ABSTRACT
The aim of this study was to construct a valid publicly available method for in silico fimH subtyping of Escherichia coli particularly suitable for differentiation of fine-resolution subgroups within clonal groups defined by standard multilocus sequence typing (MLST). FimTyper was constructed as a FASTA database containing all currently known fimH alleles. The software source code is publicly available at https://bitbucket.org/genomicepidemiology/fimtyper, the database is freely available at https://bitbucket.org/genomicepidemiology/fimtyper_db, and a service implementing the software is available at https://cge.cbs.dtu.dk/services/FimTyper. FimTyper was validated on three data sets: one containing Sanger sequences of fimH alleles of 42 E. coli isolates generated prior to the current study (data set 1), one containing whole-genome sequence (WGS) data of 243 third-generation-cephalosporin-resistant E. coli isolates (data set 2), and one containing a randomly chosen subset of 40 E. coli isolates from data set 2 that were subjected to conventional fimH subtyping (data set 3). The combination of the three data sets enabled an evaluation and comparison of FimTyper on both Sanger sequences and WGS data. FimTyper correctly predicted all 42 fimH subtypes from the Sanger sequences from data set 1 and successfully analyzed all 243 draft genomes from data set 2. FimTyper subtyping of the Sanger sequences and WGS data from data set 3 were in complete agreement. Additionally, fimH subtyping was evaluated on a phylogenetic network of 122 sequence type 131 (ST131) E. coli isolates. There was perfect concordance between the typology and fimH-based subclones within ST131, with accurate identification of the pandemic multidrug-resistant clonal subgroup ST131-H30. FimTyper provides a standardized tool, as a rapid alternative to conventional fimH subtyping, highly suitable for surveillance and outbreak detection.
KEYWORDS: fimH, Escherichia coli, typing, whole-genome sequencing analysis
INTRODUCTION
The fimH gene is part of the fim operon, which encodes a surface organelle named type 1 fimbriae found in most Escherichia coli strains (1). The FimH protein is located at the tip of the fimbrial structure and serves as a d-mannose-specific adhesin, which aids in immobilizing the bacterium on both biotic and abiotic surfaces (2, 3). Studies have shown only minor sequence variation within the fimH genes, which renders the fimH alleles feasible for use in high-resolution subtyping of multilocus sequence typing (MLST)-based E. coli clonal groups. The applicability of fimH subtyping has been shown to be particularly relevant within the highly virulent sequence type 131 (ST131) clonal group, where the resistant and multiresistant H30 subgroups carrying the fimH30 allele have been identified (4, 5). As ST131 E. coli is the most dominant human-pathogenic clonal group being reported in relation to bloodstream infections, the need to perform fimH subtyping is undisputed. Traditionally, typing of fimH alleles has been obtained through PCR amplification of the approximately 900-bp fimH gene, followed by a single Sanger sequencing run and alignment of the 489-nucleotide (nt) typing region to an fimH allele database containing the currently known fimH typing variants or alleles. This typing could be performed rapidly and easily on whole-genome sequencing (WGS) data; thus a need to develop a solution to handle WGS data in relation to fimH typing of especially pathogenic E. coli has emerged. The aim of the present study was construction and validation of a Web tool which would enable the user to obtain fimH allelic information from either simple Sanger-generated sequences or raw as well as assembled WGS data.
(Part of this work was presented previously at the 11th International Meeting on Microbial Epidemiological Markers [IMMEM XI], Estoril, Portugal, 12 March 2016.)
RESULTS AND DISCUSSION
Construction of FimTyper.
