Abstract
Nanotechnology is expected to play an increasingly important role in the diagnostics, prognostics, and management of targeted cancer treatments. While papers have described promising results for nanotechnology in experimental settings, the translation of fundamental research into clinical applications has yet to be widely adopted. In future, policy makers will need to anticipate new developments for clinical implementation and introduce technology assessments. Here we present an overview of the literature on the technology assessments that have already been undertaken on early stage nanotechnology in cancer care, with particular emphasis placed on clinical efficacy, efficiency, logistics, patient‐related features and technology dynamics.
Owing to the current stage of development of most nanotechnologies, we found only a limited number of publications describing the application of either Health Technology Assessment (HTA) or Constructive Technology Assessment (CTA). In spite of the promising conclusions of most papers concerning the benefits of clinical implementation, actual clinically relevant applications were rarely encountered, and so far only a few publications report application of systematic forms of technology assessment. Most articles consider aspects of environmental safety, regulation and ethics, often mentioning the need to investigate such issues more thoroughly. Evaluation of financial and organizational aspects is often missing. In order to obtain a realistic perspective on the translation and implementation process there is a need for a broad and systematic evaluation of nanotechnologies at early stages of development. Assessment methods taking technology dynamics into account, such as Constructive Technology Assessment (CTA) should be considered for evaluation purposes.
Keywords: Review, Nanotechnology, Genomics, Oncology, Early technology assessment, Dynamics
1. Introduction
Nanotechnology is a promising technology that is playing an increasingly important role in the diagnostics, prognostics, prediction and management of targeted cancer treatment. While most research in this field is still in its infancy, there is widespread agreement that the findings may have an enormous impact on society, with the potential to improve the quality of human life. A widely used definition for nanotechnology is: “The creation and utilization of materials, devices, and systems through the control of matter on the nanometer scale (1–100 nm), i.e., at the level of atoms, molecules, and supramolecular structures” (Jain, 2008c). Resulting from this size range, nanotechnology is suitable for manipulation at the molecular level, with potential applications in drug delivery, imaging, early detection of cancer and cancer research (National Cancer Institute, 2008, 2008, 2006). However, the translation process from a fundamental research tool into clinical practice will need to overcome many hurdles. To guarantee sustainable development, there is an urgent need to understand the impact that novel nano‐materials could have on human health, and to develop reliable methods for risk assessments (Maynard et al., 2006; Singh and Nalwa, 2007). The US Food and Drug Administration (FDA) has indicated that it views regulation of the nano‐industry as a challenge, from the aspect of safety and effectiveness (Pietzsch and Pate‐Cornell, 2008). In the early stage of development, technology dynamics plays an important role since both the technology and the environment influence each other in an interactive way. Methods for evaluating nanotechnologies need to take technology dynamics, related to the development stage, into account. Health Technology Assessment (HTA) is a frequently used evaluation approach, used primarily to enable decisions on coverage and reimbursement of new technologies (Hutton et al., 2007). However, the point at which a new technology should be assessed remains contentious (Mowatt et al., 1997). An HTA generally starts after the technology has been stabilized and proved to be valid in clinical trials. The period between drafting the research design and presentation of the results can take from 6 to 15 years. During this time many changes in existing treatments can occur, with the result that HTA can be answering outdated questions (Douma et al., 2007) (Figure 1).
Figure 1.
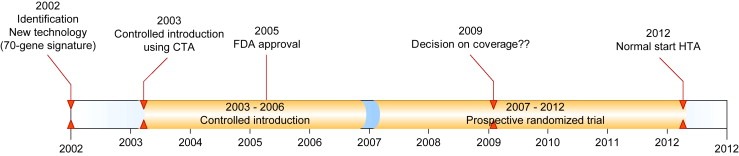
Timelines CTA and HTA The case of the 70‐gene signature.
This is a particularly important issue in the field of nanotechnology, where the pace and scope of developments has the potential to exert a far‐reaching impact on health care.
The theory of constructive technology assessment (CTA) contends that TA can be used to guide technology development in the most beneficial way. In the absence of prospective data defining benefits, clinical implementation of TA for policy decisions may be premature. If, however, we wait to perform a TA, it might very well be that valuable technology is withheld from the public (Ioannidis, 2007). Genomic knowledge is leading to the introduction of new and increasingly personalized diagnostics and treatments, which in turn are leading to even more complex and expensive evaluation designs. Technology dynamics teaches us that, during the course of technology development, choices are constantly being made about the form, function, and use of particular technologies (Schot, 1992). CTA has progressed from strictly assessing the impact of a new technology to a broader approach, including the analysis of design, development, and implementation of the new technology (Schot and Rip, 1996). At different phases of CTA, the focus will shift to the aspects most likely to change during the introduction of these new technologies. CTA covers a broad range of aspects of quality of care following the Institute of Medicine (IOM) (Institute of Medicine (IOM), 2001) recommendations as well as the criteria defined by Poulsen (Poulsen, 2000) (Table 1). Furthermore, CTA uses diffusion scenarios to monitor the dynamics and spread (diffusion) of technology implementation. Diffusion scenarios, which are commonly applied in industry to guide strategies concerning future developments, have been adapted for use in health care technology assessments (Retel et al., 2009).
