Abstract
CD19 is expressed on normal and neoplastic B cells and is a promising target for immunotherapy. However, there is still an unmet need to further develop novel therapeutic drugs for the treatment of the refractory/relapsing human CD19+ tumors. We have developed a diphtheria toxin‐based anti‐human CD19 immunotoxin for targeting human CD19+ tumors. We have constructed three isoforms of the CD19 immunotoxin: monovalent, bivalent, and foldback diabody. In vitro binding affinity and efficacy analysis demonstrated that the bivalent isoform had the highest binding affinity and in vitro efficacy. The in vivo efficacy of the CD19 immunotoxins was assessed using human CD19+ JeKo‐1 tumor‐bearing NOD/SCID IL‐2 receptor γ−/− (NSG) mouse model. In these animals, CD19 immunotoxins significantly prolonged the median survival from 31 days in controls to 34, 36, and 40 days in animals receiving the monovalent isoform, foldback diabody isoform, and bivalent isoform, respectively. The bivalent CD19 immunotoxin is a promising therapeutic drug candidate for targeting relapsing/refractory human CD19+ tumors.
Keywords: CD19+ tumor, diphtheria toxin, human CD19, immunotoxin, Pichia Pastoris
Abbreviations
- 7‐AAD
7‐aminoactinomycin D
- ATP
adenosine triphosphate
- B‐ALL
B‐cell acute lymphoblastic leukemia
- BID
bis in die
- BiscFv
bivalent single‐chain variable fragment
- CLL
chronic lymphatic lymphoma
- CSC
China Scholarship Council
- DT
diphtheria toxin
- G4S
four glycine residues and one serine residue
- His
histidine
- IACUC
Institutional Animal Care and Use Committee
- IC50
half maximal inhibitory concentration
- IP
intraperitoneal injection
- IV
intravenous injection
- kDa
kilodalton
- KD
dissociation constant
- mAb
monoclonal antibody
- MFI
mean fluorescence intensity
- MGH
Massachusetts General Hospital
- NHL
non‐Hodgkin's B‐cell lymphoma
- Ni
nickel
- nM
nanomolar
- NOD/SCID
nonobese diabetic/severe combined immunodeficiency
- NSG
NOD/SCID IL‐2 receptor γ−/−
- PBMC
peripheral blood mononuclear cell
- PE
phycoerythrin
- SA
streptavidin
- scFv
single‐chain variable fragment
- SDS/PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SD
standard deviation
- VH
heavy chain variable domain
- VL
light chain variable domain
1. Introduction
CD19 is a 95‐kDa transmembrane glycoprotein which is widely expressed on both normal and malignant B cells including chronic lymphatic lymphoma, non‐Hodgkin's B‐cell lymphoma, and B‐cell acute lymphoblastic leukemia (Turtle et al., 2016; Vallera et al., 2005). CD19 is known as a promising immunotherapy target. Anti‐human CD19 mAb drugs are already in the clinic or in clinical trials (Wang et al., 2012). However, effective antibody treatment requires the contributions of host effector mechanisms such as complement and NK cells. In contrast, immunotoxins are able to eliminate target cells even when host effector mechanisms are compromised due to chemotherapeutic agents or malnutrition. Some chemical‐conjugated anti‐human CD19 immunotoxins are already in clinical trial (Wang et al., 2012). However, these have varied degree of effectiveness and production difficulties (Vallera et al., 2005; Wayne et al., 2014). CD22‐CD19‐bispecific recombinant immunotoxin is in clinical trials (Bachanova et al., 2015; Vallera et al., 2005). However, its efficacy is dependent on target cells expressing CD22 and CD19 simultaneously. Previous attempts to produce a CD19‐specific recombinant immunotoxin have been unsuccessful (Du et al., 2008). We hypothesize that this was due to a suboptimal expression system. In this study, we developed a recombinant anti‐human CD19 immunotoxin using unique diphtheria toxin‐resistant yeast Pichia Pastoris expression system. The in vitro binding affinity and efficacy of the CD19 immunotoxin were assessed using human CD19+ mantle cell lymphoma cell line JeKo‐1. The in vivo efficacy of the CD19 immunotoxin was characterized using human CD19+ tumor‐bearing NSG mouse model.
2. Materials and methods
2.1. Cell lines and antibodies
Human CD19+ mantle cell lymphoma cell line JeKo‐1 (cat# CRL‐3006) and human CD19− cell line CCRF‐CEM (cat# CCL‐119) were purchased from ATCC (Manassas, VA, USA). Human CD19− cell line M14 and MD‐MBA‐231 were generously provided by Soldano Ferrone (Massachusetts General Hospital). mAbs used in this study are listed in Table 1.
Table 1.
