Abstract
Stroke is associated with over-production of misfolded and aggregating proteins. However, it remains largely unclear whether enhanced removal of protein aggregates following ischemic stroke is neuroprotective. Deubiquitinating enzymes (DUBs) are a large group of proteases that regulate protein degradation. The ubiquitin-specific protease 14 (USP14) is a DUB that is associated with the proteasome and negatively regulates proteasome activity. In this study, we examined the effect of IU1, a specific small molecule inhibitor of USP14, on mouse focal cerebral ischemic stroke-induced neuronal injury in mice. We found that IU1 treatment attenuated ischemic stroke-caused neuronal injury, which was reflected by increased survival rate, reduced infarct volume, as well as decreased neuronal loss in the IU1-treated mice compared to the control-treated mice. Additionally, IU1 treatment is associated with reduced protein aggregates and enhanced proteasome functionality. These data not only highlight the significance of protein homeostasis in cerebral ischemia/reperfusion-induced neuronal injury but also extend the therapeutic role of DUB inhibitors.
Keywords: stroke, ischemia, USP14, IU1, deubiquitinating enzymes, small-molecule inhibitor
INTRODUCTION
Ischemic stroke, a major health problem around the world, causes high mortality and long-term disability. However, developing effective therapeutics for stroke has been a major challenge. Neuropathologically, cerebral ischemia/reperfusion (I/R) causes oxidative stress and neuroinflammation, resulting in over-production of misfolded and aggregating proteins in the brain (Ge et al. 2007, Chen et al. 2012). To develop effective therapies for stroke, it is important to determine whether augmented removal of damaged proteins is neuroprotective.
The ubiquitin (Ub)-proteasome system (UPS) plays a critical role in the removal of abnormal proteins. Unneeded proteins that are targeted to the proteasome are ubiquitinated through the sequential covalent addition of a tract of Ub molecules (polyUb) to protein substrates, which function as tags marking proteins for degradation (Hochstrasser 1996). Ubiquitination is a regulated and reversible process. The ubiquitin chain can be trimmed or removed by a set of enzymes known as deubiquitinating enzymes (DUBs) (Lee et al. 2011).
DUBs are a large group of proteases that are classified into five families. The Ub-specific proteases (USPs) are the largest DUB family among the five families, consisting of 58 enzymes and one of them is USP14. USP14 is reversely associated with the proteasome to trim K48 Ub chains and negatively regulates proteasome activity (Lee et al. 2011). As DUBs play critical roles in protein homeostasis (also known as proteostasis) and regulation of various cellular activities, they are likely therapeutic targets for a number of diseases. Indeed, many DUB inhibitors have been tested in treating cancers (Sippl et al. 2011). Interestingly, USP14 activity is significantly upregulated in T cells-derived from elderly donors (Ponnappan et al. 2013). Inhibition of USP14 activity by a small molecule, 1-[1-(4-fluorophenyl)-2,5-dimethylpyrrol-3-yl]-2pyrrolidin-1-ylethanone (IU1), has been shown to enhance proteasome (Lee et al. 2010) and autophagy activities (Xu et al. 2016), to reduce oxidative stress-induced cell death in vitro (Lee et al. 2010), and to attenuate intrapulmonary inflammatory response during ventilator-induced lung injury (Xu & Guo 2014). As protein aggregation and proteasome dysfunction occur following cerebral I/R (Ge et al. 2007), we hypothesize that enhanced proteostasis reduces I/R-caused neuronal injury. To test the hypothesis, in this study we examined whether a USP14-specific inhibitor, IU1, offers a neuroprotective role against ischemic stroke-induced brain injury.
MATERIALS AND METHODS
Animals
Adult C57BL/6 male mice (between 2–3 months of age; mean body weight 25 g) were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Sample size calculations and power analysis were performed using the statistical software Stata (StataCorp LP, College Station, TX, USA). Animals were randomly separated into vehicle and IU1 treatment groups, or sham and surgery groups. A proteasome reporter, GFPu, transgenic mouse has previously been described (Liu et al. 2014a). Mice were maintained in a temperature and humidity controlled environment with a 12 h light/dark cycle and with ad libitum access to food and water. All animal maintenance and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota and were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Transient middle cerebral artery occlusion (MCAO)
MCAO surgery was according to a previously described method (Lu & Wang 2012). After surgery, body temperature of the mice was maintained at 37°C using a heating pad. When the mice regained complete consciousness, their neurological deficit was evaluated using a 5-point scale as previously described (Atochin et al. 2003). Only the animals with scores between 2 and 3 were included in the experiments. Those mice with score 1, indicating unsuccessful MCAO, or 4, indicating less chance of survival, were sacrificed and excluded from the experiments. Additionally, to ensure that only the mice receiving successful surgery were included in the experiments, blood flow in the MCA was monitored by a laser Doppler blood flowmeter (Vasamed, Eden Prairie, MN, USA) during and after MCAO.