Table 1.
Search terms for Technology Assessments CTA: it covers aspects of quality of care following the Institute of Medicine (IOM) and criteria defined by Poulsen and uses diffusion scenarios to monitor the dynamics.
| Clinical | Safety, efficacy, effectiveness |
| Economic | Cost‐effectiveness |
| Patient‐related | Ethical/juridical, acceptability, psychosocial reactions, patient centeredness |
| Organizational | Diffusion, adoption, implementation, timeliness, equity, skills/routines/logistics, education/training |
| Scenario/Roadmap | Diffusion scenario (using Rogers phases) |
The aim of this review is to present current literature on methods and results concerning the evaluation of nanotechnologies in cancer care at an early stage and at various stages of diffusion. Related to the early stage of development, we developed a scoring system based on the CTA‐aspects and criteria. Previously, we used these aspects to perform assessments of early implementation of new (nano) technologies in cancer care (Douma et al., 2007; Retel et al., 2008).
2. Methods
Nanotechnology in oncology encompasses many applications, making it difficult to cover all these uses in one review. We formulated a scoring‐system (based on criteria defined by Poulsen and quality aspects of the Institute of Medicine (IOM)) that included factors on clinical and economic information as well as patient‐related organizational aspects and scenarios, see Table 1 (Douma et al., 2007). These aspects were first used in two studies that performed an early technology assessment (Constructive Technology Assessment, CTA) on microarray technology for breast cancer patients. In addition, the mixed method approach of the CTA adapted diffusion scenarios, of the type commonly used in industry to guide future development, was used to monitor the dynamics (Retel et al., 2009, 2008). Since new technologies are often dynamic, even at an early stage of development, the focus of evaluation assessments shifted to the aspects most likely to change during the introduction of new technologies.
In this review, we focus on the terms “nanotechnology” and “oncology” combined with the several CTA‐aspects. References were obtained by PubMed searches using combinations of MeSH search terms, such as : “nanotechnology”/“nanobiotechnology”/“nano‐arrays”/“micro‐arrays”/“biomarkers”/“nanoparticles”, AND “Oncology”/“Cancer” AND “Evaluation”/“Assessment”/“Diffusion”/“Research”/“Effectiveness”/“Efficiency”/“Efficacy”/“Safety”/“Ethics”/“Juridical”/“Organizational”/“Cost‐effectiveness”/“Quality of life” and “Dynamics”. During the search it became apparent that several applications of nanotechnologies are also described by terms such as “nanoparticles” and “nanooncology”. We therefore decided to extend our search with these additional terms, combined with the two CTA‐aspects “safety” and “cost‐effectiveness”, which appeared to be the most relevant aspects evaluated in the field of nanotechnology. No limits were applied to the year of publication, language, or study design. In addition to formal publications and databases,(non)governmental websites, reports, and white papers on nanotechnology and technology assessments were included in the search.
3. Results
The first search using the terms “nanotechnology” AND “oncology” led to a total of 91 results, made up of 46 fundamental articles, 24 reviews, 20 other specified reports and 1 technology assessment (TA) as shown in Table 2 and Figure 2. All articles resulting from the extended search using specific aspects were duplicates of the original search for “nanotechnology” AND “oncology”. The paper explicitly directed at TA gives two examples of technology assessments on nanotechnologies which were evaluated at an early stage of development by monitoring patient‐related aspects, efficiency, and scenario drafting (Retel et al., 2008).
Table Table 2.
Search results.