Antibodies used in this study
| Antibody Name | Clone# | Source | Cat# |
|---|---|---|---|
| PE‐mouse anti‐human CD19 | FMC63 | Millipore | MAB1794H |
| APC‐mouse anti‐human CD4 | L200 | BD | 551980 |
| FITC‐mouse anti‐human CD3Ɛ | SP‐34‐2 | BD | 556611 |
| PE‐mouse anti‐human CD3Ɛ | SP‐34‐2 | BD | 556612 |
| PE‐mouse anti‐human CD19 | HIB19 | BioLegend | 302208 |
| APC‐mouse anti‐human CD8 | RPA‐T8 | BioLegend | 301014 |
| FITC‐anti‐human CD14 | M5E2 | BioLegend | 301803 |
| PE‐mouse anti‐human CD16 | 3G8 | BioLegend | 302008 |
| PE‐mouse anti‐human CD194 (CCR4) | L291H4 | BioLegend | 359412 |
| PE‐streptavidin | BioLegend | 405204 | |
| FITC‐Annexin V | BioLegend | 640906 | |
| 7‐Aminoactinomycin (7‐AAD) | Sigma | A9400 |
2.2. DNA construction
As shown in Fig. 1, anti‐human CD19 immunotoxins were constructed using DT390 (Woo et al., 2002) and the codon‐optimized scFv (FMC63) nucleotide sequences (Figure 2, Nicholson et al., 1997). A strategy previously used to generate the anti‐human CCR4 immunotoxin (Wang et al., 2015) was applied to construct the anti‐human CD19 immunotoxins. DT390 and scFv (FMC63) or bi‐scFv (FMC63) are connected by a G4S (four glycines and one serine residue) linker. The two scFv (FMC63) of the bivalent immunotoxin are joined by (G4S)3 linker. Six histidines (6x His tag) were added to the C terminus of each construct to facilitate protein purification. To construct DT390‐scFv (FMC63), the codon‐optimized scFv (FMC63) was synthesized by GenScript and cloned into pwPICZalpha‐DT390 (Wang et al., 2011, 2015) between NcoI and EcoRI sites, yielding the final construct DT390‐scFv (FMC63) in pwPICZalpha. To construct DT390‐BiscFv (FMC63), the first scFv (FMC63) was amplified using PCR primers FMC‐Nco carrying XhoI and NcoI sites + FMC‐Bam1 carrying BamHI and EcoRI sites and then cloned into pwPICZalpha between XhoI and EcoRI sites for sequencing confirmation. The insert was subsequently cut out with NcoI + BamHI as insert I. The second scFv (FMC63) was PCR‐amplified using FMC‐Bam2 carrying XhoI and BamHI sites + FMC‐Eco carrying an EcoRI site and then cloned into pwPICZalpha between XhoI and EcoRI sites for sequencing confirmation. The insert was then cut out with BamHI + EcoRI as insert II. The insert I carrying NcoI and BamHI sites + insert II carrying BamHI and EcoRI sites [NcoI‐scFv (FMC63)‐BamHI/BamHI‐scFv (FMC63)‐EcoRI] were together cloned into pwPICZalpha‐DT390 between NcoI and EcoRI, yielding the final construct DT390‐BiscFv (FMC63) in pwPICZalpha. To construct the single‐chain foldback diabody CD19 immunotoxin, it is needed to build a modified scFv (FMC63) with a short linker (one G4S) between VL and VH. The VL portion was amplified using PCR primers FMC‐Nco + Bam‐FMC carrying BamHI site and digested using BamHI. The VH portion was amplified using PCR primers BgL‐FMC carrying BglII site + FMC‐Bam1 and digested using Bgl II. The BamHI‐digested VL portion and Bgl II‐digested VH were ligated together for 4 h at room temperature as PCR template to amplify the scFv (FMC63) with short linker (one G4S) for constructing the single‐chain foldback diabody isoform following the construction procedure for DT390‐BiscFv (FMC63). All PCR primers that were used are listed in Table 2.
Figure 1.
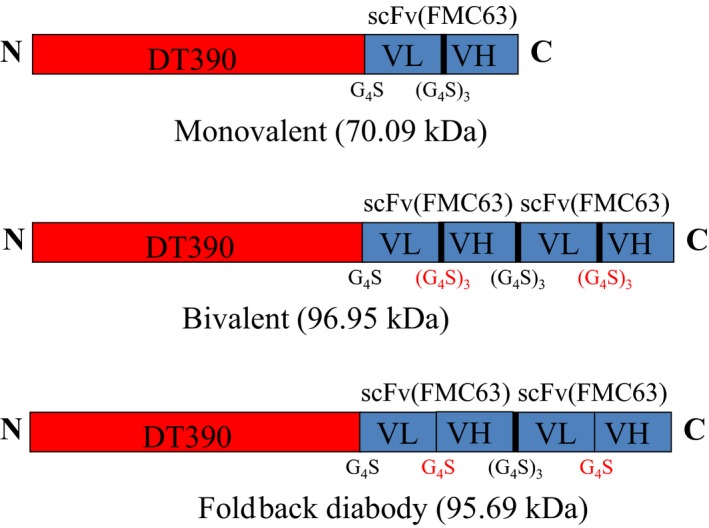
Schematic diagrams of the monovalent, bivalent, and single‐chain foldback diabody anti‐human CD19 immunotoxins.
Table 2.
PCR primers used in this study

CD19 immunotoxin expression in the diphtheria toxin‐resistant yeast Pichia Pastoris expression system and purifications were performed as previously described (Peraino et al., 2013a; Wang et al., 2011). Western blot analysis, binding and blocking analysis by flow cytometry were all performed as previously described (Peraino et al., 2013a) using a human CD19+ mantle cell lymphoma cell line JeKo‐1. DT390 was used as negative control for in vitro functional analysis. C21 immunotoxin (a nonrelated DT390‐based immunotoxin) was used as negative control for in vivo efficacy characterization. Both DT390 and C21 immunotoxin were also expressed using yeast Pichia Pastoris expression system in our laboratory. Isolation of human PBMC, in vitro binding and depletion analysis of the CD19 immunotoxins to human blood, or PBMC with flow cytometry was performed as previously described (Wang et al., 2015).
2.3. In vitro efficacy analysis
In vitro efficacy of the CD19 immunotoxins was assessed using CellTiter‐Glo® Luminescent Cell Viability Assay (Promega, cat# G7571) to human CD19+ mantle cell lymphoma cell line JeKo‐1. Briefly, the JeKo‐1 cells were added to the wells of the opaque‐walled 96‐well plate at 104 cells/well. The CD19 immunotoxin was diluted in the tissue culture medium and added to the well at final concentration of 10−6, 10−7, 10−8, 10−9, 10−10, 10−11, and 10−12 m. The final volume is 100 μL per well for both JeKo‐1 cells and the CD19 immunotoxin. The control wells containing only the tissue culture medium were included to obtain the background luminescence value. Cycloheximide (1.25 mg·mL−1) was added to the positive control wells. The plates were incubated for 24 h at 37 °C, 5% CO2. The plates were equilibrated at room temperature for approximately 30 min. 100 μL of the CellTiter‐Glo® reagent was added and mixed for 2 min on an orbital shaker to induce cell lysis. The plate was then incubated at room temperature for 10 min to stabilize the luminescent signal, and the luminescence signals were recorded using Wallac Victor2 1420 multilabel counter (Perkin Elmer, Waltham, MA, USA).
