ABSTRACT
South Africa is a country with a high incidence of tuberculosis (TB), complicated by coinfection with human immunodeficiency virus (HIV). The Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) is used in South Africa as the test for the initial diagnosis of TB, and other molecular platforms such as the m2000 (Abbott Molecular, Des Plaines, IL, USA) are widely used for molecular monitoring of HIV load. The latter platform is now also equipped with the RealTime (RT) MTB and RealTime MTB RIF/INH assays for TB and first-line drug resistance screening but has not been evaluated in settings of HIV and TB coinfection. A prospective clinical validation study was conducted at a community health center in Johannesburg, South Africa, and consenting individuals with presumptive pulmonary TB were enrolled. The performance of the Abbott assays was compared with those of the Xpert MTB/RIF, liquid culture, drug susceptibility testing, and clinical case definitions. A statistical analysis was performed on 206 individuals (73% were HIV positive). The sensitivity and specificity of the RT MTB were 82.5% (confidence interval [CI], 67.2 to 92.7) and 93.1% (CI, 86.2 to 97.2) on raw sputum and 77.5% (CI, 61.5 to 89.2) and 95.1% (CI, 88.9 to 98.4) on concentrated sputum, respectively, compared with those from liquid culture. The RT MTB correctly identified 17/35 more smear-negative culture-positive specimens than the Xpert MTB/RIF. Both the RT MTB and the Xpert MTB/RIF displayed sensitivities >70% and specificities >90% in HIV-positive individuals. The available drug resistance results concurred with MTBDRplus and drug susceptibility profiles. The RT MTB assay has similar diagnostic performance to the Xpert MTB/RIF and is suited to testing presumptive TB patients coinfected with HIV. The existing laboratory information system connectivity, training, and technical support make this a viable polyvalent option to scale up TB alongside HIV laboratory testing services in South Africa.
KEYWORDS: Abbott RealTime MTB, South Africa, diagnostics, human immunodeficiency virus, tuberculosis
INTRODUCTION
South Africa is a country with a high incidence of tuberculosis (TB) (834/100,000 [range, 539 to 1190 per 100,000]), multidrug-resistant TB (MDR-TB) (37/100,000), and 57% TB/HIV coinfection (1). To meet the End TB goals (2) and break the chain of transmission, particularly among those harboring MDR-TB (3), optimal diagnosis and treatment of all infected individuals is paramount (4). The role molecular assays play in optimizing diagnosis has been dramatic within the last 5 years, with the Xpert MTB/RIF (Cepheid, Sunnyvale, California, USA) and the Genotype MTBDRplus (Hain LifeScience, Nehren, Germany) assays at the forefront. The former is not only applicable to the diagnosis of pulmonary TB (including resistance to rifampin [RIF]), but also in the diagnosis of childhood TB and extrapulmonary TB (EPTB) (5). The MTBDRplus is recommended for the detection of RIF and isoniazid (INH) in smear-positive specimens and mycobacterial isolates (6). The MTBDRsl has also recently been recommended for the diagnosis of extensively drug-resistant TB (XDR-TB) (7).
However, gaps exist, in that earlier (more sensitive) diagnosis is still needed, universal access to drug susceptibility testing (DST) is lacking, diagnostics to accelerate active case finding are needed, and all are required in the context of HIV coinfection (8). The solutions to allow the integration of TB and HIV testing services are being addressed through the development pipeline (9) and through polyvalent platforms, which enable the testing of different assays on a single platform, either concurrently or consecutively. Among those in development are the Xpert Omni (Cepheid) (currently undergoing clinical trials [ClinicalTrials.gov no. NCT03044158]) and the Xpert MTB/RIF Ultra (Cepheid) (10); however, both will still lack the ability to identify INH resistance. The polyvalent platform approach is being applied by companies such as Cepheid and Abbott. The GeneXpert automated platform is now also available for the qualitative Xpert HIV-1 Qual (World Health Organization [WHO] prequalified [11]) (12) and the quantitative Xpert HIV-1 viral load (13, 14), especially in settings of high HIV and TB burden (14). The Abbott m2000 (Abbott, Des Plains, Illinois, USA) automated platform is well established for HIV viral load (VL) testing in many settings with a high HIV burden, including South Africa (15, 16), with many trained staff and quality procedures already in place. A molecular assay, the RealTime MTB (RT MTB; Abbott), for the diagnosis of Mycobacterium tuberculosis complex (MTBC) targeting the IS and PAB genes has now been introduced for the Abbott m2000 platform (17–20). Sputum or bronchoalveolar lavage (BAL) specimens are inactivated with an isopropanol-based inactivation buffer (Abbott) prior to DNA extraction using a guanidinium thiocyanate magnetic microparticle based technology (Abbott), either manually or using the m2000sp. Amplification and detection of the target regions are performed using real-time PCR in the m2000rt. The overall sensitivity and specificity of the RT MTB assay to date on prospectively collected clinical specimens range from 93% to 100% and 97% to 100%, respectively (17–19), although one study reported decreased sensitivity of 81% in smear-negative specimens (18). Similar sensitivity (92% over a range of specimen types and 93.5% for respiratory specimens only) and specificity (100%) have been reported from residual clinical specimens, with a decreased sensitivity of 76.1% in smear-negative specimens (21).
Specimens detected as positive for MTBC DNA by the RT MTB assay can be reflexed to the RT MTB RIF/INH (Abbott) assay using the same platform as for the detection of RIF and INH resistance, identifying mutations in the rpoB, inhA, and katG genes. Alternatively, the RT MTB RIF/INH assay can be performed as a stand-alone test (21). The RIF and INH mutations are identified within the same wild-type and mutation regions reported by the MTBDRplus assay, and comparison to date shows a 99.5% concordance (21, 22). A second study showed statistically similar performances in the detection of RIF compared with that of the Xpert MTB/RIF assay (sensitivity, 94.8% compared with 95.8%, respectively; specificity of both, 100%) and in the detection of INH compared with that of the MTBDRplus assay (sensitivity and specificity of 88.3% and 94.3%, respectively, for the RT MTB RIF/INH compared with 87.2% and 96.7%, respectively, for the MTBDRplus) (22).
