | 60/60 | 100 | 346 | 130 | 38 |
| 8 | 60/60 | 100 | 313 | 82 | 26 | |
| 5 | 58/60 | 97 | 379 | 56 | 15 | |
| B. garinii | 10 | 60/60 | 100 | 316 | 57 | 18 |
| 8 | 60/60 | 100 | 347 | 34 | 10 | |
| Negatives | 0 | 0/40 | NA | 29 | 2 | 5 |
CV, coefficient of variation; NA, not applicable.
Clinical samples.
Table 2 exhibits the T2MR results and comparison with standard tests for banked frozen whole-blood samples obtained from Marshfield Labs (n = 45) and the Gundersen Clinic (n = 21). These samples were tested by various methods, including real-time PCR of blood (n = 21), whole-cell or C6 enzyme-linked immunosorbent assay (ELISA) (n = 64), and Western blotting (n = 37). A total of 63 samples were subjected to a two-tiered testing protocol (14) and 1 sample to a modified two-tiered algorithm using a first tier of whole-cell sonicate EIA and a second tier of C6 ELISA (26). Eight patients received follow-up serologic testing, and 3 were shown to seroconvert.
TABLE 2.
Categories representing probability of LD
| Category | Description | No. positive/no. tested (%) by: |
||
|---|---|---|---|---|
| T2MR | Real-time PCR | Two-tiered serology | ||
| 1 | Confirmed LD based on seroconversion or PCR result and clinical features | 2/2 (100) | 1/1 (100) | 1/2 (50) |
| 2 | Probable early LD based on (i) EM and clinical syndrome without serologic confirmation or (ii) seroconversion without EM but with compatible clinical syndrome | 17/54 (31) | 0/20 (0) | 17/52 (33) |
| 3 | Probable early LD based on EM and clinical syndrome but antibiotics taken prior to sample collection for T2MR | 0/6 (0) | NAa | 4/6 (67) |
| 4 | Possible early LD based on clinical syndrome, but no EM rash and no seroconversion | 0/2 (0) | NA | 0/2 (0) |
| 5 | Unlikely early LD based on clinical syndrome, time of season, and/or physician decision not to treat | 0/2 (0) | NA | 0/2 (0) |
NA, not applicable.
The samples were divided into categories based upon the probability of LD (Table 2). The first category contains samples from patients with confirmed LD. All patients in this category had a solitary or multiple EM and compatible clinical syndrome. One sample in this category was tested by real-time PCR and determined to be positive for Borrelia ribosomal RNA genes. The second sample in this category demonstrated seroconversion between the original visit and a follow-up, although it was not tested by PCR. Both samples were determined to be positive by T2MR.
The second category contains samples from patients with probable LD. This includes samples from patients with solitary or multiple EM but no serologic confirmation, which can mean either that follow-up was not performed or that seroconversion did not occur. This category also contains samples from patients who did seroconvert and had a compatible clinical syndrome but who lacked an EM rash. T2MR detected B. burgdorferi in 17 of 54 samples in this category (31%). Real-time PCR testing was performed on 20 samples in this category, and 0/20 (0%) were positive by PCR, compared to 7/20 (35%) positive by T2MR. Two-tiered serology was performed on 52 samples in this category and was positive for 17/52 (33%). Agreement between T2MR and two-tiered serology was found for 34/52 (65%) samples. Two samples in this category were determined to be positive for anaplasmosis by PCR; T2MR and two-tiered serology were negative for both.
The third category includes samples from patients with probable LD who had solitary or multiple EM but had started antibiotic therapy prior to collection of the sample for T2MR detection. T2MR was negative for all of these samples (0/6 or 0%); however, two-tiered serology was positive for 4/6 (67%). No real-time PCR testing was performed on these samples. Antibody use is further detailed in Table S3.
In category 4, the probability of LD is reduced; samples in this category were from patients who had a compatible clinical syndrome but did not have an EM rash or evidence of seroconversion. For these samples, neither T2MR nor two-tiered serology (0/2) detected Borrelia. No real-time PCR was performed on these samples.
The fifth and final category includes samples from patients that are unlikely to have had LD, due to lack of a compatible clinical syndrome, the time of season, and/or a physician's decision not to treat for LD. Both T2MR and two-tiered serology in these samples were negative (0/2).