2.4. In vivo efficacy analysis
The breeding pairs of NSG mice were purchased from Jackson laboratories (Bar Harbor, Maine) and bred in our rodent barrier facilities. All animal experiments were approved by Massachusetts General Hospital (MGH) Institutional Animal Care and Use Committee (IACUC). The NSG mice were divided into following groups: (a) C21 immunotoxin control group (a nonrelated diphtheria toxin‐based immunotoxin as negative control) (n = 7); (b) monovalent CD19 immunotoxin group (n = 7); (c) bivalent CD19 immunotoxin group (n = 7); and (d) single‐chain foldback diabody CD19 immunotoxin group (n = 7). All animals were injected IV with 10 million of human CD19+ mantle cell lymphoma cells (JeKo‐1) via the tail vein. The immunotoxin was injected IP from day 0 on at 100 μg·kg−1, BID for four consecutive days as one course, four courses in total, and three‐day break between any two courses. The injected animals were observed daily for signs and symptoms of illness and scored twice weekly based on the parameters as previously reported by our laboratory (Peraino et al., 2013b and Wang et al., 2015). In addition, to assess whether the CD19 immunotoxin is toxic to the animals, NSG mice (n = 2) injected with the bivalent CD19 immunotoxin only were also included as control.
2.5. Statistical analysis
All p values were calculated using log‐rank (Mantel–Cox) test of Prism. p < 0.05 was considered as significant. IC50 was determined using nonlinear regression (curve fit) of Prism.
3. Results
3.1. Expression and purification of the CD19 immunotoxin
As shown in Fig. 1, monovalent, bivalent, and single‐chain foldback diabody versions of the anti‐human CD19 immunotoxins were constructed to find the isoform with the highest binding affinity and greatest ability to deplete human CD19+ cells. The codon‐optimized anti‐human CD19 scFv (FMC63) DNA (Fig. 2) (Nicholson et al., 1997) was cloned into the C terminus of the DT390‐containing yeast Pichia Pastoris expression vector pwPICZalpha‐DT390 between the NcoI and EcoRI sites (Wang et al., 2015). A 6xHis tag was added to the C terminus of each immunotoxin isoform to facilitate the purification. The DT390 domain was genetically linked to the scFv (FMC63) domain by a linker containing four glycine residues and a serine residue (G4S). For the monovalent and bivalent versions, the VL and VH were linked together by a long linker (G4S)3 to generate the scFv (FMC63). For the single‐chain foldback diabody isoform, the VL and VH were linked by a short linker G4S. The two scFv (FMC63) of the bivalent and the single‐chain foldback diabody isoforms were also joined by a long linker (G4S)3.
Figure 2.

Codon‐optimized anti‐human CD19 scFv (FMC63) DNA and amino acid sequence.
The CD19 immunotoxins were expressed using unique diphtheria toxin‐resistant yeast Pichia Pastoris (Liu et al., 2003) expression system in 1‐L Erlenmeyer flasks. The CD19 immunotoxins were captured using a Ni‐Sepharose fast flow resin and further purified using strong anion‐exchange resin. The final purification yield was ~ 15 mg per liter for the monovalent and ~ 6 mg per liter for the bivalent and ~ 2 mg per liter for the single‐chain foldback diabody CD19 immunotoxins of the original harvested supernatant. The purified anti‐human CD19 immunotoxins were analyzed by SDS/PAGE (Fig. 3A) and western blot using a mouse anti‐6xHis mAb (Fig. 3B) and a mouse antidiphtheria toxin mAb (Fig. 3C). SDS/PAGE and western blot analysis demonstrated that the three versions of the CD19 immunotoxins were expressed and purified with expected molecular weight of 70.09 kDa for the monovalent isoform, 96.95 kDa for the bivalent isoform, and 95.69 kDa for the single‐chain foldback diabody isoform.
Figure 3.
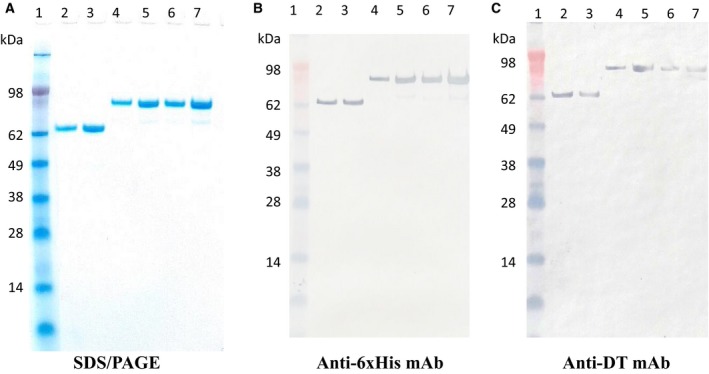
SDS/PAGE, western blot analysis of the anti‐human CD19 immunotoxins. (A) SDS/PAGE analysis (4–12% NuPAGE, Thermo Fisher Scientific); (B) western blot analysis using a mouse anti‐His mAb (clone#: 4A12E4, Thermo Fisher Scientific); (C) western blot analysis using a mouse antidiphtheria toxin mAb (clone# 3B6, Meridian). Lane 1: protein marker; Lanes 2–3: monovalent anti‐human CD19 immunotoxin [DT390‐scFv (FMC63), 70.09 kDa]; Lanes 4–5: bivalent anti‐human CD19 immunotoxin [DT390‐BiscFv (FMC63), 96.95 kDa]; Lanes 6–7: single‐chain foldback diabody anti‐human CD19 immunotoxin (95.69 kDa).