While the performance of the RT MTB and RT MTB RIF/INH assays on clinical specimens (raw sputa, concentrated sputa, BAL fluid, and tracheal aspirates) has been reported in seven publications (17–23), no data are available among HIV/TB-coinfected individuals and therefore warrants evaluation, especially since the m2000 is a polyvalent platform designed for high-throughput HIV and TB testing. This study reports the performance of the Abbott RT MTB and RT MTB RIF/INH assays in a cohort of individuals with presumptive TB attending a community health care clinic in Johannesburg, South Africa, where a rate of TB/HIV coinfection of 70% has previously been reported (24).
RESULTS
Cohort characteristics.
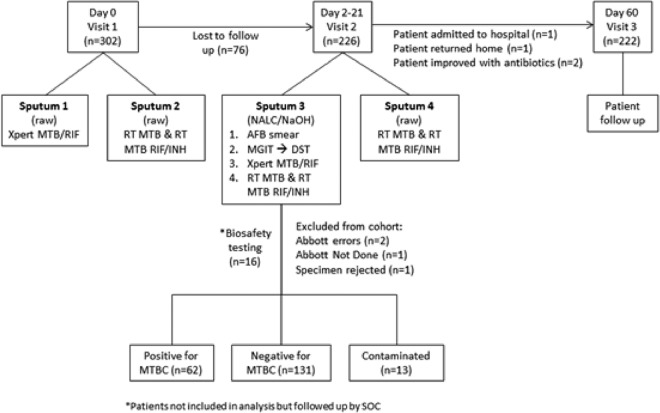
Informed consent was obtained from 302 patients at visit 1, as illustrated in Fig. 1. Seventy-six patients (25%) were lost to follow-up between visits 1 and 2, and a further four (∼1%) did not attend visit 3 but were contacted telephonically (one was admitted to a hospital, one returned home, and two improved with antibiotic therapy). Most participants (75%) returned for visit 2 within a median of 5 days (range, 1 to 31 days; mean, 5.7 days). Specimens used for biosafety testing (n = 16), specimens with incomplete results due to instrument error, patients for whom testing was not performed or the specimen was rejected prior to testing (n = 4), and patients who were lost to follow-up (n = 76) were excluded from the final data set. The statistical analysis was performed on data from 206 participants (93% of participants completing the study and 68% of all enrolled patients).
FIG 1.
Study outline indicating clinic visits, sputum laboratory pathways, and data descriptions for statistical analysis.
As listed in Table 1, the average age of the 206 patients was 34 years, with 62% of the participants being male. Almost all participants (99%) reported clinical signs and symptoms of TB disease at presentation. The HIV infection rate was 73% (151/206), and the mean CD4 count was 226 cells/μl (range, 4 to 1,140 cells). A total of 29/151 clients (19%) reported having initiated antiretroviral therapy (ART) previously (median CD4 count, 139 cells/μl). Only 15% (30/206) of clients reported a history of TB, with 96% (27/30) of these having completed their treatment. Per bacteriological classification, 13% (27/206) were smear and culture positive, 17% (35/206) were smear negative and culture positive, and 63% (129/206) were smear and culture negative. According to the most recent WHO clinical case classification (25), 46% (94/206) had any TB and 52% (108/206) had no TB, with only 4 clients classified as indeterminate for TB infection. The culture contamination rate was 6.3% (13/206); of these, the Xpert MTB/RIF detected six MTBC-positive specimens, and five of these specimens were also positive for MTBC by the RT MTB.
TABLE 1.
Cohort characteristics for clients entered into the statistical analysisa
| Characteristic | Value |
|---|---|
| Demographics | |
| Age (mean years [range]) | 34 (18–60) |
| Male sex (n [%]) | 127 (62) |
| Clinical signs and symptoms at presentation (n [%]) | |
| Weight loss | 204 (99) |
| Fever | 205 (99) |
| Cough for >2 weeks | 204 (99) |
| Night sweats | 203 (99) |
| HIV-related information | |
| HIV positive (n [%]) | 151 (73) |
| HIV negative (n [%]) | 48 (23) |
| Unknown (n [%]) | 7 (3) |
| CD4 count (mean cells/μl [range]) | 226 (4–1,140) |
| On antiretrovirals (n [%]) | 29 (19) |
| TB history (n [%]) | |
| Previously diagnosed with TB | 30 (15) |
| Previous TB treatment completed | 27 (96) |
| Bacteriological classification (n [%]) | |
| Smear and culture positive | 27 (13) |
| Smear negative and culture positive | 35 (17) |
| Smear and culture negative | 129 (63) |
| Smear negative and culture contaminated | 13 (6) |
| Smear positive and culture negative | 2 (1) |
| Clinical classification (n [%]) | |
| Definite TB | 74 (36) |
| Possible TB | 11 (5.5) |
| Probable TB | 9 (4.5) |
| Indeterminate TB (on TB drugs) | 4 (2) |
| No TB | 108 (52) |
Total of 206 study participants.
Diagnostic evaluation of the RT MTB and Xpert MTB/RIF assays for the detection of MTBC.
The performance of both molecular TB diagnostic assays (RT MTB and Xpert MTB/RIF) directly on raw sputum and decontaminated sputum (pellet) is described in Table 2. The RT MTB assay generated higher sensitivities (84% [95% confidence interval {CI}, 72 to 92%] and 89% [95% CI, 78 to 95%] on visit 1 and visit 2 specimens, respectively) than the Xpert MTB/RIF (79% [95% CI, 67 to 88%] at visit 1) when performed on raw sputum specimens, whereas the Xpert MTB/RIF generated a higher sensitivity (92% [95% CI, 82 to 97%]) than the RT MTB (86% [95% CI, 74 to 93%]) on visit 2 sputa, following sputum decontamination and concentration. Neither of these differences reached statistical significance (the 95% CIs overlapped in all cases). The sensitivity of the RT MTB assay did not vary between raw sputa and processed sputum pellets. The performances of both molecular assays showed the same trends among HIV/TB coinfected individuals, and overall were reduced in HIV/TB coinfected individuals, but these also did not reach statistical significance (95% CIs overlapped).