DISCUSSION
Direct detection is a potential pathway for early LD diagnosis where serology results demonstrate poor accuracy. However, currently available direct detection techniques have drawbacks that limit their applicability. It is not possible to produce timely results by using culture of EM lesions or blood. While PCR-based technologies can be performed rapidly for early diagnosis, standard techniques require skilled professionals and cumbersome DNA extraction methods. The clinical sensitivity of PCR differs based on the matrix tested, with synovial fluid, skin biopsy specimen, and CSF being the highest-sensitivity specimen types (9). While whole blood is easy to obtain, the reported average sensitivity for patients with early LD is low, at 14% (9). A large amount of variability exists for reported sensitivity of PCR assays, and this may be due to different targets and methods used for the test. Newer PCR methods for the detection of Borrelia in blood have been shown to improve sensitivity to 38 to 62% (21, 27).
We have developed a robust alternative to PCR methods that can be used to detect Borrelia spp. directly in whole blood, circumventing the necessity of DNA extraction. Results are highly reproducible, with no observable cross-reactivity and no false positives over the 204 negatives in the study, and freezing samples did not negatively affect detection. We report an LoD of 5 to 8 cells/ml in whole blood for the three major species involved in LD in North America, Europe, and Asia. Standard PCR assays indicate LoDs as low as 10 cells/reaction; however, due to interrogation of small sample volumes and necessity of DNA extraction, the LoD in terms of milliliters is >100 cells/ml (28, 29). Recent experiments using electrospray ionization-mass spectrometry have demonstrated LoDs with spiked DNA as low as 0.48 genome copy/ml; however, similar results have not been obtained with spiked cells (21). T2MR analytic sensitivity benefits from the testing of a large sample volume (1.75 ml), and since the sample is directly tested with minimal processing, there is no expectation of loss of nucleic acids as with DNA extraction procedures. Quantitative PCR has suggested that patients with acute LD have 414 to 56,000 spirochetes/ml in the bloodstream (28), and semiquantitative approaches have estimated <20 to >4,000 cell/ml in plasma (30); however, these calculations are subject to the PCR LoD in blood. Our reported analytic sensitivity for T2MR detection falls below 20 cells/ml, though it is possible that spirochetes may circulate at lower concentrations.
The initial test of frozen clinical samples in this work indicated that T2MR can be used to detect spirochetes in the blood of patients with LD. While only a small sample size and not indicative of the sensitivity of this assay, the samples from patients with confirmed early LD were 100% detected by T2MR (2/2 samples [Table 2, category 1]). One of these two samples was positive by real-time PCR, and since agreement exists between this method and T2MR, one can deduce that B. burgdorferi was present in this patient's blood at titers of potentially >100 cells/ml based on the known LoD of the real-time PCR assay used. The second sample within the confirmed category was from a patient who seroconverted, and T2MR classified the early sample as positive before serologic testing could detect antibodies against B. burgdorferi.
The majority of the clinical specimens included in this study were considered to be probable LD samples. For some of the probable samples, patients had taken antibiotics within 1 month prior to collection of samples for T2MR analysis (Table 2, category 3; see also Table S3). As antibiotic therapy affects spirochetes circulating in the bloodstream, it was not expected that samples from patients treated with antibiotics would be detectable by T2MR or any other direct detection method. Therefore, it is not surprising that 0/6 (0%) of these samples were positive by T2MR. In comparison, 4/6 (67%) of these samples were positive by two-tiered serology, and it is expected that serologic tests would take longer to respond to the use of antibiotics.
Of the samples collected from patients with probable LD who were not treated with antibiotics prior to collection, 17/54 (31%) were positive by T2MR (Table 2, category 2). These samples lacked either EM or definitive serologic confirmation of LD through seroconversion, and it is difficult to determine which among these samples represent true positives that were not detected by T2MR. We found 65% agreement between T2MR and serology in this category. In some cases, the spirochetes may have no longer been present in the blood and thus not detectable by T2MR or any direct detection technique. Blood culture of spirochetes has been shown to be positive for samples taken from patients with early LD with a window of bacteremia of 30 days or less (31). Spirochete circulation in the blood may be expected to drop following development of immune responsiveness in the first few weeks of disease, thereby potentially limiting the usage of T2MR detection after that period. As mentioned previously, it is entirely possible that the level of spirochetemia could be below the LoD of T2MR in some cases. However, this study has shown that T2MR is able to detect Borrelia in a larger number of samples than standard PCR, as agreement was found in 1/1 sample positive by real-time PCR and T2MR detected B. burgdorferi in an additional 7 samples which were negative by real-time PCR (out of 21 total samples tested by real-time PCR methods). The disagreement between serology and T2MR is also due to T2MR detecting B. burgdorferi in 9 samples that were negative by two-tiered serology, highlighting that T2MR may detect LD causing spirochetes prior to an immune response.