3.2. Binding affinity analysis of the CD19 immunotoxins using flow cytometry
CD19 immunotoxins were designed to deplete human CD19+ cells via binding of the anti‐human CD19 scFv (FMC63) domain of the immunotoxins on the CD19 receptor of the CD19+ cells. Following the internalization, the released DT390 domain functions to inhibit protein synthesis resulting in the cell death (Murphy, 2011); therefore, the first step to characterize the CD19 immunotoxins was to analyze their binding affinity to human CD19 receptor. The anti‐human CD19 immunotoxins were biotin‐labeled for the binding analysis to human CD19+ JeKo‐1 cells using flow cytometry. As shown in Fig. 4A, monovalent (left panel), bivalent (middle panel), and single‐chain foldback diabody (right panel) CD19 immunotoxins bound to the human CD19 in a dose‐dependent manner. The binding affinity was quantified by the dissociation constant (K D) for each CD19 immunotoxin isoform using mean fluorescence intensity (MFI) (Peraino et al., 2013a). The foldback diabody isoform (K D = 11.22 nm, Fig. 4D) bound stronger than the monovalent isoform (K D = 47.42 nm, Fig. 4B). However, the bivalent isoform exhibited the highest binding affinity (K D = 4.59 nm, Fig. 4C).
Figure 4.
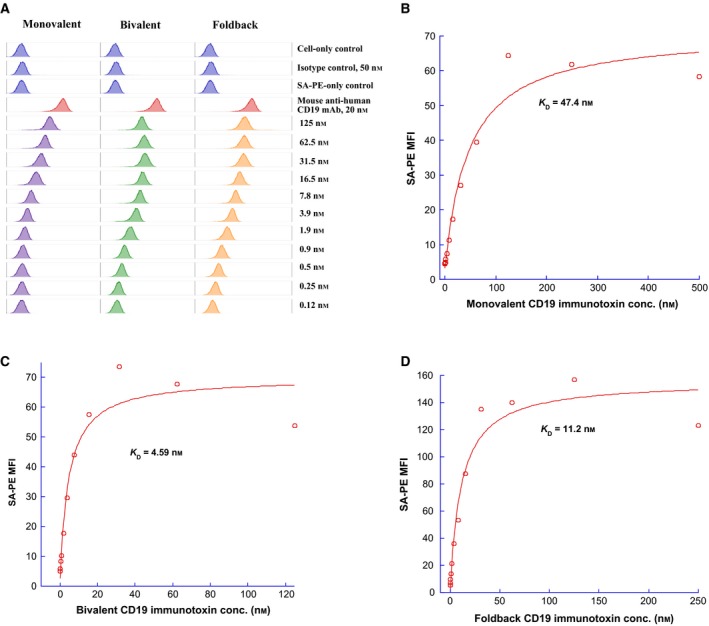
(A) Flow cytometry binding analysis of biotinylated monovalent anti‐human CD19 immunotoxin [DT390‐scFv (FMC63)] (left panel), bivalent anti‐human CD19 immunotoxin [DT390‐BiscFv (FMC63)] (middle panel), single‐chain foldback diabody anti‐human CD19 immunotoxin (right panel) to human CD19+ JeKo‐1 cells (mantle cell lymphoma cell line). Cells incubated with only the secondary staining (PE‐conjugated streptavidin) served as a negative control and PE‐mouse anti‐human CD19 mAb (clone#HIB19, BioLegend, cat#302208) for the positive control. PE‐mouse anti‐human CD194 (CCR4) (clone#L291H4, BioLegend, cat#359412) served as isotype control. Biotin‐labeled porcine CD3‐εγ (Peraino et al., 2012) was included as a negative control for background due to protein biotinylation. The data are representative of three individual experiments. (B‐D) KD determination using flow cytometry and nonlinear least‐squares fit. MFI was plotted over a wide range of concentrations of biotinylated (B) DT390‐scFv (FMC63), (C) DT390‐BiscFv (FMC63), and (D) single‐chain foldback diabody anti‐human CD19 immunotoxin. The accompanying least‐squares fits are shown based on the hyperbolic equation y = m1 + m2 * m0/(m3 + m0), where y = MFI at the given biotinylated anti‐human CD19 immunotoxin concentration; m0 = biotinylated anti‐human CD19 immunotoxin concentration; m1 = MFI of zero biotinylated anti‐human CD19 immunotoxin control; m2 = MFI at saturation; and m3 = KD. A fitted KD of 47.4 nm was obtained for DT390‐scFv (FMC63), 4.59 nm for DT390‐BiscFv (FMC63), and 11.2 nm for single‐chain foldback diabody anti‐human CD19 immunotoxin.
The binding specificity of the CD19 immunotoxins was further analyzed by blocking the binding of the parent anti‐human CD19 mAb (FMC63) to its receptor on human CD19+ JeKo‐1 tumor cells. As shown in Fig. 5, monovalent (left panel), bivalent (middle panel), and single‐chain foldback diabody (right panel) CD19 immunotoxins blocked the binding of anti‐human CD19 mAb (FMC63) to the JeKo‐1 cells in a dose‐dependent fashion. The data strongly suggest that the CD19 immunotoxins bind specifically to the human CD19 receptor.
Figure 5.
Blocking analysis of the anti‐human CD19 immunotoxins for the human CD19 receptor on human CD19+ JeKo‐1 lymphoma cells. Unlabeled anti‐human CD19 immunotoxins were each incubated with JeKo‐1 cells at a range of concentrations for 15 min at 4 °C in the dark. Subsequently, without washing the cells, anti‐human CD19 mAb (FMC63) was added to each tube containing cells in the presence of the unlabeled immunotoxins. Binding affinity of the anti‐human CD19 immunotoxins to the human CD19 receptor on JeKo‐1 cells was measured by a decrease in anti‐human CD19 mAb staining in the presence of increasing concentrations of the unlabeled immunotoxins.