TABLE 2.
Molecular assay performance compared with reference testing for the diagnosis of TBa
| Performance measurement | Percentage (95% CI) for tests performed on: |
||||||
|---|---|---|---|---|---|---|---|
| Pellets (SOC) |
Sputa (direct) |
Pellets |
|||||
| Sputum 3 |
Sputum 1 (SOC) |
Sputum 2 |
Sputum 4 |
Sputum 3 |
|||
| Smear microscopy | MGIT culture | Xpert MTB/RIF, visit 1 | Abbott RealTime MTB assay, visit 1 | Abbott RealTime MTB assay, visit 2 | Xpert MTB/RIF, visit 2 | Abbott RealTime MTB assay, visit 2 | |
| Comparison with MGIT culture (n = 193) | |||||||
| Sensitivity | 43.5 (31.7–56.7) | 79.0 (66.8–88.3) | 83.9 (72.3–92.0) | 88.7 (78.1–95.3) | 91.9 (82.2–97.3) | 85.5 (74.2–93.1) | |
| Specificity | 98.5 (94.6–99.8) | 96.9 (92.4–99.2) | 92.3 (86.3–96.2) | 94.6 (89.2–97.8) | 97.7 (93.5–99.5) | 92.4 (86.4–96.3) | |
| PPV | 93.1 (77.2–99.2) | 92.5 (81.8–97.9) | 83.9 (72.3–92.0) | 88.7 (78.1–95.3) | 95.0 (86.1–99.0) | 84.1 (7278–92.1) | |
| NPV | 78.7 (71.6–84.7) | 90.7 (84.6–95.0) | 92.3 (86.3–96.2) | 94.6 (89.2–97.8) | 96.2 (91.4–98.8) | 93.1 (87.3–96.8) | |
| Comparison with MGIT culture (HIV-positive cohort only) (n = 142)$ | |||||||
| Sensitivity | 37.5 (22.7–54.2) | 70.0 (53.5–83.4) | 75.0 (58.8–87.3) | 82.5 (67.2–92.7) | 87.5 (73.2–95.8) | 77.5 (61.5–89.2) | |
| Specificity | 98.0 (93.1–99.8) | 96.1 (90.3–98.9) | 93.1 (86.2–97.2) | 95.1 (88.9–98.4) | 97.1 (91.6–99.4) | 93.1 (86.4–97.2) | |
| PPV | 88.2 (63.6–98.5) | 87.5 (71.0–96.5) | 81.1 (64.8–92.0) | 86.8 (71.9–95.6) | 92.1 (78.6–98.3) | 81.6 (65.7–92.3) | |
| NPV | 80.0 (71.9–86.6) | 89.1 (81.7–94.2) | 90.4 (83.0–95.3) | 93.3 (86.6–97.3) | 95.2 (89.1–98.4) | 91.3 (84.2–96.0) | |
| Comparison with MGIT culture (HIV-negative cohort only) (n = 44)$ | |||||||
| Sensitivity | 55.0 (31.5–76.9) | 95 (75.1–99.9) | 100 (83.2–100) | 100 (83.2–100) | 100 (83.2–100) | 100 (83.2–100) | |
| Specificity | 100 (86.3–100) | 100 (86.3–100) | 92.0 (74.0–99.0) | 91.7 (73.0–99.0) | 100 (86.3–100) | 88.0 (68.8–97.5) | |
| PPV | 100 (71.5–100) | 100 (82.4–100) | 90.9 (70.8–98.9) | 90.9 (70.8–98.9) | 100 (83.2–100) | 87.0 (66.4–97.2) | |
| NPV | 73.5 (55.6–87.1) | 96.2 (80.4–99.9) | 100 (85.2–100) | 100 (84.6–100) | 100 (86.3–100) | 100 (84.6–100) | |
| Comparison with clinical case definition# (Any TB including definite, possible, probable TB) (n = 202) | |||||||
| Sensitivity | 31.6 (22.4–41.9) | 74.7 (63.9–83.6) | 62.8 (52.2–72.5) | 61.7* (51.1–71.5) | 64.9 (54.4–74.5) | 67 (56.6–76.4) | 66 (55.5–75.4) |
| Specificity | 100 (96.6–100) | 100 (96.5–100) | 100 (96.6–100) | 95.3* (89.4–98.5) | 95.3 (89.4–98.5) | 100 (96.6–100) | 94.4 (88.3–97.9) |
| PPV | 100 (88.4–100) | 100 (94.2–100) | 100 (93.9–100) | 92.1* (82.4–97.4) | 92.4 (83.2–97.5) | 100 (94.3–100) | 91.2 (81.8–96.7) |
| NPV | 62.4 (54.8–69.7) | 83.1 (75.2–89.2) | 75.5 (67.6–82.3) | 73.9* (65.8–81.0) | 75.6 (67.4–82.5) | 77.7 (69.9–84.3) | 76.1 (68.0–83.1) |
*, test not done (n = 1); $, of the 193 MGIT results, there were 7 patients with an unknown HIV status, and these were excluded from the HIV stratification; #, reported to minimize differences between specimen collection and testing due to limited volume and SOC, return visits, and potential use of antibiotics.