Drug treatments, infarct volume measurements, and animal behavior tests
Drug treatment experiments were carried out in a blinded manner such that the researchers who performed an animal test did not know what treatment the animal received. For acute neuronal injury tests via TTC staining, mice were intraperitoneally injected twice or once either with IU1 (400 μg/kg, Sigma, St. Louis, USA) or an equal volume of vehicle (saline) at day 0 (surgery day), as outlined in Fig. 1A and Fig. 1D, respectively. Mouse brains were allowed to re-perfuse for 24 h before being sacrificed for assessing brain injury by staining brain slices with TTC (Sigma, St. Louis, MO, USA), as previously described (Lu & Wang 2012, Liu et al. 2014b). Infarct volume was calculated as percentage of corrected infarct volume [contralateral hemisphere volume – (ipsilateral hemisphere volume – infarct volume)]/contralateral hemisphere volume × 100/100. Alternatively, the mice were allowed to survive until day 7 and received daily IU1 injection (400 μg/kg) to examine animal body weight, survival rate, and functional recovery by assessing the modified neurological severity scores (mNSSs, see Table 1) according to a previously described method (Chen et al. 2005). The mNSS includes motor, reflex, and balance tests, with a range of 0 to 14 (0, normal; maximal deficit score 14). One score point was given to a mouse for its failure in performing a specific test (Table 1). Therefore, the score is inversely correlated with brain function. The behavioral tests were performed at day 1, 3, 5 and 7 after MCAO.
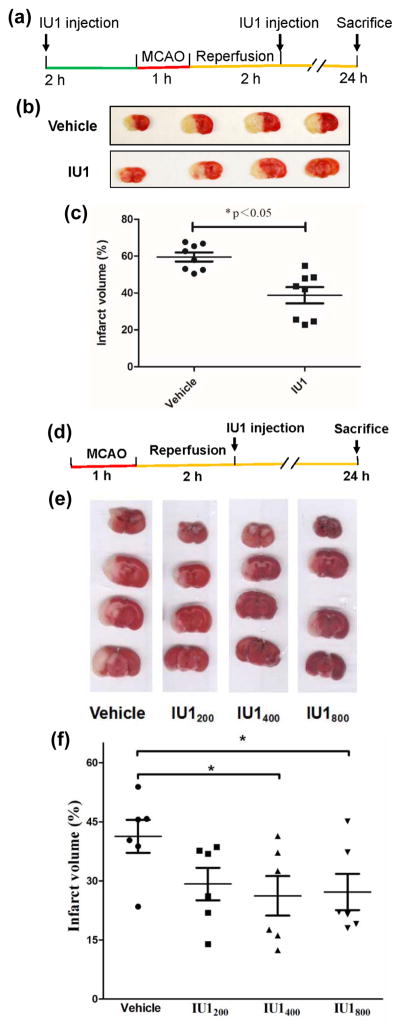
Fig. 1. IU1 treatment reduces I/R-caused infarct volume.
A. A diagram illustrating the regime for the experiments performed in 1B & 1C.
B. Representative images of TTC-stained mouse brains treated twice either with IU1 or vehicle.
C. Measurement of infarct volume. Data are shown as Mean ± SEM; n = 8 in each group; *p < 0.05 (Student’s t test).
D. A diagram illustrating the regime for the experiment performed in 1E & 1F.
E. Representative images of TTC-stained mouse brains treated once either with vehicle or IU1.
F. Measurement of infarct volume. Data are shown as Mean ± SEM; n = 6 in each group; *p < 0.05 (Tukey’s test).
Table 1.