None of the clinical samples collected from patients who were less likely to have LD, due to lack of an EM rash or lack of epidemiologic risk factors, were positive by T2MR (Table 2, categories 4 and 5). Total agreement was found between two-tiered serology and T2MR.
This initial study of the application of T2MR to the detection of Borrelia spp. is not without limitations. Sample-to-sample variability may influence the analytical sensitivity results, although we designed experiments in a manner so as to limit the influence. Lot-to-lot variation was apparent in donated negative blood as determined by complete blood count (CBC), although the effects of variable hematocrit and cell composition on the Borrelia assay were not observed or thoroughly characterized at this point. LoDs were determined over several lots of donated blood, with similar positivity rates across lots. The assay itself is not fully automated as presented in this study, and variability can be introduced by the operator. The reproducibility of our automated cell counting method was studied over multiple glycerol stocks and cultures and compared to manual counts with a hemocytometer. The clinical samples tested in this study have limitations as well. Some samples were from incompletely characterized patients, and not all samples were subjected to the same testing methods. Few samples were from patients that had follow-up testing in convalescence, thus making the determination of seroconversion and the basis of confirmation of LD difficult. In the future, different sample matrices may be used in this type of method, and while 1.75 ml is not large for blood, it may be difficult to obtain for matrices like cerebrospinal fluid. Smaller sample volumes may be used in this assay; however, the sample volume used in this study was driven by the desired analytic sensitivity. A concomitant rise in LoD would be expected for smaller sample volumes.
In conclusion, we have demonstrated a method to detect Borrelia spp. in whole-blood samples collected from patients with early LD. Low limits of detection were observed for spiked samples, and encouraging results were obtained for clinical remainder samples. The T2MR assay represents an opportunity for rapid and robust detection of early LD.
MATERIALS AND METHODS
Sample preparation.
Whole-blood samples spiked with cultured B. burgdorferi sensu lato isolates were prepared for the determination of analytical sensitivity and specificity of the T2MR Borrelia detection assay. B. burgdorferi (ATCC 35210), B. afzelii (ATCC 51992), and B. garinii (ATCC 51991) were obtained from the ATCC as glycerol stocks and were initially grown in 150 ml of BSK-H medium (Sigma), using a BD GasPak anaerobe growing system in a 34°C incubator. Cell morphology and culture quality were evaluated using a Nikon Eclipse Ci microscope in dark-field mode. Cells were stained with 1× SYBR green (Thermo Fisher Scientific) and counted on a Cellometer X2 cytometer (Nexcelom Bioscience). Glycerol stocks with 15% glycerol were prepared and frozen at −80°C. A second growth was performed to prepare samples for spiking by inoculating 13 ml of BSK-H media with 0.5 ml of initial glycerol stock and growing to 5e6 to 1e7 cells/ml prior to dilution 100× to 1,000× in BSK-H and subculture. Glycerol stocks prepared with 15% glycerol were frozen at −80°C and set aside for cell spiking. The typical duration of culture incubation ranged from 3 to 5 days. For each spiked sample, a glycerol stock was diluted 1:10 in phosphate-buffered saline (PBS) and a cell count was obtained with the Cellometer X2. Additional serial dilutions were performed such that the spirochetes could be added as a 1% addition to human whole blood from healthy donors (e.g., 300-μl spike in 30 ml of blood). All samples were tested on the same day as spiking. Negatives were prepared from matching lots of donor blood.
T2MR detection.