| Aspects | Total hits PubMed | Fundamental articles | Review articles | Other Articles | TA articles | Relevant references | ||
|---|---|---|---|---|---|---|---|---|
| Nanotechnology | Oncology | 90 | 46 | 24 | 19 | 1* | Retel, 2008*; Jain, 2008c | |
| Nanobiotechnology | Oncology | 3 | – | 2 | – | * | Retel, 2008 | |
| Nanotechnology | Oncology | Evaluation | 11 | 9 | – | 2 | – | |
| Nanotechnology | Oncology | Assessment | 4 | 1 | – | 2 | * | Retel, 2008 |
| Nanotechnology | Oncology | Diffusion | 3 | 2 | – | – | * | Retel, 2008 |
| Nanotechnology | Oncology | Research | 64 | 36 | 17 | 9 | * | Retel, 2008; Hede&Huilgol, 2006; Wilson, 2006 |
| Nanotechnology | Oncology | Effectiveness | 7 | 4 | 3 | – | * | Retel, 2008 |
| Nanotechnology | Oncology | Efficiency | 4 | 4 | – | – | – | |
| Nanotechnology | Oncology | Efficacy | 12 | 6 | 5 | – | * | Retel, 2008 |
| Nanotechnology | Oncology | Safety | 5 | 3 | 1 | – | * | Retel, 2008 |
| Nanotechnology | Oncology | Dynamics | 0 | – | – | – | – | Retel, 2008 |
| Nanooncology | 2** | – | 2 | – | – | Jain, 2008b; Jain, 2008c | ||
| Nanoarrays | Cancer | 4** | 2 | 2 | – | – | ||
| Microarrays | Cancer | Cost‐effectiveness | 8** | 3 | 3 | – | 2* | Retel, 2008; Retel, 2009 |
| Nano particles | Oncology | Cost‐effectiveness | 9** | – | – | – | 9 | Capri, 2003; Forbes, 2002; Fountzilas, 2006; Hjortsberg, 1999; Main, 2006; Pietzsch, 2008; Portney, 2006; Nanotechnology: Horizon Scanning Appraisal, 2006; Wang, 2008 |
Other: Meeting reports, columns, TA: Technology Assessment * Appeared from one article ** Appeared from extended search.
Figure 2.
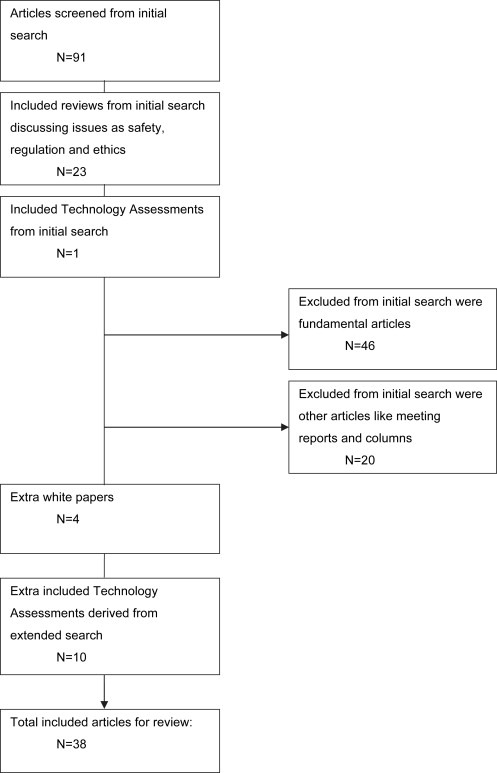
Search method Included and excluded papers from initial and extended search.
Most reviews debate the assessment of safety risks on theoretical grounds, with no actual safety analyses or systematic risk assessments undertaken. Most reviews summarize results of studies reporting the potential for clinical implementation, while the possible implications are often described in the discussion, specifying the need for a form of technology assessment. The major areas where nanomedicine is currently being developed in cancer are early detection and diagnostics and drug‐delivery devices. The results of the search have been structured according to Jain's classification in the Handbook of Nanomedicine (Jain, 2008c) and include a short description of the technology involved.
3.1. Nanotechnology‐based detection
Nanotechnology‐based detection includes cancer detection, biomarkers, and diagnostics.
Photodynamic therapy (PDT) provides one example of cancer detection, also offering the potential for treatment, and involving three key components, a photosensitizer, light and oxygen. 5‐Aminolaevulinic acid (ALA) is an endogenous cellular component that is metabolized within the haem biosynthetic pathway to produce protoporphyrin IX (PpIX), a potent endogenous photosensitizer. Following exogenous administration of 5‐ALA, PpIX is generated intra cellularly, and can then activated by visible light for PDT treatment (Yang et al., 2007). A cost‐effectiveness analysis of PDT as a treatment for advanced head and neck tumors was performed by Hopper et al. (2004) (Hopper et al., 2004) and a TA description of the implementation process was performed by Retel et al. (2008) (Retel et al., 2008).
The second example of cancer detection is Rapid Detection of Single Nucleotide Polymorphism (SNP), an emerging technology in the field of biomarkers using a Nano Magnetic Device. Here DNA microarrays labeled with gold nanoparticles (Au‐np) are used to make the detection of SNPs, known to be associated with hereditary conditions and cancers, more efficient and less time consuming. It is, however, not clear what costs will be involved and what the exact application of this field will be (Cox et al., 2007; Easton et al., 2007; Miller et al., 2008). While there are eight articles describing what the cost efficiency of SNP should be relative to other cancer detection methods, no solid cost‐effectiveness analyses have been undertaken on the subject.