Modified neurologic severity scores (mNSS) (Chen et al. 2005)
| Behavioral Tests
|
Sore points
|
|---|---|
| Raising the mouse by the tail | |
| • Flexion of forelimb | 1 |
| • Flexion of hindlimb | 1 |
| • Head moved more than 10° to the vertical axis within 30 seconds | 1 |
| Walking on the floor (normal = 0; deficits = 1–3) | |
| • Normal walk | 0 |
| • Inability to walk straight | 1 |
| • Circling toward the paretic side | 2 |
| • Falling down to the paretic side | 3 |
| Beam balance tests (normal = 0; deficits = 1–6) | |
| • Balances with steady posture | 0 |
| • Grasps side of beam | 1 |
| • Hugs the beam and one limb falls down from the beam | 2 |
| • Hugs the beam and two limbs fall down from the beam, or spins on beam for more than 30 seconds | 3 |
| • Attempts to balance on the beam but falls off >20 seconds | 4 |
| • Attempts to balance on the beam but falls off >10 seconds | 5 |
| • Falls off: No attempt to balance or hang on to the beam >10 seconds | 6 |
| Reflexes absence (normal = 0; deficits =1–2) | |
| • Absence of pinna reflex (normal, head shake upon touching the auditory meatus) | 1 |
| • Absence of corneal reflex (normal, an eye blink upon gently touching the cornea with cotton) | 1 |
|
| |
| Maximum score points | 14 |
Isolation and assessment of Triton-X100 insoluble protein aggregates and western blotting
A previously described method was used to isolate Triton-X100 (TX)-insoluble protein aggregates from brains (Ge et al. 2007). To assess protein aggregates, the TX-insoluble pellets were re-suspended in 1X protein sample loading buffer (containing 10% glycerol, 60 mM Tris/HCl, pH 6.8, 2% SDS, 0.01% bromophenol blue, 1.25% β-mercaptoethanol) and sonicated for 3 X 5 seconds. The protein samples were boiled for 10 min before being separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred onto a nitrocellulose membrane for western blot analysis.
Western blotting
Brain tissue lysates or isolated protein aggregates were sonicated and then separated in 10–12% SDS PAGE before being transferred onto a nitrocellulose membrane for immunoblotting analysis as previously described (Dong et al. 2012). The primary and secondary antibodies were previously described (Liu et al. 2014a).
Proteasome activity assay
The chymotrysin-like proteasome activity was measured using a previously described method (Liu et al. 2014a). Briefly, 20 μg of cell lysate proteins collected above were added to a 96-well microtiter plate and a final concentration of 40 μM of the fluorogenic substrate Suc-Leu-Leu-Val-Tyr-AMC (Enzo Life Sciences, Farmingdale, NY, USA) was then added to lysates. Fluorescence (380-nm excitation, 460-nm emission) was monitored on a microplate fluorometer every 5 min for 1 h at 25°C.
Histological assessment of neuronal loss by Nissl staining
Mouse brains were fixed, cryoprotected, and sectioned as previously described (Liu et al. 2014b). For Nissl staining, the brain sections were rinsed in distilled water for 5 minutes and stained in 1% Cresyl violet (Sigma, St. Louis, MO, USA) in dH2O for 5 minutes under an agitation condition. The brain sections were then soaked in 95% ethanol, dehydrated in 100% alcohol, and cleared in xylene for 5 minutes before applying DPX mountant. The morphologies of damaged neurons were imaged with an upright microscope equipped with a digital camera and software (Amscope, Irvine, CA, USA).
Statistical analysis
Student’s t-test was used for comparison between two groups. For those more than two groups, data were analyzed with the one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test using the statistical analysis software PRISM 7.0 (GraphPad Software, La Jolla, CA). P < 0.05 was regarded as statistically significant.
RESULTS
IU1 reduces infarct volume induced by cerebral ischemia/reperfusion (I/R)
The UPS function is impaired following I/R (Ge et al. 2007) and USP14 is a negative regulator of proteasome activity (Lee et al. 2010). To determine whether a USP14-specific inhibitor, IU1, has a neuroprotective role in cerebral ischemia/reperfusion (I/R) induced by MCAO in mice, we first examined the effect of different doses of IU1 on mouse brain infarct volume and found that IU1 showed a significant protective effect when mice were treated with 400 μg/kg of IU1 twice, with one injection performed 2 h in prior to 1 h-MCAO and the other treatment carried out 2 h immediately after the MCAO (Fig. 1A). As shown in Figs. 1B & 1C, compared to the vehicle-treated animals, IU1-treated mice showed significant reduction of infarct volume. To further determine whether a post-ischemic IU1 treatment of the stroke mice is sufficient to suppress I/R-induced brain injury, we performed one-time post-MCAO treatment to each animal (Fig. 1D) at different does. When the mice were treated with IU1 at a dose of 400 or 800 μg/kg, they showed significant attenuation of infarct volume (Figs. 1E & 1F). These results indicate that the USP14 inhibitor, IU1, alleviates I/R-induced neuronal injury.