3.3. In vitro efficacy analysis of the CD19 immunotoxin
In vitro efficacy of the CD19 immunotoxins was assessed using CellTiter‐Glo® Luminescent Cell Viability Assay (Promega) in a human CD19+ JeKo‐1 tumor cell line. As shown in Fig. 6, all of the three CD19 immunotoxin isoforms (monovalent, bivalent, and foldback diabody) were effective. The foldback diabody isoform (IC50 = 1.7 × 10−11 m) was better than the monovalent isoform (IC50 = 2 × 10−10 m), while the bivalent isoform (IC50 = 2 × 10−12 m) was greatest. This findings correlate well with the corresponding binding affinity analysis.
Figure 6.
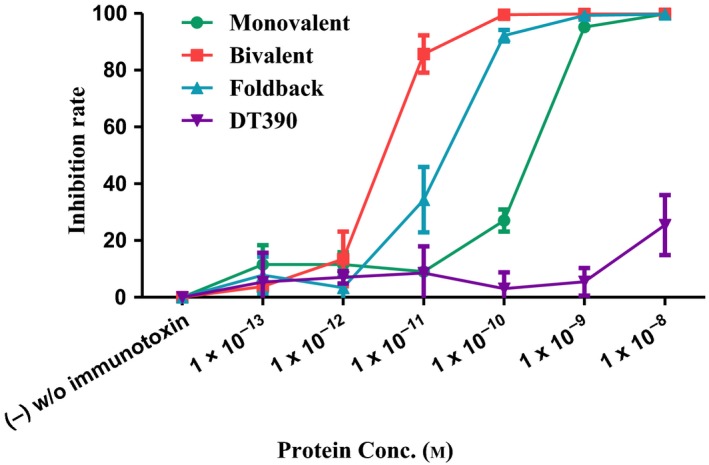
In vitro efficacy analysis of the CD19 immunotoxins using CellTiter‐Glo® Luminescent Cell Viability Assay (Promega, cat# G7571) to human CD19+ mantle cell lymphoma cell line JeKo‐1. (a) Monovalent anti‐human CD19 immunotoxin [DT390‐scFv (FMC63), green line]; (b) bivalent anti‐human CD19 immunotoxin [DT390‐BiscFv (FMC63), red line]; (c) single‐chain foldback diabody anti‐human CD19 immunotoxin (blue line); (d) DT390 alone (purple line). Y‐axis: inhibition rate of the cell viability by determining the number of viable cells based on the quantification of the ATP present. X‐axis: plated anti‐human CD19 immunotoxin concentration. Cycloheximide (1.25 mg·mL−1) was used as a positive control. The negative control contained cells without immunotoxin. p < 0.0001 by log‐rank (Mantel–Cox) test of Prism (n = 3). Error bars indicate ±SD. Data are representative of multiple assays.
3.4. In vitro binding and depletion analysis of the CD19 immunotoxins to human blood or PBMC
CD19 is expressed on normal human B cells. To further characterize the CD19 immunotoxins, we performed in vitro binding and depletion analyses of the CD19 immunotoxins in human blood or PBMC. As shown in Fig. 7A, all three isoforms of the biotinylated CD19 immunotoxins bound to CD19+ human blood in a dose‐dependent fashion. The foldback diabody isoform bound more strongly than the monovalent isoform, while the bivalent isoform was best, which is consistent with the previous binding analysis using the human CD19+ JeKo‐1 cell line. We then further performed in vitro depletion analysis in CD19+ human PBMC using the CD19 immunotoxins. As shown in Fig. 7B, CD19+ human PBMC was depleted in a dose‐dependent manner following incubation of the CD19 immunotoxin with the human PBMC for 48 h. The bivalent and foldback diabody isoforms were better than the monovalent isoform, while the bivalent isoform was the most effective.
Figure 7.
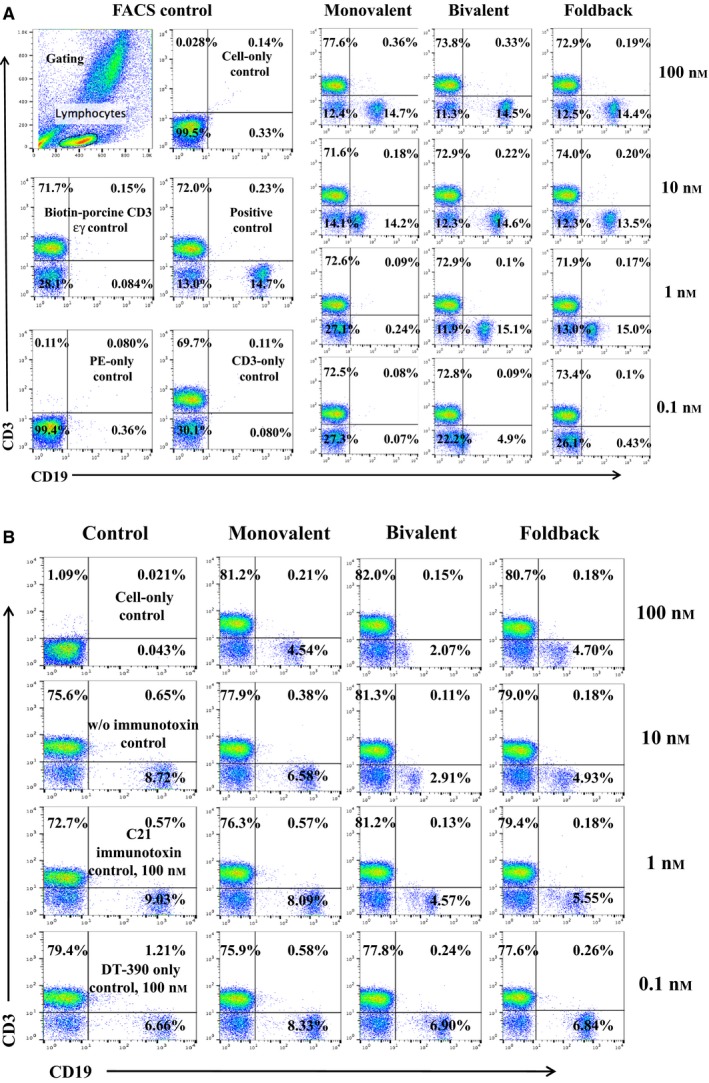
In vitro binding and depletion analysis of the anti‐human CD19 immunotoxins to human CD19+ blood or PBMC. (A) Flow cytometry binding analysis of the anti‐human CD19 immunotoxins to the CD19+ cells within human blood. Monovalent: monovalent anti‐human CD19 immunotoxin [DT390‐scFv (FMC63)]; bivalent: bivalent anti‐human CD19 immunotoxin [DT390‐BiscFv (FMC63)]; foldback: single‐chain foldback diabody anti‐human CD19 immunotoxin. PE‐SA: PE‐conjugated streptavidin; bio‐pCD3εγ: biotin‐labeled porcine CD3εγ (Peraino et al., 2012). (B) In vitro depletion of the CD19+ cells within human PBMC using the anti‐human CD19 immunotoxins. C21 immunotoxin and DT390 alone were included as negative controls. Data of both (A) and (B) are representative of three individual experiments.