In comparison to the clinical case definition, liquid cultures displayed the greatest sensitivity (75% [95% CI, 64 to 84%]) compared with 66% (95% CI, 56 to 75%) and 67% (95% CI, 57 to 76%) for the RT MTB and Xpert MTB/RIF assays, respectively, on sputum pellets. The RT MTB assay displayed slightly lower specificities (95% [95% CI, 89 to 99%] on raw sputum and 94% [95% CI, 88 to 98%] on sputum pellets) than the other assays (all 100%). The positive predictive values (PPVs) of the RT MTB were also lower (92.4% [95% CI, 83 to 98%] on raw sputum and 91.2% [95% CI, 82 to 97%] on sputum pellets), while the other assays had 100% PPVs. The negative predictive values (NPVs) for the RT MTB were similar to those of the Xpert MTB/RIF (RT MTB, 75.6% [95% CI, 67 to 83%] and 76.1% [95% CI, 68 to 83%]; Xpert MTB/RIF, 75.5% [95% CI, 68 to 82%] and 77.7% [95% CI, 70 to 84%] on raw sputa and sputum pellets, respectively), which were lower than the NPV of 83.1% (95% CI, 75 to 89%) obtained by mycobacterial growth indicator tubes (MGIT) culture.
As described in Table 1, 35 cases (27 HIV-positive) were reported as smear negative culture positive. The Xpert MTB/RIF correctly identified 25.7% (9/35) as MTBC positive, and the RT MTB correctly identified 74.3% (26/35) as MTBC positive. Overall, the RT MTB assay detected 48% (17/35) more MTBC-positive cases than the Xpert MTB/RIF. Among those that were smear negative and culture negative (n = 129), the Xpert MTB/RIF identified 3% (4/129) as MTBC positive, of which none had a history of TB and all of which had initiated TB treatment, and the RT MTB identified 7% (9/129), of which 6 cases were HIV infected and none had a history of TB, but only 2 patients were considered for treatment initiation.
Figure 2 graphically represents the quantitative values for all assays from 62 patient specimens reported as positive for MTBC by liquid culture. Trends are visible between increasing smear grades, decreasing liquid culture times to positivity, and reductions in both the cycle threshold (CT) and cycle number (CN) values of the Xpert MTB/RIF and RT MTB assays, respectively, indicating an overall increase in bacterial burden, reflected by all the assays.
FIG 2.
Scatter plot of quantitative values reported by each assay (y axis: CT of probe C for the Xpert MTB/RIF, CN for the RT MTB, liquid culture time to detection [ttd], and smear grade [0 to 3]). The dotted horizontal line illustrates the CN cutoff of 35, above which the RT MTB assay that generated a positive result could not report a result from the RT MTB RIF/INH reflex assay.
Evaluation of drug resistance.
The dashed horizontal line in Fig. 2 indicates the threshold of CN values above which the RT MTB RIF/INH reflex assay does not generate a result, as the concentration of extracted MTBC DNA in the specimen is below the limit of detection (LOD). The median CN value of the RT MTB assay where the reflex RT MTB RIF/INH test reported below the LOD was 29 (minimum, 17; maximum, 40), among 24% (32/133) of the raw sputum samples processed and 30% (20/66) of the pellet specimens processed. This is in contrast to the Xpert MTB/RIF in which 1 (<2%) MTBC-positive specimen yielded an RIF indeterminate result.
Overall, results were generated from six patients in whom RIF and or INH drug resistance was identified by the current standard-of-care (SOC) testing (MTBDRplus performed on MTB-positive liquid culture). RIF resistance concurred in all cases between the Xpert MTB/RIF (performed on pellets) and the SOC testing. The RT MTB RIF/INH concurred with the MTBDRplus for both RIF and INH in all cases where the RT MTB RIF/INH generated a reportable result.
Qualitative performance of the Abbott MTB and MTB RIF/INH assays.
In total, 715 specimens were tested using the Abbott m2000 platform. These were managed in 17 runs (2 specimens reported an error) for the RT MTB assay and in 12 runs for the RT MTB RIF/INH assay. The RT MTB assay was easy to perform as the process is automated, with very little hands-on time required after specimen preparation (i.e., adding inactivation reagent and aliquoting 1.7 ml to the m2000 sample tube, with a total hands-on time of ∼75 min for 93 specimens). We determined that, including the specimen inactivation, a full RT MTB run (93 specimens and 3 controls) can be completed in ∼8 h (2 h and 15 min for specimen inactivation and preparation [75 min hands-on time], 3 h for specimen processing, including instrument set-up, ∼30 min for master mix addition, and 2 h for amplification and reporting). An additional 2.5 h is required for reflex RT MTB RIF/INH resistance testing (automated), indicating that the results, including primary resistance testing, would be available the day after sputum collection. The manual set-up (only required in this study due to software version differences) for the RT MTB RIF/INH assay was neither difficult nor time consuming, although attention to detail is required. One further advantage of the RT MTB assay, particularly for low-volume facilities, is that unused prepared master mix can be stored at 2 to 8°C for up to 14 days. The qualitative performance of the Xpert MTB/RIF assay has been extensively described elsewhere (26, 27). Within an 8-h period, the GeneXpert IV can report 16 results, the GeneXpert XVI can report 64 results, and the GeneXpert 80 can report 320 results compared with 93 results reported from the m2000 platform (excluding the RT MTB RIF/INH result).
DISCUSSION
The national use of the Xpert MTB/RIF across 207 testing facilities in South Africa's National TB program (28) has reduced the TB diagnostic positivity from 16% in 2011 to 11% in 2016 (29), but this cohort of individuals in central Johannesburg still showed a high TB and HIV coinfection rate (73%), with patients presenting late (CD4 count, 226 cells/μl). In addition, 63% of presumptive cases were smear and culture negative, but a total of 46% had TB per the WHO clinical case definition (25). The polyvalent ability of more sensitive molecular diagnostic testing platforms may therefore contribute to the integration of HIV and TB laboratory services to improve the diagnosis and care of coinfected individuals.