IU1 enhances functional recovery
Following I/R procedure, we monitored animal body weight daily from day 0 to day 7 in mice treated daily either with 400 μg/kg of IU1 or vehicle. Following the surgery, both the IU1- and control-treated mice rapidly lost their body weights (Fig. 2A), due to acutely impaired motor functions. The average body weight of the two types of treatment did not show a significantly difference within 7 days after the MCAO procedure (Fig. 2A). However, IU1 treatment promoted animal survival compared to the control treatment. The median survival time (the time with probability of survival of 0.5) for those on vehicle treatment was 5 days, while the median survival time for those on IU1 treatment were more than 7 days (Fig. 2B). To evaluate whether IU1 alters animal functional recovery, we assessed animal behaviors following I/R using the mNSS system that inversely reflects not only animal motor functions but also reflex and balance capabilities (Table 1) (Chen et al. 2005). As showed in Fig. 2C, mice treated with IU1 showed more rapid functional recovery than those treated with vehicle: IU1 treatment significantly enhanced functional recovery after 3 days, which persisted until day 7. Therefore, suppression of USP14 by IU1 improves animal survival and enhances functional recovery.
Fig. 2. IU1 treatment improves survival and functional recovery following I/R.
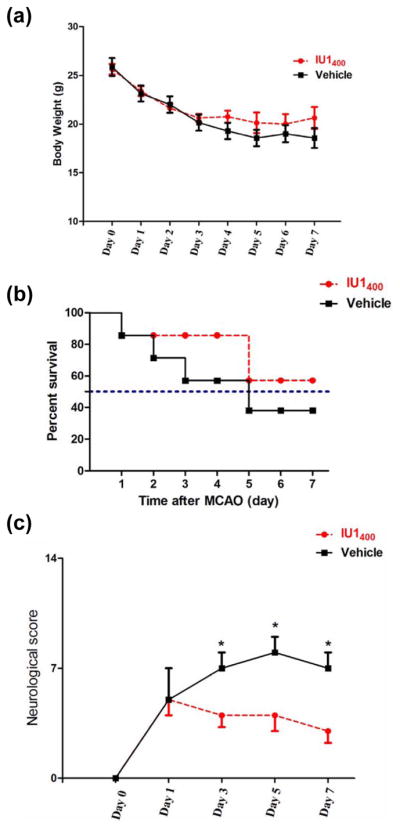
A. Body weight of IU1- and vehicle-treated mice. Mice were treated either with IU1 (400 μg/kg) or vehicle 2 h after 1 h-MCAO. Mice were then allowed to survive for 7 days and received the same dose of daily treatment after the surgery. Data are shown as Mean ± SEM; n = 7 – 8 for each group of mice.
B. IU1-treated mice show improved survival rate compared to the vehicle-treated mice. n = 7 – 8 in each group.
C. IU1-treated mice show more rapid functional recovery than the vehicle-treated mice. Data are shown as median value ± SEM; n = 7–8; *p < 0.05 (Tukey’s test).
IU1 reduces neuronal loss following I/R
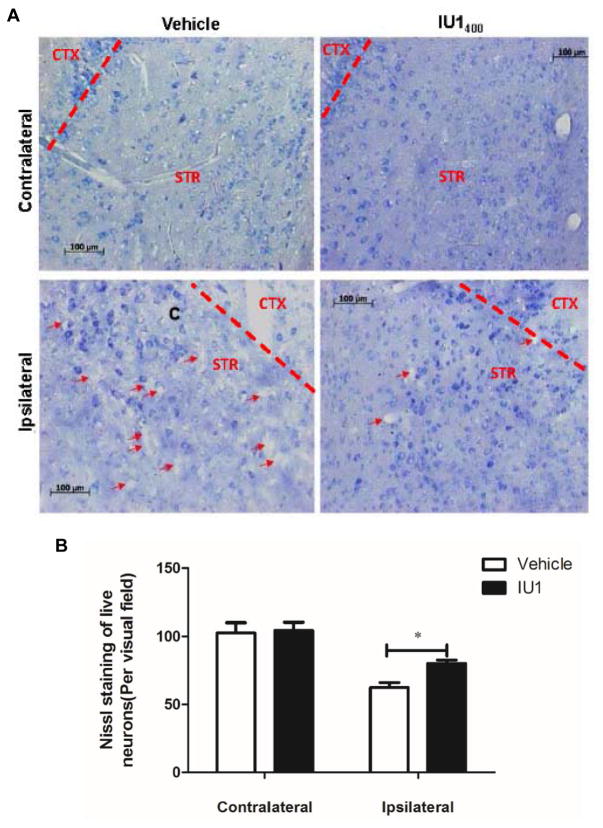
To determine whether improved functional recovery by IU1 was attributed to reduced neuronal loss, we sacrificed the IU1- and control-treated mice and performed histological analysis of neuronal loss by Nissl staining of mouse brains. Our results revealed remarkable loss of neurons in the striatum of vehicle-treated mice (Fig. 3, lower left panel). However, the neuronal loss was evidently reduced in the brain regions of IU1-treated mice (Fig. 3, the lower right panel), indicating that IU1 treatment reduces neuronal loss following I/R.