Whole-blood samples (1.75 ml) were lysed with a detergent-based lysis buffer, and centrifugation was used to concentrate target cells. The supernatant was discarded and the tube walls and pellet were rinsed with 150 μl of Tris-EDTA buffer (TE), pH 8.0, containing 4 copies/μl of the inhibition control (IC). This buffer was removed after centrifugation, and 100 μl of the IC-containing TE was added prior to bead beating. The lysate (50 μl) was mixed with a total of 50 μl of an amplification master mix and a proprietary formulation of a whole-blood-compatible thermophilic DNA polymerase (T2 Biosystems). The master mix contained primers directed toward a single conserved gene residing on a required plasmid for each species and the IC sequence. The genes targeted were as follows: an outer membrane protein (OMP) gene on linear plasmid lp54 for B. burgdorferi, a phosphotransferase system (PTS) gene on circular plasmid cp26 for B. afzelii, and the oligopeptide permease OppA gene on lp54 for B. garinii. A Mastercycler Pro-S (Eppendorf) thermocycler was used for denaturing (a total of 15 min at 95°C) and 46 cycles consisting of 20 s at 95°C, 30 s at 58°C, and 30 s at 68°C, with a final extension at 68°C for 10 min. Asymmetric DNA amplification was used to preferentially generate a single-stranded amplicon from the Borrelia spp. or IC.
Carboxylated superparamagnetic particles (T2 Biosystems) were functionalized with oligonucleotide capture probes and characterized as previously described (22). The performance of the probes was verified in TE buffer with 2.5 ng/μl of salmon sperm carrier DNA using dilutions of a control oligonucleotide bearing the sequence of the amplified Borrelia gene. Agglomeration of the particles was induced by hybridization between the particles and the single-stranded DNA amplicon at 62°C. Particles that clustered in the presence of the target sequence exhibited high T2MR signals. The baseline signal of nonclustered particles had T2 values of 29 to 34 ms by titration with a sodium phosphate hybridization buffer (SSPE).
T2MR analysis was performed in a highly automated manner. After amplification, samples were mixed with particle suspensions and hybridized at 62°C for 30 min while being vortexed at 140 rpm (VorTemp; Labnet International). Samples were then transferred to a high-throughput robot (Epson), which vortexed each tube for 10 s, incubated the tubes at 37°C for 50 s, and placed each tube in the T2MR reader for 8 s. The T2MR signal of each sample was obtained using a CPMG pulse sequence. For analysis purposes, samples with a T2MR signal of <45 ms were considered negative and samples with a T2MR signal of ≥45 ms were considered positive. This cutoff was set >5 standard deviations above the T2MR signal of the blank samples, as determined from analysis of negative samples. Both signals for target and inhibition control were measured, and samples in which the inhibition control was <85 ms were considered to be invalid.
Contamination controls are a necessary component of any highly sensitive molecular diagnostic assay, and the contamination control methods for this assay were designed as part of the technology development for T2Candida. For manual experiments, all sample processing is performed in laminar flow environments and pre- and postamplification laboratory areas are segregated. Once automated, this workflow uses the contamination control methods on the T2Dx instrument, namely, dedicated disposables, a walled-off laminar airflow environment from all other samples, and automation compatible tube caps. Specificity as high as 99.9% was achieved using these contamination controls for a different automated assay on the T2Dx (32).
Blood samples from patients with suspected LD.
Remainder EDTA whole-blood specimens were collected from patients that were tested for tick-borne diseases at the Marshfield Clinic (Marshfield, WI) from 2011 to 2013 and stored at −80°C according to protocols approved by the Marshfield Clinic Research Foundation Institutional Review Board (IRB). Patient electronic medical records were screened for eligibility using the following inclusion criteria: age of ≥18 years with illness onset within 30 days of specimen collection and documented evidence of single or multiple erythema migrans (EM) lesions. Descriptions of EM were adjudicated by a board-certified dermatologist. Pregnant or immunosuppressed patients or patients with active connective tissue disease were excluded from the study.
Blood from suspected LD patients was collected in EDTA purple-top tubes at the Gundersen Clinic (La Crosse, WI) and stored at 4°C according to protocols approved by the Gundersen Health System IRB prior to serologic testing. Samples were stored at −80°C until testing by real-time PCR or T2MR.
Serologic testing.
Laboratory diagnosis for LD was performed according to the CDC-recommended two-tiered serologic testing protocol (n = 63) or a modified two-tiered protocol consisting of a first-tier whole-cell sonicate EIA and a second-tier C6 ELISA (n = 1) (14, 26). Samples for serologic testing were collected at the same time as the whole-blood samples studied in this work. Vidas Lyme IgG and IgM whole-cell lysate EIA (bioMérieux), C6 Lyme ELISA (Immunetics), and IgG and IgM ViraStrip immunoblot (ViraLab) tests were used in this study.