The RT MTB assay performed well against the SOC Xpert MTB/RIF in this population, with a higher sensitivity on raw specimens (89% [95% CI, 78 to 95%]), although the Xpert MTB/RIF had a higher sensitivity on decontaminated pellets (92% [95% CI, 82 to 97%]), which did not reach statistical significance. The noninferiority of the RT MTB assay compared with the Xpert MTB/RIF assay is in line with the study by Wang and colleagues (20). However, among the smear-negative culture-positive cases, the RT MTB detected 48% (17/35) more cases than the Xpert MTB/RIF. A diagnostic gap in the market is the continuing need for liquid culture to identify presumptive TB in HIV-positive patients who are Xpert MTB/RIF negative. The pooled median sensitivity of the Xpert MTB/RIF among these individuals is 79% (30). Alternate nucleic acid amplification tests (NAATs), such as the RT MTB assay, could potentially identify a number of these patients and minimize empirically based treatment initiation. However, the reduced PPV of the RT MTB assay (92.5% [95% CI, 83 to 98%] on raw sputum) does raise some concerns, since molecular tests identify viable and nonviable bacterial DNA. This may complicate the diagnostic algorithm, especially in settings of low TB prevalence, and would need to take into account a patient's history of TB, but would also be an advantage for identifying patients earlier in their disease course. Both molecular assays performed well among HIV-positive individuals, with sensitivities of 83% (95% CI, 67 to 93%) and 88% (95% CI, 73 to 96%) for the RT MTB and the Xpert MTB/RIF, respectively, also with no statistically significant difference between the overall group and HIV-positive individuals. However, liquid culture remains the most sensitive diagnostic by detecting an additional 9% positivity compared with both molecular diagnostic tests. The advantages of these molecular platforms over culture are speed (2 days versus 1 to 6 weeks), the simultaneous detection of RIF and/or INH mutations, not being affected by contaminating bacteria, and the potential use of the CN and CT values to quantitate bacterial input concentrations. Overall, the m2000 platform was easy to use within the laboratory environment and generated few errors, and the quality management system is already in place within South Africa, making this platform a real option for expanding integrated HIV and TB testing services.
Although the drug resistance rate among this population is not high, the performance of both assays to detect RIF (Xpert MTB/RIF) and RIF/INH (RT MTB RIF/INH) was good and concurred with current DST technology. A disadvantage of the RT MTB RIF/INH found in this study was the high percentage (33%) of specimens that could not generate a reflex result, as the DNA concentration was below the LOD. A number of reasons are postulated to explain the lower than expected number of available resistance results. First, the resistance assay was delayed, which resulted in the prolonged storage of DNA eluates and manual rather than automated preparation. A second explanation may be the differences in software versions used to run the two assays, as version 1.0 software was used on the RT MTB assay. The upgraded version 2.0 software is now available for the RT MTB assay and is recommended for use in conjunction with the RT MTB RIF/INH resistance assay (version 1.0). Recently published studies used the updated software and the direct reflex protocol, and a greater sensitivity was attained (21, 22).
A diagnostic gap left by the Xpert MTB/RIF assay is that it currently only generates information on RIF resistance, complementing the current WHO recommendations that all patients with RIF resistance be treated for MDR-TB (31). However, it has been reported that up to 10% of patients on MDR-TB treatment could benefit from the inclusion of INH (32, 33) and that the use of high-dose INH or the exclusion of ethambutol may also be preferable, depending on the genetic cause of INH resistance (34, 35). Of even greater concern are the ∼14% of patients with RIF-susceptible TB who have INH mono-resistant TB, and it is vital that these patients are identified to avoid suboptimal treatment (32, 33). DST beyond the Xpert MTB/RIF result remains laboratory based, relying on genotypic line probe assays (such as the MTBDRplus used in South Africa) or phenotypic (culture-based) assays. The inclusion of identifying drug resistance in novel NAATs is of high importance in the TB diagnostic pipeline (9), hence the need for assays such as the RT MTB and RT MTB RIF/INH, which also address increasing testing volume needs with the m2000 high-throughput platform.
Although this study focused on centralized testing, where 93 specimen results were generated within 2 days and patients returned to the clinic for their result outcomes and treatment management within 5 days, the diagnostic delay for some was still as much as 31 days. This further highlights the need for expanded (tiered) testing options all the way to POC testing. The mean patient age of 34 years and 62% being male also highlights the need for strengthening health care in ways beyond diagnostics. This is further evidenced by the 25% of enrolled individuals whom were lost to follow-up despite the dedicated staff in this study, illustrating that improving diagnostic assay sensitivity is only one component in the health system targeting global disease improvements. The importance of active case finding, intensive patient history taking, particularly in relapse cases, and a strong patient follow-up protocol are vital to the goal of TB elimination.
Conclusion.
The Abbott RT MTB assay has similar diagnostic performance to the Xpert MTB/RIF and is suited to testing patients with presumptive TB coinfected with HIV. The added advantages of the Abbott RT MTB RIF/INH assay, namely, the reporting of both RIF and INH drug resistance within the same reporting time frame, its connectivity to the laboratory information system and training, and the technical support already in place (16), make this a viable polyvalent option to scale up TB alongside HIV laboratory testing services in South Africa.
MATERIALS AND METHODS
Ethics approval.
Ethics approval for this study was obtained from the University of the Witwatersrand Human Ethics Review Committee (M110139).
Clinical study design.
Participants were recruited for the clinical evaluation of the RT MTB and the RT MTB RIF/INH assays at the Hillbrow Community Health Centre between September 2014 and June 2015 using a dedicated study nurse. All patients with suspected MTBC infection, whether or not they elected to participate in the study, were diagnosed and managed according to South African TB management guidelines (36). Individuals were offered HIV diagnosis and management according to the South African HIV treatment guidelines in use at the time of study commencement in 2014 (37). Persons with suspected MTBC infection who were over the age of 18 and able to give sputum specimens were informed about the study as described in Fig. 1. Persons who agreed to participate were asked to provide two sputum specimens for the purposes of the study at two visits (1 week apart). Patients histories were documented at visit 1 and included information on the presence of a cough for >2 weeks, weight loss, night sweats, fever, chest pain, and suspected extrapulmonary involvement, as well as details on previous MTBC episodes (to ensure there was no TB episode in the last 6 months), HIV status, CD4 count, and ART. If indicated, participants were started on antibiotics at this visit. The sputa were couriered to the Clinical Laboratory Services (CLS) (10 min from the clinic) where an Xpert MTB/RIF test (1 ml) was performed as part of SOC (for sputum 1). Sputum 2 was inactivated using Abbott inactivation reagent (IR) buffer in a 3:1 (IR buffer to specimen) ratio for a minimum of 60 min and then couriered to the research diagnostics laboratory (10 min from the CLS) for RT MTB testing.