For cancer diagnostics, Quantum Dots (QDs), coated with a polyacrylate cap and covalently linked to antibodies, have been used for immunofluorescence labeling of the breast cancer marker Her‐2 (Wu et al., 2003). An article by Hardman (2006) was identified concerning QDs in general and the potential for toxicity for humans (see also below) (Hardman, 2006).
Microarray analysis, used for gene expression profiling, offers another diagnostic and prognostic approach. An example is the 70‐gene signature, identified at the Netherlands Cancer Institute (NKI‐AVL) in Amsterdam, with a performed early cost‐effectiveness analysis regarding the potential benefits and policy implications of gene expression profiling in clinical practice (Oestreicher et al., 2005). Furthermore, a Constructive Technology Assessment (CTA) appeared to be a helpful approach to monitor, evaluate and anticipate the early introduction of this new technology in daily practice. Moreover, the CTA method was helpful in Coverage with the Evidence Development (CED) procedure (Retel et al., 2009).
3.2. Nanotechnology‐based imaging
Quantum Dots (QDs) Aided Lymph Node Mapping is an improved method for performing sentinel lymph node (SLN) biopsy, where the QDs emit NIR light that is used to identify lymph nodes during surgery (Kim et al., 2004). SLN mapping has already revolutionized cancer surgery and the introduction of NIR QDs offers the possibility to improve the technique further. However, since QDs are composed of heavy metals they pose potential risks to human health and the environment, and therefore have yet to be approved for human applications (Hardman, 2006).
3.3. Nanotechnology‐based drug delivery
Nanoscale devices can serve as targeted drug‐delivery vehicles carrying chemotherapeutic agents or therapeutic genes directly into malignant cells. Examples of such drug‐delivery devices for breast or non‐small‐cell lung cancer include albumin‐bound 130 nm particle formulation of paclitaxel for injectable suspension (‘Abraxane®’, Abraxis BioScience, Inc.), approved by the FDA for metastatic breast cancer, and doxorubicin‐loaded, long‐circulating, polyethylene glycol‐coated liposomes (‘Doxil®’, ALZA Corp.). A phase III trial evaluating use of Abraxane® as a vehicle showed it eliminated solvent‐related toxicities and overcame the need for steroid and antihistamine premedication (Green et al., 2006). An economic evaluation of albumin‐bound paclitaxel versus Docetaxel has been performed, with a favorable result for albumin‐bound paclitaxel (Dranitsaris et al., 2008).
The second FDA approved nanoparticle formulation for drug delivery is the folate‐linked liposomal doxorubicin (Doxil), a reformulated version of Doxorubicin. Doxil has been validated in a phase III trial for multiple myeloma patients and is also indicated for metastatic ovarian cancer and AIDS‐related Kaposi's sarcoma (Hussein et al., 2002). Nine cost‐effectiveness analyses were performed regarding pegylated liposomal doxorubicin, and two cost‐minimization analyses (Capri and Cattaneo, 2003; Ojeda et al., 2003). CEA's concerning ovarian cancer (Forbes et al., 2002; Main et al., 2006; Smith et al., 2002), multiple myeloma (Porter and Rifkin, 2007), AIDS‐related Kaposi's sarcoma (Hjortsberg et al., 1999; Vanni et al., 2006), and head and neck cancer (Fountzilas et al., 2006) all found in favor of the new technology. It should, however, be noted that most of the economic evaluations dealt with only with one good quality randomized controlled trial (RCT), and as a result most evaluations concluded that more evidence was needed to provide a clearer picture of clinical effectiveness.
3.4. Nanoparticles
Nanoparticles have been used in several applications such as imaging, targeting tumors, drug delivery and in combination with other physical agents for tumor ablation, such as brachytherapy (Jain, 2008c). BrachySil™ a nanoengineered Silicon for Brachytherapy, was shown to be safe and effective in a phase IIa trial for primary liver cancer (Goh et al., 2007). Faunce, however, has raised major concerns regarding highly reactive and mobile engineered nanoparticles (ENPs), suggesting that they may present health risks when used in medical applications. Disturbingly, there appears to be no effective methods for monitoring ENP exposure in patients or health care workers (Faunce, 2007). Wang et al. (2008) raised critical questions, such as whether there might be changes in the safety profile of nanoparticles after conjugation, that they say need to be addressed before further clinical development. Hede & Huilgol have reported on various applications of nanotechnology in oncology, particularly on those that are already in clinical trial and those which are in the pipeline for commercialization, like radioactive nanoparticles (ongoing phase II, 2006) and nanoparticles of Paclitaxel (ongoing phase I, 2006). They state that these nanoparticle ionizing radiation and chemotherapeutic agents are the only nanotechnology innovations that at present seem to be feasible for implementation in clinical practice in terms of ‘’improvised” treatment and cost‐effectiveness. They conclude that extensive studies on environmental safety aspects should be conducted and predictive models must be developed to forecast long‐term toxicities (Hede and Huilgol, 2006). Jain has reported on several applications of nanooncology (Jain, 2008, 2008, 2005, 2007, 2003), pointing out that there are still many unanswered questions concerning the introduction of nanoparticles into the living body. Empirical evidence for the basis of those concerns, however, is not provided. One recent development, the use of nanoparticles in oncoproteomics, although promising, has yet to be translated from bench to bedside (Jain, 2008a). Jain described safety concerns relating to the potential toxic effects of in vivo nanoparticles, raising questions about the environmental effects of releasing nanoparticles during the manufacturing process (Jain, 2007).