3.5. Off‐target analysis of the CD19 immunotoxin
To rule out the off‐target effect of the CD19 immunotoxin, three human CD19− tumor cell lines were analyzed with biotinylated bivalent human CD19 immunotoxin using flow cytometry: (a) CCRF‐CEM, (b) M14, and (c) MD‐MBA‐231. The results demonstrated that there was no binding activity with the three human CD19− tumor cell lines (Fig. 8A). The viability of the three human CD19− tumor cell lines was also analyzed by flow cytometry using 7‐aminoactinomycin D and Annexin V staining following incubation with the bivalent CD19 immunotoxin for 18 h. Their viability was not affected by this treatment (Fig. 8B).
Figure 8.
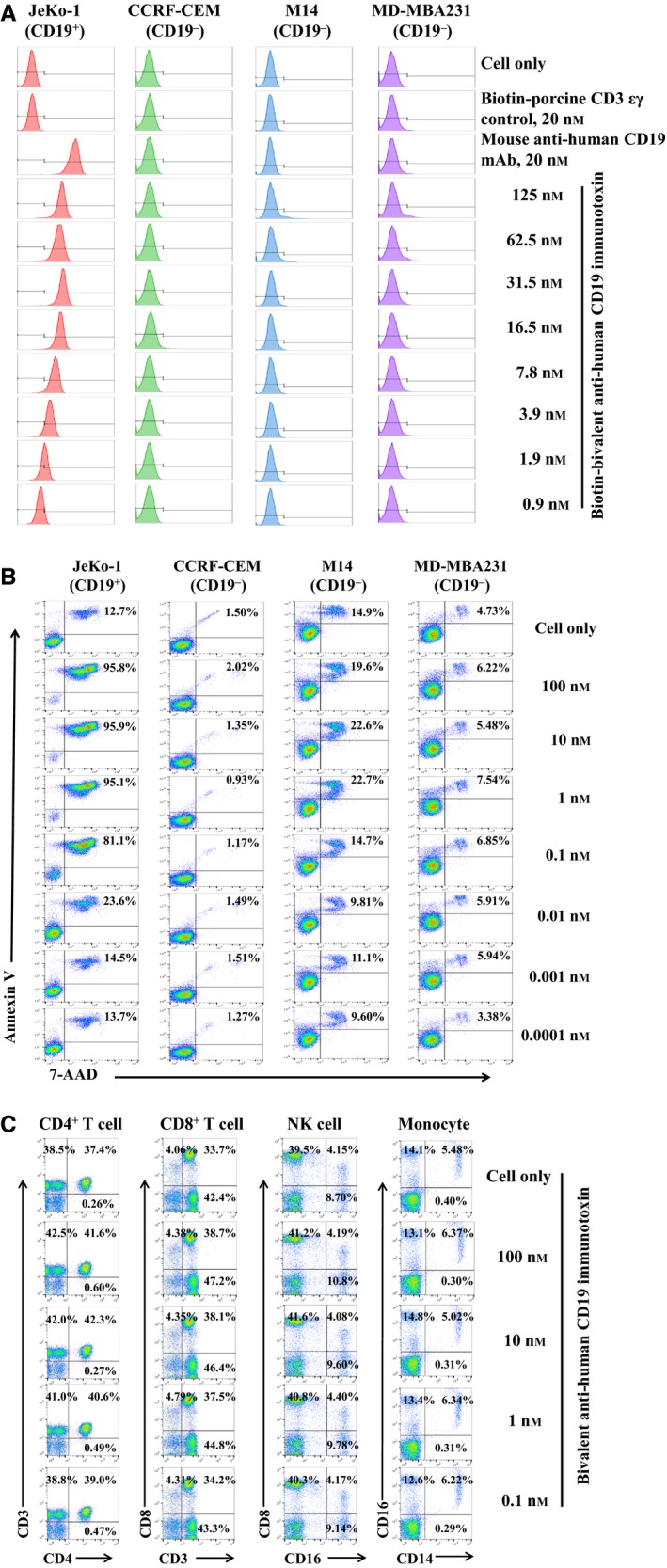
Off‐target analysis of the CD19 immunotoxin. (A) Flow cytometry binding analysis of the biotinylated bivalent anti‐human CD19 immunotoxin to three human CD19− tumor cells (CCRF‐CEM, M14, and MD‐MBA‐231). PE‐mouse anti‐human CD19 mAb was included as positive antibody control, and biotinylated porcine CD3‐εγ (Peraino et al., 2012) as a negative control for background due to protein biotinylation. (B) Cell viability analysis of the three human CD19− tumor cell lines by staining with 7‐ADD and Annexin V following incubation with the bivalent anti‐human CD19 immunotoxin for 18 h. (C) Depletion analysis by flow cytometry for the human CD19− subpopulations within human PBMC following incubation with the bivalent anti‐human CD19 immunotoxin for 48 h. The data are representative of three individual experiments for all of the data in figure.