A second visit (visit 2) occurred approximately 1 week later, when the Xpert MTB/RIF results were available, and patients were asked to provide two sputum samples. A chest X-ray was performed if deemed necessary (smear negative and unwell) by the study nurse and was read by a pulmonologist who advised on further management. TB treatment was commenced where indicated and was based on the treatment guidelines (36). The sputa collected at visit 2 were couriered to the CLS where the SOC sputum (sputum 3) was decontaminated and prepared for routine testing (smear and liquid culture). Residual pellets (SOC) were tested using the Xpert MTB/RIF (0.5 ml) and the remainder of the samples was inactivated using Abbott IR buffer in a 3:1 (IR buffer-to-specimen) ratio for at least 1 h, which was then couriered to the research diagnostics laboratory for RT MTB testing. Sputum 4 was inactivated using Abbott IR buffer in a 3:1 (IR buffer-to-specimen) ratio for a minimum of 60 min and then couriered to the research diagnostics laboratory for RT MTB testing.
A third (follow-up) visit (visit 3) occurred when the tuberculosis culture results were available (approximately 6 weeks after visit 1), when the patients were reassessed and started on MTBC treatment if indicated; if treatment was started at visit 2, the response to treatment was assessed. Body weights were measured at each visit, with body temperatures measured at visits 1 and 2. Results from the RT MTB and the RT MTB RIF/INH assays were not used for clinical patient management. Cases which did not have a microbiological diagnosis of MTBC at this stage were assessed by a pulmonologist and classified as clinical tuberculosis if they had persistent symptoms without a cause other than tuberculosis and compatible radiology (either chest X-ray or abdominal ultrasound features suggestive of tuberculosis). Patients who had been started on empirical tuberculosis treatment at their second visit and had shown a clinical response with improvement of symptoms and weight gain were considered to have clinical tuberculosis. Patients in whom a diagnosis other than tuberculosis was made that could explain their clinical condition and patients that improved clinically without empirical tuberculosis treatment were considered to not have tuberculosis.
Standard of care laboratory testing.
SOC tests were performed by the CLS and the results were used for patient management. Sputum decontamination was performed using N-acetyl-l-Cysteine (NALC)-NaOH concentration, followed by centrifugation at 3,000 × g for 15 min and resuspension in phosphate buffer (pH 6.8) to a volume of 3 ml. The pellets were processed for smear microscopy (auramine O fluorescence staining) and graded per international guidelines. For liquid culture, 500 μl of the concentrated pellet was added to prepared MGIT (Becton Dickinson & Co., Franklin Lakes, NJ, USA) and loaded into the Bactec MGIT 960 mycobacterial detection system (Becton Dickinson & Co., NJ, USA) until either a positive result was noted or 42 days had passed (MGIT culture negative). Positive cultures were confirmed using Ziehl-Neelsen staining and culture contamination was reported. Species identification and genotypic first-line DST was performed using the GenoType MTBDRplus version 2 assay (Hain LifeScience GmbH, Nehren, Germany) either on smear-positive sputum (direct) or on culture for culture-positive specimens. The presence/absence of MTBC and resistance/susceptibility to RIF and INH were visually reported. First-line MGIT DST (Bactec MGIT 960 SIRE [streptomycin, isoniazid, rifampin, and ethambutol] and pyrazinamide [PZA]; Becton Dickinson & Co., NJ, USA) was performed on positive cultures. If resistance to any of these drugs was identified, then further phenotypic drug susceptibility (second-line) testing was performed. The Xpert MTB/RIF (Cepheid, Sunnyvale, California, USA) was performed using 1 ml of raw sputum (2:1 of sample reagent [SR] buffer and sputum) and 0.5 ml (3:1 of SR buffer and pellet) pellet. The CT was documented as a measure of mycobacterial concentration (38).
Comparator Abbott RealTime MTB and RealTime RIF/INH testing.
The biosafety of the IR buffer was determined according to published biosafety studies (23) using sputum specimens from the first 16 enrolled patients (not compromising their SOC). The biosafety results were in accord with this study (23) and are therefore not further reported. The Abbott RealTime MTB assay (Abbott Molecular, Des Plains, IL, USA) was performed according to the manufacturer's instructions, using the automated m2000 platform, software version 1.0, and has been described in detail previously (18). Briefly, sputum specimens (∼3 ml raw sputum or 500 μl NALC-decontaminated sputum pellet) were inactivated using the supplied IR buffer (10 mM NaOH-isopropanol-Tween 20) using a 3:1 (IR buffer-to-specimen) ratio for a minimum of 60 min. This was performed in a biosafety level 3 (BSL-3) facility (although this level is not required for the RT MTB/RIF inactivation of raw sputum), after which the specimens could be safely processed under standard laboratory conditions. After inactivation, nucleic acids were extracted from 1.7 ml specimens using the m2000sp instrument and prepared for amplification (25 μl) and fluorescent detection on the m2000rt instrument. The provided positive (one for the RT-MTB assay and one for the RT MTB/RIF assay) and negative (a single negative control for both assays) controls for the RT MTB and the RT MTB RIF/INH assays were included in every run. The CN was recorded for each result as an indication of bacterial concentration.
Extracted DNA from specimens classified as “MTB detected” by the RT MTB were reflex tested for RIF and INH resistance using the RT MTB RIF/INH as per the manufacturer's instructions. The RT MTB RIF/INH uses three different master mixes in separate wells, targeting the most common MTB regions that confer resistance to RIF (rpoB) and INH (katG and upper inhA promoter region). The detection of RIF and INH drug resistance is determined through the combined interpretation of different target probe signals. If the PCR for one of the resistance-determining regions fails, the other two are not compromised and a result is still obtained. The RT MTB RIF/INH kit was not available at the time that the study was started, and all DNA eluates were stored in sealed 96-deep-well plates (Abbott) at −80°C for testing at a later time. The PCR plates were manually prepared for the RT MTB RIF/INH resistance assay per the manufacturer's instruction and transferred to the m2000rt instrument for amplification and detection.