3.5. Regulation of nanotechnologies in general
In the Journal of Law, Medicine and Ethics, Wilson states that it is unclear whether and to what degree nanotechnology is safe, suggesting that the response should be to the real rather than the perceived or theoretical risks (Wilson, 2006). In another article in the same journal, Faunce and Shats argue that a broader approach to the regulation of nanotherapeutics needs to be taken, and that issues such as workplace safety and environmental impact should not be ignored. Many individuals, they add, are concerned that “nanoparticles could become the asbestos of the 21st century” (Faunce and Shats, 2007).
3.6. Ethics
Ethical issues most often appear in “general health” articles about nanotechnologies, for example those concerning food manipulation, and are not specific for the oncology field.
Quality of life issues are not yet reported, but have on occasion been mentioned briefly in reviews.
3.7. Additional reports
Besides the PubMed search, relevant white papers were found such as Ontario, a Horizon Scanning Appraisal (The Medical Advisory Secretariat, 2006), a Technology Assessment on nanotechnologies from TA‐Swiss (Baumgartner et al., 2003), a RAND report (Silberglitt et al., 2006), and an FDA report (US Food and Drug Administration Nanotechnology Taskforce, 2007). The papers, which descriptively review the recent literature, identified promising technologies and conclude that clinical implementation and research is still rare, and that no systematic TA had been performed.
4. Discussion
The aim of this review was to present an abridged interpretation of the current literature on methods and results of studies evaluating nanotechnologies in cancer care. While the literature regarding fundamental research on nanotechnologies can appear overwhelming, reports on technology assessments of actual clinical applications and implementation processes are scarce. We found that while most articles focus on the theoretical aspects of regulation and (environmental) safety, they lack empirical data, and provided no structured evaluation of dynamics, health economics or organizational aspects.Abraxane and Doxil are two nanotechnology‐based products that have received FDA approval for treating cancer. CEAS concerning these products have concluded that the technologies are less costly than current approaches, but require further high‐quality randomized controlled trials to provide a clearer picture of clinical effectiveness. Discussions on theoretical safety issues seem to dominate the debate on clinical translation and implementation, with few papers concerning clinical effectiveness and cost. The paucity of research addressing these issues appears to have halted progress on broader evaluation. At the level of the technology, aspects of technical feasibility, clinical utility, and potential areas of application are being studied, all of which may steer further technological development. Evidently, knowledge about biological interaction and function is needed to understand the underlying mechanisms. At a societal level, studies focus on ethical considerations and the environmental impact of nanotechnology to public health, with such research supporting policy making with respect to law and regulation. Even though the first treatments based on nanotechnology have received FDA approval there has been little sign of any moves to introduce legal regulation, despite growing concerns that “nanoparticles could become the asbestos of the 21st century”. A more comprehensive type of technology assessment, as conducted by a Constructive Technology Assessment, can improve the pro‐active fine tuning of the decision‐making processes of both governmental policy makers and technological developers. Regulation can then take the traditional safety issues into account, in addition to issues such as workplace safety and environmental impacts as suggested by Faunce & Shats (Faunce and Shats, 2007). What has been lacking in the current research is an analysis of the effects of nanotechnology at the level of health care organization. For instance, if new devices or selective/targeted therapies are to be introduced, health care processes are likely to undergo radical changes, affecting patients as well as health care professionals. Nanotechnology is likely to impact the organization of care, and in its turn, the organizational context will influence how nanotechnology can be applied to the new processes of care. Hospital‐based technology assessment will be required, evaluating the consequences of using specific technologies in organizational settings, which should consider aspects such as the diffusion rate of the technology, implementation, and logistics. In hospital‐based technology assessments perhaps the first place to start would be an evaluation of devices such as lab‐on‐a‐chip or single nucleotide polymorphisms. Ultimately in the hospital setting, nanotechnology is likely to have an impact on patient communication, guidelines, safety protocols and investments in staffs and other resources. For a start to be made on the assessment process it is important to leave theoretical considerations to one side and focus attention first on actual early stage technology. In addition to consideration of effectiveness and safety, it will be necessary to monitor and evaluate organizational aspects of nanotechnology including adoption, routines and logistics, and to observe the environment in which the technology is being utilized. Initially in the early phases of introduction it is likely that just a few experts will adopt the technology, but it is important to consider potential implications of wider use, such as whether the technology is difficult to understand or to implement in daily routines and whether it might prove controversial. As the technology adopted by more user sites it will be important to canvass patient opinion and to consider the financial implications. In addition it may be valuable to consider future scenarios that may be helpful in detecting potential areas for concern.