Real-time PCR.
DNA from blood samples was recovered by using a QIAamp DNA blood minikit (Qiagen). Briefly, 1 ml of whole blood was centrifuged at 2,500 × g for 10 min. Following centrifugation, 200 μl of red blood cells (RBCs) and buffy coat was combined with 20 μl of protease, 200 μl of buffer AL, and 5 μl of an exogenous DNA control. Samples were then processed as recommended by the manufacturer.
The DNA was detected by a real-time PCR that used primers Bb23Sf (5′-CGAGTCTTAAAAGGGCGATTTAGT-3′) and Bb23Sr (5′-GCTTCAGCCTGGCCATAAATAG-3′) and probe Bb23Sp-FAM (5′-6-carboxyfluorescein [FAM]-AGATGTGGTAGACCCGAAGCCGAGTG-BHQ1a-FAM-3′) (29). Five microliters of extracted DNA was combined with 20 μl of master mix that contained 12.5 μl of B buffer mix (10× AmpliTaq Gold buffer, 2.5 mM MgCl2, deoxynucleoside triphosphates [dNTPs], 4.5 μl of Bb23S primer-probe mix (20 μM), 2.5 μl of exogenous control primers and probe, and 0.5 μl of AmpliTaq Gold DNA polymerase (1.5 U). The DNA was then amplified using an Mx3000P real-time thermal cycler (Stratagene) under the following conditions: 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s, 60°C for 1 min, and 72°C for 30 s, and 1 cycle at 25°C for 5 s.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by T2 Biosystems, of which J.L.S., H.G., C.B.-G., J.T., B.E.J., R.S., and T.J.L. are employees. J.A.B. is a consultant for T2 Biosystems, and salary support was provided to A.M.S.
We thank the T2 Biosystems team for their efforts and L. A. Neely and H. U. Thomann for their discussions and support in assay design. We also thank P. Mundt and L. Larson of the Marshfield Clinic for assistance with patient chart reviews.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00510-17.
REFERENCES
- 1.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 29:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehn BM. 2013. CDC estimates 300,000 US cases of Lyme disease annually. JAMA 310:1110. doi: 10.1001/jama.2013.278331. [DOI] [PubMed] [Google Scholar]
- 4.Hubálek Z. 2009. Epidemiology of Lyme borreliosis. Curr Probl Dermatol 37:31–50. doi: 10.1159/000213069. [DOI] [PubMed] [Google Scholar]
- 5.Markowicz M, Ladstatter S, Schotta AM, Reiter M, Pomberger G, Stanek G. 2015. Oligoarthritis cause by Borrelia bavariensis, Austria, 2014. Emerg Infect Dis 21:1052–1054. doi: 10.3201/eid2106.141516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golovchenko M, Vancova M, Clark K, Oliver JH, Grubhoffer L, Rudenko N. 2016. A divergent spirochete strain isolated from a resident of the southeastern United States was identified by multilocus sequence typing as Borrelia bissettii. Parasit Vectors 9:68. doi: 10.1186/s13071-016-1353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collares-Pereira M, Couceiro S, Franca I, Kurtenbach K, Schafer SM, Vitorino L, Goncalves L, Baptista S, Vieira ML, Cunha C. 2004. First isolation of Borrelia lusitaniae from a human patient. J Clin Microbiol 42:1316–1318. doi: 10.1128/JCM.42.3.1316-1318.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritt BS, Mead PS, Hoang Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schiefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry L, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM. 2016. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis 16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borchers AT, Keen CL, Huntley AC, Gershwin ME. 2015. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun 57:82–115. doi: 10.1016/j.jaut.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 12.Bratton RL, Whiteside JW, Hovan MJ, Engle RL, Edwards FD. 2008. Diagnosis and treatment of Lyme disease. Mayo Clin Proc 83:556–571. doi: 10.4065/83.5.566. [DOI] [PubMed] [Google Scholar]