The ease of use, skill level required to perform the RT MTB and the RT MTB RIF/INH assays, the time to reportable results, and reported errors were documented.
Statistical analysis.
The statistical analysis was performed using Stata version 13 (StataCorp LLC, College Station, TX, USA). Patients were recorded as definite MTBC infection (culture positive) or no MTBC infection (culture negative). Patients for whom cultures were contaminated or for whom the Xpert MTB/RIF results or the RT MTB results were unavailable (assay error) were excluded from the statistical data analysis. The data included smear microscopy, MGIT culture, MTBDRplus, Xpert MTB/RIF, and RT MTB assay results, and clinical case definitions. The sensitivity, specificity, PPV, and NPV of the RT MTB and the Xpert MTB/RIF were calculated (with 95% CIs) using the culture as the gold standard. HIV status further stratified this analysis. Limitations to the study design to evaluate diagnostic technologies include the SOC protocol (the Xpert MTB/RIF is already well implemented in South Africa as the initial diagnostic), delayed return visits, and the potential use of antibiotics. In effort to minimize complications with statistical analysis for this study design, the sensitivity, specificity, PPV, and NPV of smear, liquid culture, Xpert MTB/RIF, and RT MTB assays were compared to clinical case definitions as defined by the WHO: bacteriologically confirmed TB, clinically confirmed TB, and no TB (25). The CN of the RT MTB and the CT of the Xpert MTB/RIF, both as indicators of mycobacterial burden, were compared to the number of days to positive liquid culture and to smear grade.
ACKNOWLEDGMENTS
We thank Abbott Molecular for providing the test kits used in this study free of charge.
Research by L.S. and W.S. is supported by funding received from the South African Medical Research Council and with funds received from the South African National Department of Health, the UK Medical Research Council, the UK Government's Newton Fund under the UK/South Africa Newton Fund (no. 015NEWTON TB), and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (under award number R21AI116015).
A.B. received a salary from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) Health Systems Strengthening grant.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the funding bodies.
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report 2016. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf Accessed 20 December 2016. [Google Scholar]
- 2.World Health Organization. 2015. The end TB strategy: global strategy and targets for tuberculosis prevention, care and control after 2015. World Health Organization, Geneva, Swizerland: http://www.who.int/tb/strategy/End_TB_Strategy.pdf Accessed 20 December 2016. [Google Scholar]
- 3.Van Rie A, Warren RM. 2015. MDR tuberculosis control: time to change the dogma? Lancet Respir Med 3:907–909. doi: 10.1016/S2213-2600(15)00477-4. [DOI] [PubMed] [Google Scholar]
- 4.Kendall EA, Fofana MO, Dowdy DW. 2015. Burden of transmitted multidrug resistance in epidemics of tuberculosis: a transmission modelling analysis. Lancet Respir Med 3:963–972. doi: 10.1016/S2213-2600(15)00458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 2013. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Policy update. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf Accessed 20 December 2016. [PubMed] [Google Scholar]
- 6.World Health Organization. 2016. The use of molecular line probe assay for the detection of resistance to isoniazid and rifampicin: policy update. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/250586/1/9789241511261-eng.pdf Accessed 19 December 2016. [Google Scholar]
- 7.World Health Organization. 2016. The use of molecular line probe assays for the detection of mutations associated with resistance to fluoroquinolones (FQs) and second-line injectable drugs (SLIDs). Policy guidance. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/WHOPolicyStatementSLLPA.pdf Accessed 18 August 2017. [Google Scholar]
- 8.Scott L, da Silva P, Boehme CC, Stevens W, Gilpin CM. 2017. Diagnosis of opportunistic infections: HIV co-infections - tuberculosis. Curr Opin HIV AIDS 12:129–138. doi: 10.1097/COH.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. 2015. UNITAID tuberculosis diagnostics technology and market landscape, 4th ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 10.Schumacher SG, Nabeta P, Boehme CC, Ellner J, Alland D, Dorman SE, Denkinger CM. 2017. A multicenter, diagnostic accuracy study of the Xpert Ultra for tuberculosis diagnosis, abstr 76LB. Conf Retrovir Oppor Infect (CROI) 2017, WA, USA, 13 to 16 February 2017. [Google Scholar]
- 11.World Health Organization. 2016. WHO prequalification of in vitro diagnostics public report. Product: Xpert HIV-1 Qual assay. PQDx 0259-070-00 World Heath Organization, Geneva, Switzerland: http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/160613PQPublicReport_0259-0700-00_XpertQualHIV_v2.pdf Accessed 11 September 2016. [Google Scholar]
- 12.Michaeli M, Wax M, Gozlan Y, Rakovsky A, Mendelson E, Mor O. 2016. Evaluation of Xpert HIV-1 Qual assay for resolution of HIV-1 infection in samples with negative or indeterminate Geenius HIV-1/2 results. J Clin Virol 76:1–3. doi: 10.1016/j.jcv.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Mor O, Gozlan Y, Wax M, Mileguir F, Rakovsky A, Noy B, Mendelson E, Levy I. 2015. Evaluation of the RealTime HIV-1, Xpert HIV-1, and Aptima HIV-1 Quant Dx assays in comparison to the NucliSens EasyQ HIV-1 v20 assay for quantification of HIV-1 viral load. J Clin Microbiol 53:3458–3465. doi: 10.1128/JCM.01806-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gous N, Scott L, Berrie L, Stevens W. 2016. Options to expand HIV viral load testing in South Africa: evaluation of the GeneXpert(R) HIV-1 viral load assay. PLoS One 11:e0168244. doi: 10.1371/journal.pone.0168244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott L, Carmona S, Gous N, Horsfield P, MacKay M, Stevens W. 2012. Use of a prequalification panel for rapid scale-up of high-throughput HIV viral load testing. J Clin Microbiol 50:4083–4086. doi: 10.1128/JCM.01355-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona S, Peter T, Berrie L. 2017. HIV viral load scale-up: multiple interventions to meet the HIV treatment cascade. Curr Opin HIV AIDS 12:157–164. http://journals.lww.com/co-hivandaids/Abstract/2017/03000/HIV_viral_load_scale_up___multiple_interventions.9.aspx. [DOI] [PubMed] [Google Scholar]