FimTyper was constructed to perform fimH subtyping using sequencing data originating from PCR and subsequent Sanger sequencing (assembled and saved in FASTA format), raw reads obtained directly from sequencing platforms such as Illumina, Ion Torrent, or Roche 454, or de novo-assembled draft (or complete) genomes. The FimTyper tool contains all currently known fimH alleles and is a BLAST-based publicly available Web-based service hosted by the Center for Genomic Epidemiology (CGE [https://cge.cbs.dtu.dk/services/FimTyper]). The default settings for FimTyper were set to a minimum identity (ID) of 95% and minimum length of 60% compared to the reference sequence to avoid noise from, e.g., gene fragments; however, FimTyper allows the user to specify similarity from 55% to 100% identity. The best-matching hit from the database was given as output, including the percent identity (%ID) between the hit in the genome and in the database and the length of the hit compared to the database record of the fimH allele. Additionally, the contig in which the hit was found, followed by the position in the contig, and the accession number of the fimH allele were reported. A detailed description of the output of FimTyper can be found at the CGE website.
Using FimTyper on Sanger sequences from PCR products.
To evaluate the performance of the FimTyper Web tool versus conventional typing, multiple analysis strategies were employed. Initially, the tool was evaluated on preassembled pairs of Sanger sequences from a data set consisting of 42 samples (data set 1) that had already been subtyped in relation to their fimH alleles by conventional typing methods prior to the current study. The Sanger sequences covered 13 different variants of fimH subtypes (Table 1). The FimTyper identified fimH subtypes from all 42 assembled Sanger sequences correctly at a 100% identity match.
TABLE 1.
fimH subtype prediction by conventional typing versus FimTyper
| Data set no. | No. of samplesa |
Concordance between conventional and FimTyper results (%)b | ||
|---|---|---|---|---|
| Total | Positive by conventional typing | Detected by FimTyper | ||
| 1 | 42 | 42 | 42 | 100 |
| 2 | 243 | ND | 230 | NA |
| 3 | 40 | 37 | 37 | 100 |
ND, not determined.
NA, not available.
Thus, an excellent concordance between conventional typing and the FimTyper tool was found, suggesting an equally good performance for the FimTyper tool as for conventional typing in analyzing preassembled Sanger sequences uploaded as FASTA files.
Using FimTyper on whole-genome sequencing data.
The FimTyper Web tool successfully analyzed all 243 draft genomes of third-generation-cephalosporin-resistant E. coli isolates (data set 2). FimTyper was able to identify an fimH allele in 230 of the 243 draft genome data sets. The 13 fimH-negative isolates were further verified as negative by BLAST against the complete fim operon, including part of its flanking regions (9,754 nt in total) from E. coli K-12 MG1655. All 13 fimH-negative isolates showed BLAST hits to the upstream and downstream regions of the fim operon but no hits to any of the genes of the fim operon, including fimH, suggesting that these isolates were missing not only the fimH gene but the complete fim operon.
Among the 492 fimH alleles in the FimTyper database, 32 different alleles were found to match the sequences of the 243 draft genomes, including one new allele (Table 2). The most abundant hits were to the fimH30 allele (n = 98), the fimH27 allele (n = 42), the fimH5 allele (n = 17), and the fim41 allele (n = 15). The new allele was assigned number 517 (fimH517) and added to the database.
TABLE 2.
Distribution of fimH subtypes identified among 243 draft genomes of Escherichia coli isolates using the FimTyper Web tool
| fimH subtype | No. of isolates with the subtype |
|---|---|
| fimH30 | 98 |
| fimH27 | 42 |
| fimH5 | 18 |
| fimH41 | 15 |
| fimH54 | 9 |
| fimH24 | 5 |
| fimH106 | 4 |
| fimH29 | 4 |
| fimH2 | 3 |
| fimH35 | 3 |
| fimH65 | 3 |
| fimH31 | 3 |
| fimH38 | 2 |
| fimH64 | 2 |
| fimH34 | 2 |
| fimH22 | 1 |
| fimH517a | 1 |
| fimH103 | 1 |
| fimH142 | 1 |
| fimH63 | 1 |
| fimH25 | 1 |
| fimH32 | 1 |
| fimH39 | 1 |
| fimH58 | 1 |
| fimH60 | 1 |
| fimH43 | 1 |
| fimH215 | 1 |
| fimH445 | 1 |
| fimH97 | 1 |
| fimH483 | 1 |
| fimH10 | 1 |
| fimH15 | 1 |
| fimH negative | 13 |
New fimH subtype identified by FimTyper.