Fig. 3. IU1 treatment attenuates neuronal loss following I/R.
A. Mice were treated either with vehicle or IU1 and were then allowed to survive for 7 days to receive the same dose of daily treatment before being sacrificed for Nissl staining of brain sections. Note the dead cells pointed by red arrows.
B. Quantitation of Nissl-stained neurons. Data are shown as Mean ± SEM; n = 4, *p < 0.05 (Student’s t test).
IU1 reduces protein aggregates and enhances proteasome activity following I/R
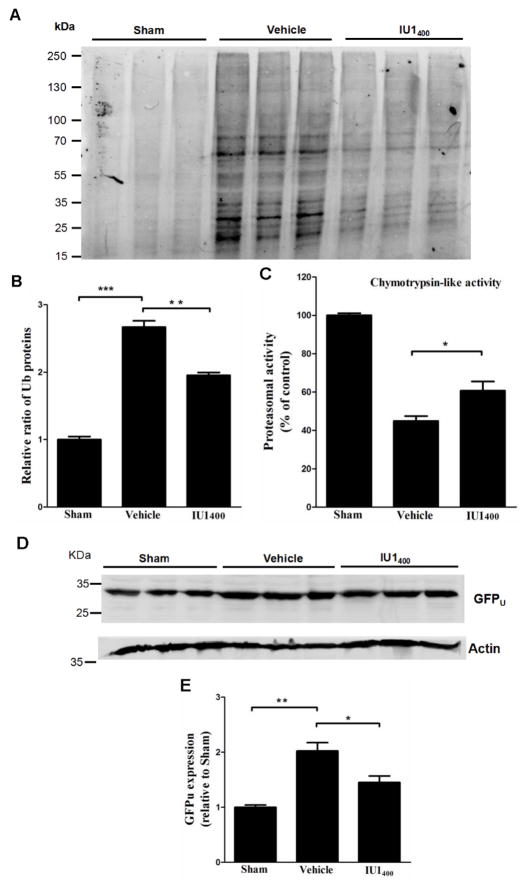
As I/R is associated with oxidative stress, protein and organelle damages, as well as accumulation of ubiquitinated protein aggregates (Ge et al. 2007, Hayashi & Abe 2004), we next examined whether inhibition of USP14 by IU1 alters the accumulation of protein aggregates. Accordingly, we isolated the Triton-X100-insoluble aggregates from either IU1- (400 μg/kg) or the control-treated mouse brains 24 h following the I/R procedure. In the vehicle-treated animals, I/R induced remarkable accumulation of protein aggregates positive in ubiquitin (Figs. 4A & 4B). In contrast, IU1-treated mice showed pronounced-decrease of protein aggregates compared to the vehicle-treated animals (Figs. 4A & 4B). To further test whether the reduced protein aggregates were associated with enhanced activation of the proteasome, we measured the chymotrypsin-like activity, one major peptidase activity of the 20S core of the proteasome, in the brain homogenates derived from the mice treated either with vesicle or IU1. Our results indicate that, compared to the control treatment, IU1 treatment significantly enhanced chymotrypsin-like activity (Fig. 4C). To confirm this result, we also performed the same surgery procedures and treatments to a UPS functionality reporter mouse. This UPS functionality reporter mouse expresses a green fluorescent protein (GFP) transgene fused with the degradation signal CL1 at its C-terminus which targets the fusion protein for ubiquitination and degradation by the UPS (Liu et al. 2014a, Su et al. 2011). The modified GFP is referred to as GFPu. Because GFPu serves as a surrogate substrate for the UPS, its level inversely reflects UPS functionality. As shown in Figs. 4D & 4E, I/R induced significant accumulation of GFPu in the control (vehicle)-treated mouse brains, whereas IU1 treatment reduced GFPu accumulation. Thus, these data indicate that IU1 enhances proteasome functionality and reduces damaged protein accumulation.