To conclude, in this paper we have established that a chasm exists between the potential for clinical use of nanotechnology and the actual evidence base derived from technology assessments. Performing HTA or CTA at an early stage as possible should help decide on the priorities to be set both the development of nanotechnology and also in defining our subsequent approach to assessments.
Acknowledgements
Special acknowledgements to Jacques Neefjes, Theo Ruers and Fiona Stewart for their input.
Retèl Valesca P., Hummel Marjan J.M., van Harten Wim H., (2009), Review on early technology assessments of nanotechnologies in oncology, Molecular Oncology, 3, doi: 10.1016/j.molonc.2009.05.001.
References
- Baumgartner, W. , Jackli, B. , Schmithusen, B. , Weber, F. , Borrer, C. , Bucher, C. , Hausmann, M. , 2003. Nanotechnology in Medicine TA-SWISS, Centre for Technology Assessment; Bern: 25–35. Ref Type: Report [Google Scholar]
- Capri, S. , Cattaneo, G. , 2003. Cost-minimization analysis of pegylated liposomal doxorubicin versus topotecan for the treatment of ovarian cancer in Italy. Clin. Ther.. 25, (6) 1826–1845. [DOI] [PubMed] [Google Scholar]
- Cox, A. , 2007. A common coding variant in CASP8 is associated with breast cancer risk. Nat. Genet.. 39, (3) 352–358. [DOI] [PubMed] [Google Scholar]
- Douma, K.F. , Karsenberg, K. , Hummel, M.J. , Bueno-de-Mesquita, J.M. , van Harten, W.H. , 2007. Methodology of constructive technology assessment in health care. Int. J. Technol. Assess. Health Care. 23, (2) 162–168. [DOI] [PubMed] [Google Scholar]
- Dranitsaris, G. , Cottrell, W. , Spirovski, B. , Hopkins, S. , 2008. Economic analysis of albumin-bound paclitaxel for the treatment of metastatic breast cancer. J. Oncol. Pharm. Pract.. [DOI] [PubMed] [Google Scholar]
- Easton, D.F. , 2007. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 447, (7148) 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faunce, T. , Shats, K. , 2007. Researching safety and cost-effectiveness in the life cycle of nanomedicine. J. Law Med.. 15, (1) 128–135. [PubMed] [Google Scholar]
- Faunce, T.A. , 2007. Nanotherapeutics: new challenges for safety and cost-effectiveness regulation in Australia. Med. J. Aust.. 186, (4) 189–191. [DOI] [PubMed] [Google Scholar]
- Forbes, C. , Wilby, J. , Richardson, G. , Sculpher, M. , Mather, L. , Riemsma, R. , 2002. A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer. Health Technol. Assess.. 6, (23) 1–119. [DOI] [PubMed] [Google Scholar]
- Fountzilas, G. , 2006. Paclitaxel and gemcitabine vs. paclitaxel and pegylated liposomal doxorubicin in advanced non-nasopharyngeal head and neck cancer. An efficacy and cost analysis randomized study conducted by the Hellenic Cooperative Oncology Group. Ann. Oncol.. 17, (10) 1560–1567. [DOI] [PubMed] [Google Scholar]
- Goh, A.S. , 2007. A novel approach to brachytherapy in hepatocellular carcinoma using a phosphorous32 (32P) brachytherapy delivery device–a first-in-man study. Int. J. Radiat. Oncol. Biol. Phys.. 67, (3) 786–792. [DOI] [PubMed] [Google Scholar]
- Green, M.R. , Manikhas, G.M. , Orlov, S. , Afanasyev, B. , Makhson, A.M. , Bhar, P. , Hawkins, M.J. , 2006. Abraxane, a novel cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol.. 17, (8) 1263–1268. [DOI] [PubMed] [Google Scholar]
- Hardman, R. , 2006. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ. Health Perspect.. 114, (2) 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hede, S. , Huilgol, N. , 2006. “Nano”: the new nemesis of cancer. J. Cancer Res. Ther.. 2, (4) 186–195. [DOI] [PubMed] [Google Scholar]
- Hjortsberg, C. , Persson, U. , Lidbrink, E. , Bennett, C. , 1999. Cost-effectiveness analysis of pegylated-liposomal doxorubicin and liposomal daunorubicin treatments in patients with Kaposi's sarcoma. Acta Oncol.. 38, (8) 1063–1067. [DOI] [PubMed] [Google Scholar]