Among the 40 randomly chosen isolates from data set 2 that were additionally subjected to conventional fimH typing (comprising data set 3), three of the samples did not yield any PCR products; these results were in agreement with the FimTyper results on whole-genome sequence data, where fimH-negative results were predicted for the same three samples. For the remaining 37 samples, the conventional typing using DNA alignment and the FimTyper predictions using both assembled Sanger sequences and whole-genome sequence data were in 100% agreement.
MLST versus fimH subtype.
Subtyping of fimH is especially relevant for the major E. coli clonal group ST131. Therefore, the 122 E. coli isolates from data set 2 previously predicted by Roer et al. (6) to belong to ST131 by the Achtman MLST scheme (7) were further analyzed in relation to their fimH subtype. All 122 ST131 E. coli isolates harbored an fimH allele, with fimH30 being the most frequent (n = 95, 78%) and representing the pandemic multidrug-resistant clonal group ST131-H30, followed by fimH27 (n = 14, 11%), fimH41 (n = 11, 9%), fimH22 (n = 1, <1%), and fimH35 (n = 1, <1%). In a study by Johnson et al. (8), the same five fimH alleles were among the seven fimH subtypes found in a collection of 352 historical and recent ST131 E. coli isolates, subtyped by conventional typing. Two infrequent subtypes found by Johnson et al., the fimH15 (1/352) and fimH94 (1/352) alleles, were not found among the 122 ST131 E. coli isolates tested in the current study.
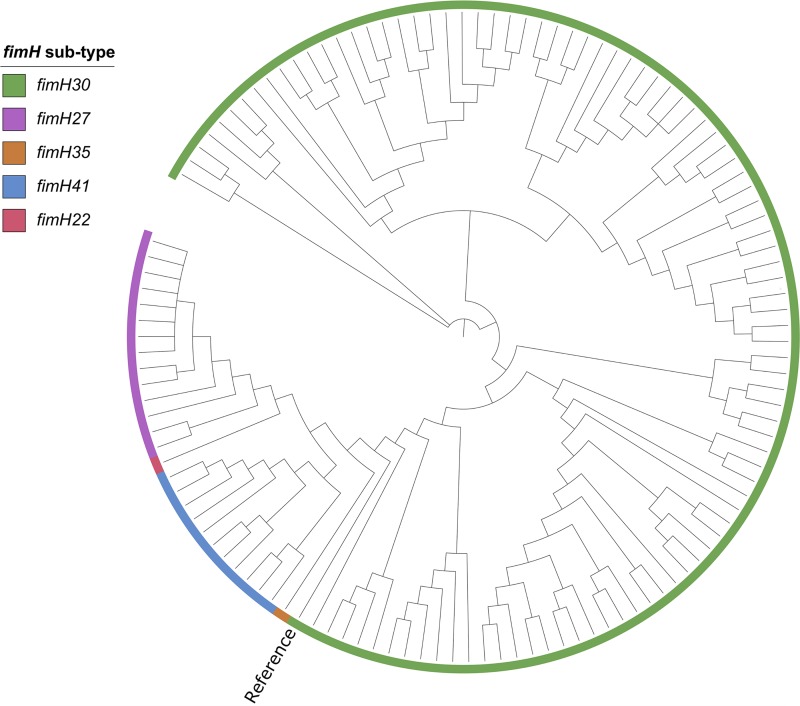
In addition to the analysis above, the phylogenetic relationship between the 122 ST131 E. coli isolates was constructed from single nucleotide polymorphism (SNP) analysis and compared to the fimH subtypes, as depicted in Fig. 1. From this analysis, a clear overlap between the structure of the phylogenetic relationship and the fimH subtype was observed. All fimH30 isolates clustered together in a distinct ST131-H30 clade, as did the fimH41 isolates and the fimH27 isolates. The two single isolates with fimH35 and fimH22 clustered in between the clades of the other fimH subtypes. Miyoshi-Akiyama et al. reported a similar correlation between SNP-based phylogeny and fimH subtype for a collection of global ST131 E. coli isolates (9). However, in their study, two fimH30 isolates clustered within the distinct fimH41 clade, whereas a clear grouping was observed in our study. This difference could be a result of mistyping of the two fimH30 isolates or of the differences in the methods used for calling SNPs, reconstructing the phylogenetic tree, and choice of genome reference. We did not have access to either the data or the custom script for SNP concatemers used by Miyoshi-Akiyama et al.; however, to investigate the possible differences caused by the reference, a new phylogenetic reconstruction was created with E. coli SE15 used by Miyoshi-Akiyama et al. (GenBank accession no. NC_013654.1) as a reference (data not shown). The reference was subtyped as an fimH41 isolate and clustered together with all our fimH41 isolates in the phylogenetic reconstruction. The overall topology of the tree once again clustered according to their fimH subtypes, eliminating the choice of reference as a parameter for differences between the two studies.
FIG 1.