Fig. 4. IU1 treatment reduces accumulation of protein aggregates and enhances proteasome activity.
A. IU1 reduces accumulation of protein aggregates. Western blot analysis of protein aggregates isolated either from sham surgery mouse brains or MCAO mouse brains treated either with vehicle or IU1. Mice were allowed to survive for 24 h before being sacrificed for isolating protein aggregates and for western blot analysis with an anti-Ub antibody.
B. Quantitation of ubiquitinated protein aggregates. Data are shown as Mean ± SEM; n = 3; **p < 0.01; ***p < 0.001 (Tukey’s test).
C. IU1 enhances proteasome activity. Chymotrypsin-like proteasome activity was measured either from sham surgery mouse brains or MCAO mouse brains treated either with vehicle or IU1 2 h following 1 h-MCAO. Mice were allowed to survive for 24 h before being sacrificed for the test. Data are shown as Mean ± SEM; n = 3; *p < 0.05 (Tukey’s test).
D. IU1 enhances degradation of the GFPu, a proteasome functionality reporter.
E. Quantitation of GFPu protein levels. Data are shown as Mean ± SEM; n = 3; *p < 0.05; **p < 0.01 (Tukey’s test).
DISCUSSION
Using a mouse stroke model, we demonstrated that pre-MCAO treatment of mice with a USP14-specific inhibitor, IU1, confers a neuroprotective effect on the animals, which was in accordance with previous observations (Doeppner et al. 2013). We then showed that post-ischemic treatment of animals with IU1 also attenuates I/R-induced neuronal injury, reduces animal mortality, and enhances animal functional recovery. The neuroprotective effect exerted by IU1 is likely through its activation of proteasome, thereby promoting degradation of damaged proteins after I/R. These data support that IU1, a USP14-specific inhibitor, is a potential drug for treating ischemic stroke.
Given that I/R damages the endothelial cells of cerebral blood vessels, the compound should be readily cross the brain blood barrier to enter the brain following its administration. On the other hand, IU1 is a cell-permeable chemical that can easily cross the plasma membrane to enter the cytoplasm as it is a small organofluorine compound (Zhou et al. 2016, Lee et al. 2010). Indeed, cell culture studies have confirmed this possibility (Lee et al. 2010, Xu et al. 2016). Following post-ischemic peripheral injections, we observed not only alleviated neuronal injury but also enhanced survival rate and functional recovery. These data suggest that the compound can cross the blood-brain barrier and functions in post-ischemic brains, thus further enhancing its promise as drug candidate worthy of further testing in clinical trials.
Our results support that enhanced proteostasis is likely responsible for IU1-induced neuroprotection observed in the post-ischemic mouse brains. IU1 was originally identified as a USP14-specific inhibitor and a proteasome activity enhancer (Lee et al. 2010). Following IU1 treatment, chymotrypsin-like proteasome activity was increased in the mouse brain, which was associated with reduced accumulation of both ubiquitinated protein aggregates and a proteasome functionality reporter, GFPu. These data strongly support this possibility. On the other hand, it is also possible that the IU1-induced neuroprotection in the post-ischemic mouse brains is a synergistic effect of multiple pathways activated by suppression of USP14 through IU1, since recent results suggest that inhibition of USP14 by IU1 in vitro elevates autophagy activity (Xu et al. 2016). Additionally, emerging studies have suggested that stimulation of Atg is neuroprotective in ischemic stroke models (Wang et al. 2012, Viscomi et al. 2012, Li et al. 2014). It is further conceivable that the enhanced proteasome and Atg activities facilitate the recycling of damaged proteins and organelles, thereby promoting neuronal survival and accelerating functional recovery following I/R.
Acknowledgments
We would like to thank Drs. Yanying Liu and Xiao Peng for technical assistance, Fangfang Qiao for assistance in blinded animal tests, and Dr. Xuejun Wang for providing the GFPu transgenic mouse. This work was supported by the National Institute of Neurological Disorders and Stroke under research grants R03NS084340 and R01NS088084 (HW).