- Hopper, C. , Niziol, C. , Sidhu, M. , 2004. The cost-effectiveness of Foscan mediated photodynamic therapy (Foscan-PDT) compared with extensive palliative surgery and palliative chemotherapy for patients with advanced head and neck cancer in the UK. Oral. Oncol.. 40, (4) 372–382. [DOI] [PubMed] [Google Scholar]
- Hussein, M.A. , Wood, L. , Hsi, E. , Srkalovic, G. , Karam, M. , Elson, P. , Bukowski, R.M. , 2002. A Phase II trial of pegylated liposomal doxorubicin, vincristine, and reduced-dose dexamethasone combination therapy in newly diagnosed multiple myeloma patients. Cancer. 95, (10) 2160–2168. [DOI] [PubMed] [Google Scholar]
- Hutton, J. , Trueman, P. , Henshall, C. , 2007. Coverage with evidence development: an examination of conceptual and policy issues. Int. J. Technol. Assess. Health Care. 23, (4) 425–432. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM), 2001. Crossing the Quality Chasm: a New Health System for the 21st Century National Academy Press; [PubMed] [Google Scholar]
- Ioannidis, J.P. , 2007. Is molecular profiling ready for use in clinical decision making?. Oncologist. 12, (3) 301–311. [DOI] [PubMed] [Google Scholar]
- Jain, K.K. , 2008. Innovations, challenges and future prospects of oncoproteomics. Mol. Oncol.. 153–160. no. 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, K.K. , 2008. Nanomedicine: application of nanobiotechnology in medical practice. Med. Princ. Pract.. 17, (2) 89–101. [DOI] [PubMed] [Google Scholar]
- Jain, K.K. , 2008. Recent advances in nanooncology. Technol. Cancer Res. Treat.. 7, (1) 1–13. [DOI] [PubMed] [Google Scholar]
- Jain, K.K. , 2008. The Handbook of Nanomedicine Springer/ Humana Press; Totowa, USA: [Google Scholar]
- Jain, K.K. , 2005. Role of nanobiotechnology in developing personalized medicine for cancer. Technol. Cancer Res. Treat.. 4, (6) 645–650. [DOI] [PubMed] [Google Scholar]
- Jain, K.K. , 2007. Applications of nanobiotechnology in clinical diagnostics. Clin. Chem.. 53, (11) 2002–2009. [DOI] [PubMed] [Google Scholar]
- Jain, K.K. , 2003. Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert Rev. Mol. Diagn.. 3, (2) 153–161. [DOI] [PubMed] [Google Scholar]
- Kim, S. , 2004. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol.. 22, (1) 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main, C. , Bojke, L. , Griffin, S. , Norman, G. , Barbieri, M. , Mather, L. , Stark, D. , Palmer, S. , Riemsma, R. , 2006. Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation. Health Technol. Assess.. 10, (9) 1–132. [DOI] [PubMed] [Google Scholar]
- Maynard, A.D. , 2006. Safe handling of nanotechnology. Nature. 444, (7117) 267–269. [DOI] [PubMed] [Google Scholar]
- Miller, M.R. , Dunham, J.P. , Amores, A. , Cresko, W.A. , Johnson, E.A. , 2008. Rapid and cost-effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genomic. Res.. 17, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt, G. , Bower, D.J. , Brebner, J.A. , Cairns, J.A. , Grant, A.M. , McKee, L. , 1997. When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies. Health Technol. Assess.. 1, (14) i–149. [PubMed] [Google Scholar]
- National Cancer Institute, 2008. Nanotechnology in Cancer: Tools to Relieve Human Suffering Available at: http://nano.cancer.gov/resource_center/tech_backgrounder.asp Ref Type: Generic [Google Scholar]
- National Cancer Institute, 2008. A Snapshot of Nanotechnology Available at: http://planning.cancer.gov/disease/Nanotechnology-Snapshot.pdf Ref Type: Report [Google Scholar]
- Oestreicher, N. , Ramsey, S.D. , Linden, H.M. , McCune, J.S. , Van't Veer, L.J. , Burke, W. , Veenstra, D.L. , 2005. Gene expression profiling and breast cancer care: what are the potential benefits and policy implications?. Genet Med.. 7, (6) 380–389. [DOI] [PubMed] [Google Scholar]