- 17.Chen JH, She KK, Kwong TC, Wong OY, Siu GK, Leung CC, Chang KC, Tam CM, Ho PL, Cheng VC, Yuen KY, Yam WC. 2015. Performance of the new automated Abbott RealTime MTB assay for rapid detection of Mycobacterium tuberculosis complex in respiratory specimens. Eur J Clin Microbiol Infect Dis 34:1827–1832. doi: 10.1007/s10096-015-2419-5. [DOI] [PubMed] [Google Scholar]
- 18.Tang N, Frank A, Pahalawatta V, Lampinen J, Coblenz-Korte A, Dunn C, Li C, Cloherty G, Abravaya K, Leckie G. 2015. Analytical and clinical performance of Abbott RealTime MTB, an assay for detection of Mycobacterium tuberculosis in pulmonary specimens. Tuberculosis (Edinb) 95:613–619. doi: 10.1016/j.tube.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Vinuesa V, Navarro D, Poujois S, Zaragoza S, Borras R. 2016. Performance characteristics of the new Abbott Real Time MTB assay for detection of Mycobacterium tuberculosis complex in respiratory specimens. Diagn Microbiol Infect Dis 84:212–214. doi: 10.1016/j.diagmicrobio.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang SF, Ou XC, Li Q, Zheng HW, Wang YF, Zhao YL. 2016. The Abbott RealTime MTB assay and the Cepheid GeneXpert assay show comparable performance for the detection of Mycobacterium tuberculosis in sputum specimens. Int J Infect Dis 45:78–80. doi: 10.1016/j.ijid.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann-Thiel S, Molodtsov N, Antonenka U, Hoffmann H. 2016. Evaluation of the Abbott RealTime MTB and RealTime MTB INH/RIF assays for direct detection of Mycobacterium tuberculosis complex and resistance markers in respiratory and extrapulmonary specimens. J Clin Microbiol 54:3022–3027. doi: 10.1128/JCM.01144-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostera J, Leckie G, Tang N, Lampinen J, Szostak M, Abravaya K, Wang H. 2016. Analytical and clinical performance characteristics of the Abbott RealTime MTB RIF/INH resistance, an assay for the detection of rifampicin and isoniazid resistant Mycobacterium tuberculosis in pulmonary specimens. Tuberculosis (Edinb) 101:137–143. doi: 10.1016/j.tube.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Qi C, Wallis C, Pahalawatta V, Frank A, Ramdin N, Viana R, Abravaya K, Leckie G, Tang N. 2015. Evaluation of the efficiency of the sample inactivation reagent in the Abbott RealTime MTB assay for inactivation of Mycobacterium tuberculosis. J Clin Microbiol 53:3001–3002. doi: 10.1128/JCM.00598-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, Sanne I, Venter WF, Duse A, Stevens W. 2011. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Med 8:e1001061. doi: 10.1371/journal.pmed.1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. 2014. Definitions and reporting framework for tuberculosis – 2013 revision (updated December 2014). World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf Accessed 18 October 2016. [Google Scholar]
- 26.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-Lopez EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. 2010. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens WS, Scott L, Noble L, Gous N, Dheda K. 2016. Impact of the GeneXpert MTB/RIF technology on tuberculosis control. Microbiol Spectr 5:TBTB2–0040-2016. doi: 10.1128/microbiolspec.TBTB2-0040-2016. [DOI] [PubMed] [Google Scholar]
- 29.Stevens W. 2016. Mapping laboratory capacity, systems and networks in Africa (Bill and Melinda Gates Foundation seminar). Afr Soc Lab Med Conf (ASLM) 3 to 8 December 2016, Durban, South Africa. [Google Scholar]
- 30.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. 2014. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014:CD009593. doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 2016. WHO treatment guidelines for drug-resistant tuberculosis: 2016 update. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/areas-of-work/drug-resistant-tb/MDRTBguidelines2016.pdf Accessed 3 January 2017. [Google Scholar]
- 32.Niehaus AJ, Mlisana K, Gandhi NR, Mathema B, Brust JC. 2015. High prevalence of inhA promoter mutations among patients with drug-resistant tuberculosis in KwaZulu-Natal, South Africa. PLoS One 10:e0135003. doi: 10.1371/journal.pone.0135003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. 2012. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis 16:203–205. doi: 10.5588/ijtld.11.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. 2008. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 12:139–145. [PubMed] [Google Scholar]
- 35.Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 36.South African National Department of Health. 2014. National tuberculosis management guidelines 2014. South African National Department of Health, Pretoria, South Africa: https://www.health-e.org.za/wp-content/uploads/2014/06/NTCP_Adult_TB-Guidelines-27.5.2014.pdf Accessed 15 December 2016. [Google Scholar]
- 37.South African National Department of Health. 2013. The South African antiretroviral treatment guidelines 2013. South African National Department of Health, Pretoria, South Africa: http://www.sahivsoc.org/Files/2013%20ART%20Treatment%20Guidelines%20Final%2025%20March%202013%20corrected.pdf Accessed 24 November 2016. [Google Scholar]
- 38.Scott L, Schnippel K, Ncayiyana J, Berrie L, Berhanu R, Van Rie A. 2015. The use of national Xpert MTB/RIF's cycle threshold (CT) as an audit indicator for program and laboratory performance. 46th Union World Conference on Lung Health, 2 to 6 December 2016 Cape Town, South Africa. [Google Scholar]