SNP-based phylogeny of the 122 ST131 Escherichia coli isolates. Phylogenetic reconstruction of the 122 ST131 E. coli isolates was performed with E. coli JJ1886 as the reference genome. The tree was constructed from 13,155 SNPs, and results are represented as a cladogram. The fimH subtype is marked at the branch tip for each isolate according to the color legend on the figure.
However, both studies illustrate the high diversity within the ST131 clonal clade and underline the benefit of, including fimH analysis as a fast tool to subtype beyond the level of MLST.
In the present study, a Web tool to identify fimH alleles from either simple Sanger-generated sequences or raw or assembled WGS data from E. coli genomes has been developed, thus enabling researchers and primary investigators to rapidly detect the fimH allele in their data sets. The software source code for the tool is publicly available at https://bitbucket.org/genomicepidemiology/fimtyper, and the database hosted by the Center for Genomic Epidemiology (CGE) is freely available at https://bitbucket.org/genomicepidemiology/fimtyper_db. A publicly available Web service implementing the software can be found at https://cge.cbs.dtu.dk/services/FimTyper.
MATERIALS AND METHODS
Development of a Web tool for fimH subtyping.
An fimH allele database was created to contain all previously identified fimH allele variants (n = 492) collected at the State University of New York and used for conventional typing. The database was constructed as a single FASTA file and implemented in a BLAST-based PERL script, originally developed by Zankari et al. for in silico detection of acquired resistance genes (10). The default setting for minimum percent identity and minimum length of a hit to be reported by BLAST was chosen as 95% and 60%, respectively, to reduce false-positive hits caused by reporting of small fragments unrelated to the fimH gene. Perfect identity hits (%ID of 100) reports the corresponding fimH allele whereas nonperfect hits (100 > %ID >95) report an “unknown or presumptive new variant,” and the user is encouraged to contact the curator of FimTyper for updating the database with this new variant.
The new stand-alone Web tool, called FimTyper, has been made publicly available as a component of the CGE Web tools (http://cge.cbs.dtu.dk/services/).
Data sets for validation.
To validate the FimTyper Web tool, two different data sets and a subset of one of these were used, covering a total of 32 fimH subtypes. Data set 1 was comprised of paired Sanger sequences of 42 E. coli isolates, where the fimH allele variants had previously been determined by the conventional typing method. The data set covered 13 different fimH subtypes. Data set 2 comprised draft genomes obtained from whole-genome sequencing using a 250-bp paired-end Illumina data set of 243 third-generation-cephalosporin-resistant E. coli isolates originating from blood infections and submitted to Statens Serum Institut in 2014 as part of the surveillance of third-generation-cephalosporin-resistant E. coli (6). These 243 E. coli isolates covered 49 different STs, of which 122 isolates belonged to ST131 (11). Data set 3 was comprised of a randomly chosen subset from data set 2 of 40 E. coli isolates belonging to 28 different STs and covering 29 different fimH subtypes and an fimH-negative fraction.