- 13.Wright WF, Riedel DJ, Talwani R, Gilliam BL. 2012. Diagnosis and management of Lyme disease. Am Fam Physician 85:1086–1093. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 15.Bakken LL, Callister SM, Wand PJ, Schell RF. 1997. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists Proficiency Testing Program. J Clin Microbiol 35:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binnicker MJ, Jespersen DJ, Harring JA, Rollins LO, Bryant SC, Beito EM. 2008. Evaluation of two commercial systems for automated processing, reading, and interpretation of Lyme borreliosis Western blots. J Clin Microbial 46:2216–2221. doi: 10.1128/JCM.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson J, Guy E, Andrews N, Wilske B, Anda P, Granstrom M, Hauser U, Moosmann Y, Sambri V, Schellekens J, Stanek G, Gray JA. 2000. European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J Clin Microbiol 38:2097–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilton E, DeVoti J, Benach JL, Halluska ML, White DJ, Paxton H, Dumler S. 1999. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 106:404–409. doi: 10.1016/S0002-9343(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Vigliotti VS, Vigliotti JS, Jones W, Pappu S. 2010. Increased sensitivity and specificity of Borrelia burgdorferi 16S ribosomal DNA detection. Am J Clin Pathol 133:569–576. doi: 10.1309/AJCPI72YAXRHYHEE. [DOI] [PubMed] [Google Scholar]
- 20.Priem S, Rittig MG, Kamradt T, Burmester GR, Krause A. 1997. An optimized PCR leads to rapid and highly sensitive detection of Borrelia burgdorferi in patients with Lyme borreliosis. J Clin Microbiol 35:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN. 2012. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neely LA, Audeh M, Phung NA, Min M, Suchocki A, Plourde D, Blanco M, Demas V, Skewis LR, Anagnostou T, Coleman JJ, Wellman P, Mylonakis E, Lowery TJ. 2013. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci Transl Med 5:182ra54. doi: 10.1126/scitranslmed.3005377. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller MA, Wolk DM, Lowery TJ. 2016. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol 11:103–117. doi: 10.2217/fmb.15.111. [DOI] [PubMed] [Google Scholar]
- 24.Lowery T. 2009. Nanomaterials-based magnetic relaxation switch biosensors. Nanotechnologies for the life sciences. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim, Germany. [Google Scholar]
- 25.Agresti A, Coull BA. 1998. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52:119–126. [Google Scholar]
- 26.Branda JA, Strle F, Strle K, Sikand N, Ferraro MJ, Steere AC. 2013. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin Infect Dis 57:333–340. doi: 10.1093/cid/cit235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liveris D, Schwartz I, McKenna D, Nowakowski J, Nadelman RB, DeMarco J, Iyer R, Cox ME, Holmgren D, Wormser GP. 2012. Quantitation of cell associated borrelial DNA in the blood of Lyme disease patients with erythema migrans. Eur J Clin Microbiol Infect Dis 31:791–795. doi: 10.1007/s10096-011-1376-x. [DOI] [PubMed] [Google Scholar]
- 28.Liveris D, Schwartz I, McKenna D, Nowakowski J, Nadelman R, DeMarco J, Iyer R, Bittker S, Cooper D, Holmgren D, Wormser GP. 2012. Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagn Microbiol Infect Dis 73:243–245. doi: 10.1016/j.diagmicrobio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. 2004. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol 42:3164–3168. doi: 10.1128/JCM.42.7.3164-3168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman JL, Bradley JF, Ross AE, Gollner P, Lagus A, Vitale B, Berger BW, Luger S, Johnson RC. 1995. Bloodstream invasion in early Lyme disease: results from a prospective, controlled, blinded study using polymerase chain reaction. Am J Med 99:6–12. doi: 10.1016/S0002-9343(99)80097-7. [DOI] [PubMed] [Google Scholar]
- 31.Nowakowski J, McKenna D, Nadelman RB, Bittker S, Cooper D, Pavia C, Holmgren D, Visintainer P, Wormser GP. 2009. Blood cultures for patients with extracutaneous manifestations of Lyme disease in the United States. Clin Infect Dis 49:1733–1735. doi: 10.1086/648076. [DOI] [PubMed] [Google Scholar]
- 32.Mylonakis E, Clancy CJ, Ostroski-Zeichner L, Garey KW, Alangaden GJ, Vazquez JA, Groeger JS, Judson MA, Vinagre Y-M, Heard SO, Zervou FN, Zacharioudakis IM, Kontoyiannis DP, Pappas PG. 2015. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 60:892–899. doi: 10.1093/cid/ciu959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.