To further rule out the off‐target effect of the CD19 immunotoxin, we also analyzed the human CD19− PBMC populations following incubation with the bivalent CD19 immunotoxin for 48 h: CD4 T cell (CD3+CD4+), CD8 T cell (CD3+CD8+), NK cells (CD16+CD8+), and monocyte (CD14+CD16+). The results demonstrated that there was no effect on human CD19− PBMC populations (Fig. 8C).
3.6. In vivo efficacy analysis of the CD19 immunotoxins
In vivo efficacy of the CD19 immunotoxins was assessed using human CD19+ JeKo‐1 mantle cell lymphoma‐bearing immunodeficient NSG mouse model. Human CD19+ JeKo‐1 cells (1 × 107) were injected IV and the CD19 immunotoxins were injected IP at 100 μg·kg−1, BID, for four consecutive days as one course, four courses in total, and three‐day break between any two courses. As shown in Fig. 9, the C21 immunotoxin was included as a negative control (n = 7). These data demonstrated that all three CD19 immunotoxin isoforms prolonged animal survival significantly from a median of 31 days in the negative control group (C21 immunotoxin) to 34 days in the monovalent isoform treatment group (n = 7, p < 0.0001), to 36 days in the foldback diabody isoform treatment group (n = 7, p < 0.0001), to 40 days in the bivalent isoform treatment group (n = 7, p < 0.0001) (Fig. 9). Mice receiving the CD19 immunotoxin only did not show any toxicity (data not shown). All animals injected with the human CD19+ JeKo‐1 cells succumbed to tumors (data not shown).
Figure 9.
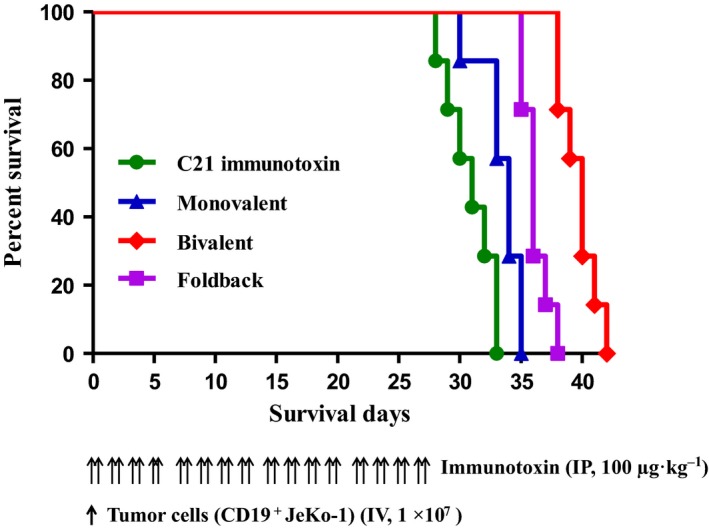
In vivo efficacy analysis of the anti‐human CD19 immunotoxins. NSG mice were injected IV with 1.0 × 107 human CD19+ JeKo‐1 lymphoma cells and treated from day 0 on with the anti‐human CD19 immunotoxin (IP injection) at 100 μg·kg−1 BID for four consecutive days as one course, four courses in total, and three‐day break between any two courses. (a) C21 immunotoxin control group (a nonrelated DT390‐based immunotoxin as negative control) (n = 7, green curve) with a median survival time of 31 days; (b) monovalent anti‐human CD19 immunotoxin group (n = 7, blue curve) with a median survival time of 34 days; (c) bivalent anti‐human CD19 immunotoxin group (n = 7, red curve) with a median survival time of 40 days; (d) single‐chain foldback diabody anti‐human CD19 immunotoxin group (n = 7, purple curve) with a median survival time of 36 days. The schedule of drug and tumor cell injection is pictured in the schematic below the survival curve. The vertical arrows indicate the days on which the tumor cells or anti‐human CD19 immunotoxins were injected.
4. Discussion
Several attempts to develop recombinant anti‐human CD19 immunotoxins have been unsuccessful (Du et al., 2008). We speculate that the use of an inappropriate expression system was part of the problem. Diphtheria toxin‐resistant yeast Pichia Pastoris expression system is suitable for DT390‐based immunotoxin development. We speculate that eukaryotic expression systems such as yeast Pichia Pastoris expression system might be better than prokaryotic expression system such as E. coli.
Based on our previous experience, the foldback diabody immunotoxin isoform is superior to both bivalent and monovalent isoforms in terms of the in vitro binding affinity and efficacy (Kim et al., 2007; Wang et al., 2015). However, the foldback diabody isoform in this study did not improve the in vitro binding affinity and efficacy comparing with the bivalent isoform. Therefore, it is case by case for the immunotoxin development to find the best isoform (Wei et al., 2014).
The in vivo dosing schedule (Fig. 9) in this study has not been optimized. Our focus was simply to assess whether the CD19 immunotoxin functions in vivo. There is still room to increase the dose and duration to improve the animal survival. It is also necessary to characterize the in vivo efficacy further using established xenograft tumor‐bearing NSG mouse model. Furthermore, analyzing the residue tumor cells in vitro to determine whether they are still sensitive to the CD19 immunotoxin is warranted. If they remain sensitive, higher doses and multiple course of treatment could improve animal survival further.