Conventional fimH subtyping.
The 40 E. coli isolates of data set 3 were subjected to conventional fimH subtyping, performed as previously described (12). Briefly, fimH PCR amplification was conducted using a Qiagen Multiplex PCR kit (Qiagen, Aarhus, Denmark) with the following two fimH primers: fimH-F, CACTCAGGGAACCATTCAGGCA (binds 50 to 72 nucleotides upstream of the fimH start), and fimH-R, CTTATTGATAAACAAAAGTCAC (spans the last 21 nucleotides of fimH). The thermocycler program for the PCRs consisted of 1 cycle of 94°C for 5 min for heat activation, followed by 30 cycles of 94°C for 30 s (denaturation), 57°C for 90 s (annealing), and 72°C for 60 s (extension), with 1 cycle of 72°C for 60 s as a final extension. The resulting PCR products were applied on an Illustra ExoProStar 1-Step kit (GE Healthcare), a BigDye Terminator, version 3.1, cycle sequencing kit (Thermo Fisher), and a BigDye XTerminator purification kit (Thermo Fisher), followed by sequencing with an Applied Biosystems 3130 XL genetic analyzer (Thermo Fisher). Contigs were assembled based on the paired chromatograms using CLC Genomics Workbench, version 9.5.1 (Qiagen).
Validation of FimTyper.
Individual FASTA assemblies of the paired Sanger sequences of data set 1 from the 42 isolates that had been subjected to conventionally fimH subtyping prior to the current study were analyzed with the newly developed FimTyper Web tool presented in this study (https://cge.cbs.dtu.dk/services/FimTyper-1.0/). The output results were compared with the results previously obtained by conventional typing using manual alignment analysis toward the fimH database.
Draft genome sequences of the 243 third-generation-cephalosporin-resistant E. coli isolates from data set 2 were analyzed directly using the FimTyper Web tool. In situations where FimTyper did not report BLAST hits with an identity of >95%, the draft genome sequences were additionally analyzed by BLAST against the complete fim operon of E. coli K-12 MG1655 (GenBank accession no. U00096, bases 4540457 to 4550210) including flanking regions with 500 bp upstream of fimB and 500 bp downstream of fimH, to confirm the absence of one of more fim-related genes. Finally, as the fimH subtypes of data set 2 had not been examined previously by conventional fimH subtyping, 40 randomly chosen isolates (data set 3) were subjected to conventional typing with PCR and Sanger sequencing and analyzed manually by multiple-alignment analysis with the known fimH sequences. The results were evaluated and compared to the results from FimTyper on Sanger sequences and whole-genome sequence data.
Clonal variation within ST131 analyzed by SNP analysis.
SNP variants were called using NASP, version 1.0 (http://biorxiv.org/content/early/2016/01/25/037267), by aligning whole-genome sequence data from the 122 ST131 E. coli isolates against the chromosome of JJ1886 (GenBank accession no. NC_022648.1) using the Burrows-Wheeler aligner (BWA) after removal of duplicated regions in the reference using NUCmer. Variants were identified using the GATK Unified Genotyper, and SNPs that did not pass a minimum coverage of 10 or SNPs that were not present in a minimum of 90% of the base calls were excluded. Phylogenetic analyses of the identified SNPs were performed by maximum-likelihood approximation with the generalized time-reversible model in FastTree, version 2.1.5 (13).
ACKNOWLEDGMENTS
We thank Karin Sixhøj Pedersen for excellent laboratory assistance.
This work was partly supported by the Danish Ministry of Health and Prevention as part of the Integrated Surveillance of ESBL/AmpC-producing E. coli and Carbapenemase-Producing Bacteria and partly by the Center for Genomic Epidemiology (www.genomicepidemiology.org).