- Ojeda, B. , de Sande, L.M. , Casado, A. , Merino, P. , Casado, M.A. , 2003. Cost-minimisation analysis of pegylated liposomal doxorubicin hydrochloride versus topotecan in the treatment of patients with recurrent epithelial ovarian cancer in Spain. Br. J. Cancer. 89, (6) 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzsch, J.B. , Pate-Cornell, M.E. , 2008. Early technology assessment of new medical devices. Int. J. Technol. Assess. Health Care. 24, (1) 36–44. [DOI] [PubMed] [Google Scholar]
- Porter, C.A. , Rifkin, R.M. , 2007. Clinical benefits and economic analysis of pegylated liposomal doxorubicin/vincristine/dexamethasone versus doxorubicin/vincristine/dexamethasone in patients with newly diagnosed multiple myeloma. Clin. Lymphoma Myeloma.. 7, (Suppl. 4) S150–S155. [DOI] [PubMed] [Google Scholar]
- Portney, N.G. , Ozkan, M. , 2006. Nano-oncology: drug delivery, imaging, and sensing. Anal. Bioanal. Chem.. 384, (3) 620–630. [DOI] [PubMed] [Google Scholar]
- Poulsen, P.B. , 2000. Health Technology Assessment and Diffusion of Health Technology: 2000 Odense University Press; Denmark: [Google Scholar]
- Retel, V.P. , 2009. Constructive Technology Assessment (CTA) as a tool in coverage with evidence development: the case of the 70-gene prognosis signature for breast cancer diagnostics. Int. J. Technol. Assess. Health Care. 25, (1) 73–83. [DOI] [PubMed] [Google Scholar]
- Retel, V.P. , Hummel, M.J. , van Harten, W.H. , 2008. Early phase technology assessment of nanotechnology in oncology. Tumori. 94, 284–290. [DOI] [PubMed] [Google Scholar]
- Schot, J. , Rip, A. , 1996. The past and future of constructive technology assessment. Technol. Forecast. Soc. Change. 54, 251–268. [Google Scholar]
- Schot, J.W. , 1992. Constructive technology assessment and technology dynamics: the case of clean technologies. Sci. Technol. Hum. Values. 17, (1) 36–56. [Google Scholar]
- Silberglitt, R. , Anton, P. , Howell, D. , Wong, A. , 2006. The Global Technology Revolution 2020 1–28 RAND Corporation; Pittsburgh: Ref Type: Report [Google Scholar]
- Singh, S. , Nalwa, H.S. , 2007. Nanotechnology and health safety–toxicity and risk assessments of nanostructured materials on human health. J. Nanosci. Nanotechnol.. 7, (9) 3048–3070. [DOI] [PubMed] [Google Scholar]
- Smith, D.H. , Adams, J.R. , Johnston, S.R. , Gordon, A. , Drummond, M.F. , Bennett, C.L. , 2002. A comparative economic analysis of pegylated liposomal doxorubicin versus topotecan in ovarian cancer in the USA and the UK. Ann. Oncol.. 13, (10) 1590–1597. [DOI] [PubMed] [Google Scholar]
- The Medical Advisory Secretariat, 2006. OMoHaL-TC. Nanotechnology, Horizon Scanning Appraisal 1–40 The Medical Advisory Secretariat; Ref Type: Report [Google Scholar]
- US Food and Drug Administration Nanotechnology Taskforce, 2007. Nanotechnology, a Report of the U.S. Food and Drug Administration Nanotechnology Task Force Public Health Service; Ref Type: Report [Google Scholar]
- Vanni, T. , Fonseca, B.A. , Polanczyk, C.A. , 2006. Cost-effectiveness analysis comparing chemotherapy regimens in the treatment of AIDS-related Kaposi's sarcoma in Brazil. HIV Clin. Trials. 7, (4) 194–202. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Yang, L. , Chen, Z.G. , Shin, D.M. , 2008. Application of nanotechnology in cancer therapy and imaging. CA Cancer J. Clin.. 58, (2) 97–110. [DOI] [PubMed] [Google Scholar]
- Wilson, R.F. , 2006. Nanotechnology: the challenge of regulating known unknowns. J. Law Med. Ethics. 34, (4) 704–713. [DOI] [PubMed] [Google Scholar]
- Wu, X. , Liu, H. , Liu, J. , Haley, K.N. , Treadway, J.A. , Larson, J.P. , Ge, N. , Peale, F. , Bruchez, M.P. , 2003. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol.. 21, (1) 41–46. [DOI] [PubMed] [Google Scholar]
- Yang, T.-H. , Chen, C.-T. , Wang, C.-P. , Lou, P.-J. , 2007. Photodynamic therapy suppresses the migration and invasion of head and neck cancer cells in vitro. Oral. Oncol.. 43, 358–365. [DOI] [PubMed] [Google Scholar]