Conventional chemotherapy and targeted mAb therapy have contributed significantly to the treatment for CD19+ B‐cell leukemia/lymphoma. However, there is still an unmet need to develop novel targeted therapies against the relapsing/refractory patients with CD19+ leukemia/lymphoma following the chemotherapy and targeted mAb therapy. We believe that CD19 immunotoxin will provide a novel alternative therapy for the refractory/relapsing CD19+ tumor patients.
Author contributions
QZ and Zhaohui Wang primarily performed the experiments and data analysis as well as participated in writing the manuscript; HZ participated in the experiments; QH partially participated in the experiments; JCM, DHS, and CAH participated in the project design, data analysis, and writing the manuscript; Zhirui Wang primarily designed the project, analyzed the data, and wrote the manuscript.
Acknowledgements
We would like to acknowledge the Joint Ph.D. Student Scholarship for Qian Zheng from China Scholarship Council (CSC). We would like to thank Angimmune LLC for kindly providing the diphtheria toxin‐resistant yeast Pichia Pastoris strain and the codon‐optimized DT390 DNA.
References
- Bachanova V, Frankel AE, Cao Q, Lewis D, Grzywacz B, Verneris MR, Ustun C, Lazaryan A, McClune B, Warlick ED et al (2015) Phase I study of a bispecific ligand‐directed toxin targeting CD22 and CD19 (DT2219) for refractory B‐cell malignancies. Clin Cancer Res 21, 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Beers R, Fitzgerald DJ and Pastan I (2008) Differential cellular internalization of anti‐CD19 and ‐CD22 immunotoxins results in different cytotoxic activity. Cancer Res 68, 6300–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GB, Wang Z, Liu YY, Stavrou S, Mathias A, Goodwin KJ, Thomas JM and Neville DM Jr (2007) A fold‐back single‐chain diabody format enhances the bioactivity of an anti‐monkey anti‐CD3 recombinant diphtheria toxin based immunotoxin. Protein Eng Des Sel 20, 425–432. [DOI] [PubMed] [Google Scholar]
- Liu YY, Woo JH and Neville DM Jr (2003) Targeted introduction of a diphtheria toxin resistant mutation into the chromosomal EF‐2 locus of Pichia pastoris and expression of immunotoxin in the EF‐2 mutants. Protein Expr Purif 30, 262. [DOI] [PubMed] [Google Scholar]
- Murphy JR (2011) Mechanism of diphtheria toxin catalytic domain delivery to the eukaryotic cell cytosol and the cellular factors that directly participate in the process. Toxins 3, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM, Zola H and Hohmann AW (1997) Construction and characterization of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukemia and lymphoma. Mol Immunol 34, 1157–1165. [DOI] [PubMed] [Google Scholar]
- Peraino JS, Hermanrud CE, Springett L, Zhang H, Li G, Srinivasan S, Gusha A, Sachs DH, Huang CA and Wang Z (2012) Expression and characterization of recombinant soluble porcine CD3 ectodomain molecules: mapping the epitope of an anti‐porcine CD3 monoclonal antibody 898H2‐6‐15. Cell Immunol 276, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino JS, Schenk M, Li G, Zhang H, Farkash EA, Sachs DH, Huang CA, Duran‐Struuck R and Wang Z (2013b) Development of a diphtheria toxin‐based recombinant porcine IL‐2 fusion toxin for depleting porcine CD25+ cells. J Immunol Methods 398–399, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino JS, Schenk M, Zhang H, Li G, Hermanrud CE, Neville DM Jr, Sachs DH, Huang CA, Duran‐Struuck R and Wang Z (2013a) A truncated diphtheria toxin based recombinant porcine CTLA‐4 fusion toxin. J Immunol Methods 391, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle CJ, Riddell SR and Maloney DG (2016) CD19‐Targeted chimeric antigen receptor‐modified T‐cell immunotherapy for B‐cell malignancies. Clin Pharmacol Ther 100, 252–258. [DOI] [PubMed] [Google Scholar]
- Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A and Chen H (2005) A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B‐cell leukemia/lymphoma. Clin Cancer Res 11, 3879–3888. [DOI] [PubMed] [Google Scholar]
- Wang Z, Duran‐Struuck R, Crepeau R, Matar A, Hanekamp I, Srinivasan S, Neville DM, Sachs DH and Huang CA (2011) Development of a diphtheria toxin based antiporcine CD3 recombinant immunotoxin. Bioconjug Chem 22, 2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wei G and Liu D (2012) CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol 1, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wei M, Zhang H, Chen H, Germana S, Huang CA, Madsen JC, Sachs DH and Wang Z (2015) Diphtheria‐toxin based anti‐human CCR4 immunotoxin for targeting human CCR4+ cells in vivo . Mol Oncol 9, 1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne AS, Fitzgerald DJ, Kreitman RJ and Pastan I (2014) Immunotoxins for leukemia. Blood 123, 2470–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Marino J, Trowell A, Zhang H, Peraino JS, Rajasekera PV, Madsen JC, Sachs DH, Huang CA, Benichou G et al (2014) Diphtheria toxin‐based recombinant murine IL‐2 fusion toxin for depleting murine regulatory T cells in vivo . Protein Eng Des Sel 27, 289–295. [DOI] [PubMed] [Google Scholar]
- Woo JH, Liu YY, Mathias A, Stavrou S, Wang Z, Thompson J and Neville DM Jr (2002) Gene optimization is necessary to express a bivalent anti‐human anti‐T cell immunotoxin in Pichia pastoris . Protein Expr Purif 25, 270. [DOI] [PubMed] [Google Scholar]