We have no conflicts of interest to declare.
REFERENCES
- 1.Klemm P, Christiansen G. 1987. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet 208:439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- 2.Bhomkar P, Materi W, Semenchenko V, Wishart DS. 2010. Transcriptional response of E. coli upon FimH-mediated fimbrial adhesion. Gene Regul Syst Bio 4:1–17. doi: 10.4137/GRSB.S4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cookson AL, Cooley WA, Woodward MJ. 2002. The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. Int J Med Microbiol 292:195–205. doi: 10.1078/1438-4221-00203. [DOI] [PubMed] [Google Scholar]
- 4.O'Hara JA, Hu F, Ahn C, Nelson J, Rivera JI, Pasculle AW, Doi Y. 2014. Molecular epidemiology of KPC-producing Escherichia coli: occurrence of ST131-fimH30 subclone harboring pKpQIL-like IncFIIk plasmid. Antimicrob Agents Chemother 58:4234–4237. doi: 10.1128/AAC.02182-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers BA, Ingram PR, Runnegar N, Pitman MC, Freeman JT, Athan E, Havers S, Sidjabat HE, Gunning E, De Almeida M, Styles K, Paterson DL, ASID CRN. 2015. Sequence type 131 fimH30 and fimH41 subclones amongst Escherichia coli isolates in Australia and New Zealand. Int J Antimicrob Agents 45:351–358. doi: 10.1016/j.ijantimicag.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Roer L, Hansen F, Thomsen MCF, Knudsen JD, Hansen DS, Wang M, Samulioniené J, Justesen US, Røder BL, Schumacher H, Østergaard C, Andersen LP, Dzajic E, Søndergaard TS, Stegger M, Hammerum AM, Hasman H. 22 March 2017. WGS-based surveillance of third-generation cephalosporin-resistant Escherichia coli from bloodstream infections in Denmark. J Antimicrob Chemother doi: 10.1093/jac/dkx092. [DOI] [PubMed] [Google Scholar]
- 7.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MCJ, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, Riddell K, Rogers P, Qin X, Butler-Wu S, Price LB, Aziz M, Nicolas-Chanoine M-H, Debroy C, Robicsek A, Hansen G, Urban C, Platell J, Trott DJ, Zhanel G, Weissman SJ, Cookson BT, Fang FC, Limaye AP, Scholes D, Chattopadhyay S, Hooper DC, Sokurenko EV. 2013. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J Infect Dis 207:919–928. doi: 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyoshi-Akiyama T, Sherchan JB, Doi Y, Nagamatsu M, Sherchand JB, Tandukar S, Ohmagari N, Kirikae T, Ohara H, Hayakawa K. 2016. Comparative genome analysis of extended-spectrum-β-lactamase-producing Escherichia coli sequence type 131 strains from Nepal and Japan. mSphere 1:e00289-16. doi: 10.1128/mSphere.00289-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bager F, Birk T, Høg BB, Jensen LB, Jensen AN, Knegt L de, Korsgaard H, Dalby T, Hammerum AM, Hoffmann S, Kuhn KG, Larsen AR, Laursen M, Nielsen EM, Olsen SS, Petersen A, Sönksen UW. 2014. DANMAP 2014—use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Danish Integrated Antimicrobial Resistance Monitoring and Research Programme, Copenhagen, Denmark: http://www.danmap.org/~/media/projekt%20sites/danmap/danmap%20reports/danmap%202014/danmap_2014.ashx. [Google Scholar]
- 12.Weissman SJ, Johnson JR, Tchesnokova V, Billig M, Dykhuizen D, Riddell K, Rogers P, Qin X, Butler-Wu S, Cookson BT, Fang FC, Scholes D, Chattopadhyay S, Sokurenko E. 2012. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl Environ Microbiol 78:1353–1360. doi: 10.1128/AEM.06663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2: approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]