Abbreviations
- I/R
ischemia/reperfusion
- DUB
Deubiquitinating enzyme
- UPS
ubiquitin-proteasome system
- USP14
Ub-specific protease 14
- IU1
1-[1-(4-fluorophenyl)-2,5-dimethylpyrrol-3-yl]-2pyrrolidin-1-ylethanone
- MCAO
middle cerebral artery occlusion
- TTC
2,3,5-triphenyltetrazolium chloride
- mNSS
modified neurological severity score
- Ub
ubiquitin
- TX
Triton-X100
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- GFP
green fluorescence protein
- K48
lysine 48-linked polyubiquitin chain
- Atg
autophagy
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke; a journal of cerebral circulation. 2003;34:1299–1303. doi: 10.1161/01.STR.0000066870.70976.57. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural regeneration research. 2012;7:376–385. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeppner TR, Doehring M, Bretschneider E, et al. MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta neuropathologica. 2013;126:251–265. doi: 10.1007/s00401-013-1142-5. [DOI] [PubMed] [Google Scholar]
- Dong G, Callegari EA, Gloeckner CJ, Ueffing M, Wang H. Prothymosin-alpha interacts with mutant huntingtin and suppresses its cytotoxicity in cell culture. J Biol Chem. 2012;287:1279–1289. doi: 10.1074/jbc.M111.294280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Luo Y, Liu CL, Hu B. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke; a journal of cerebral circulation. 2007;38:3230–3236. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Abe K. Ischemic neuronal cell death and organellae damage. Neurological research. 2004;26:827–834. doi: 10.1179/016164104X3770. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annual review of genetics. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- Lee BH, Lee MJ, Park S, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Lee BH, Hanna J, King RW, Finley D. Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Molecular & cellular proteomics : MCP. 2011;10:R110003871. doi: 10.1074/mcp.R110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang T, Wang J, Zhang Z, Zhai Y, Yang GY, Sun X. Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochemical and biophysical research communications. 2014;444:182–188. doi: 10.1016/j.bbrc.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hettinger CL, Zhang D, Rezvani K, Wang X, Wang H. The proteasome function reporter GFPu accumulates in young brains of the APPswe/PS1dE9 Alzheimer's disease mouse model. Cellular and molecular neurobiology. 2014a;34:315–322. doi: 10.1007/s10571-013-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lu L, Hettinger CL, Dong G, Zhang D, Rezvani K, Wang X, Wang H. Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. J Neurosci. 2014b;34:2813–2821. doi: 10.1523/JNEUROSCI.3541-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang H. Transient focal cerebral ischemia upregulates immunoproteasomal subunits. Cellular and molecular neurobiology. 2012;32:965–970. doi: 10.1007/s10571-012-9854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnappan S, Palmieri M, Sullivan DH, Ponnappan U. Compensatory increase in USP14 activity accompanies impaired proteasomal proteolysis during aging. Mechanisms of ageing and development. 2013;134:53–59. doi: 10.1016/j.mad.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippl W, Collura V, Colland F. Ubiquitin-specific proteases as cancer drug targets. Future oncology. 2011;7:619–632. doi: 10.2217/fon.11.39. [DOI] [PubMed] [Google Scholar]
- Su H, Li F, Ranek MJ, Wei N, Wang X. COP9 signalosome regulates autophagosome maturation. Circulation. 2011;124:2117–2128. doi: 10.1161/CIRCULATIONAHA.111.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscomi MT, D'Amelio M, Cavallucci V, et al. Stimulation of autophagy by rapamycin protects neurons from remote degeneration after acute focal brain damage. Autophagy. 2012;8:222–235. doi: 10.4161/auto.8.2.18599. [DOI] [PubMed] [Google Scholar]
- Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- Xu D, Shan B, Sun H, et al. USP14 regulates autophagy by suppressing K63 ubiquitination of Beclin 1. Genes & development. 2016;30:1718–1730. doi: 10.1101/gad.285122.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YH, Guo NL. USP14 inhibitor IU1 prevents ventilator-induced lung injury in rats. Cellular and molecular biology. 2014;60:50–54. [PubMed] [Google Scholar]
- Zhou Y, Wang J, Gu Z, Wang S, Zhu W, Acena JL, Soloshonok VA, Izawa K, Liu H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chemical reviews. 2016;116:422–518. doi: 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]