Abstract
Adults with homozygous sickle cell anemia have, on average, lower cognitive function than unaffected controls. The mechanisms underlying cognitive deterioration in this population are poorly understood, but cerebral small vessel disease (CSVD) is likely to be implicated. We conducted a systematic review using the Prisma Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines of articles that included both measures of cognitive function and magnetic resonance imaging (MRI) neuroimaging markers of small vessel disease. While all five studies identified small vessel disease by MRI, only two of them found a significant relationship between structural changes and cognitive performance. Differences in methodologies and small sample sizes likely accounted for the discrepancies between the studies. We conclude that while MRI is a valuable tool to identify markers of CSVD in this population, larger studies are needed to definitely establish a link between MRI-detectable abnormalities and cognitive function in sickle cell anemia.
Keywords: Cerebral small vessel disease (CSVD), cerebrovascular, cognition, neuroimaging, sickle cell anemia
Introduction
Sickle cell anemia is characterized by the homozygous inheritance of a mutated hemoglobin (Hb) (Hb S or HBB: c.20A>T) that leads to hemolysis and vascular dysfunction affecting virtually all organs (1–4). Vascular occlusion, ischemia/reperfusion injury and infarction eventually lead to loss of organ function. In the brain, the main manifestations of sickle cell anemia (SCA) include overt stroke, silent cerebral infarction and cognitive impairment (CI) (5). Both overt stroke and silent cerebral infarction have been associated with CI in children (6), but the link between cerebral ischemia detectable by magnetic resonance imaging (MRI) and CI is not clear in adults with sickle cell anemia (7). Elucidating the cerebrovascular pathology associated with CI in adults with sickle cell anemia is important, since this complication is associated with significant disability and its incidence is expected to rise as patients with sickle cell anemia live longer.
In other populations without sickle cell anemia, mounting evidence supports vascular mechanisms of CI (8,9). For example, cerebrovascular disease, including stroke and cerebral small vessel disease (CSVD), is the second most common cause of acquired CI and dementia, after Alzheimer’s disease (10,11). Specifically, pathological vascular processes that limit blood flow to the brain can lead to ischemic vascular disease and manifest as subtle to clinically overt CI (10–13). Neuroimaging and epidemiological studies also report that CSVD is a major contributor to the growing burden of CI and dementia in elderly subjects (14,15), and young to middle-aged adult patients with type 1 diabetes (12,16–21).
Several lines of evidence point to a possible vascular pathophysiology of CI in adult patients with sickle cell anemia. Strokes and silent cerebral infarcts occur in 3.0–38.0% of children with sickle cell anemia (22–24) and are associated with CI (6), and CSVD has also been documented in sickle cell anemia post-mortem studies (25). This review examines the literature describing the relationship between neuroradiological markers of CSVD and brain structural abnormalities, obtained concurrently with measures of cognitive performance in adults with sickle cell anemia.
Materials and methods
Studies were included if the MRI and neurocognitive exam were collected within 2 years on a population of adults with sickle cell anemia without overt stroke (i.e. the emergence of a sudden focal neurological symptom in a patient with radiological evidence of cerebral infarction) and with more than five subjects. Sickle cell anemia was defined as homozygous Hb S (βS/βS) disease. Studies were excluded if they were not in humans, only included adults with sickle cell trait, if the study design was a drug trial, randomized control trial, a single case study, or were not published in English.
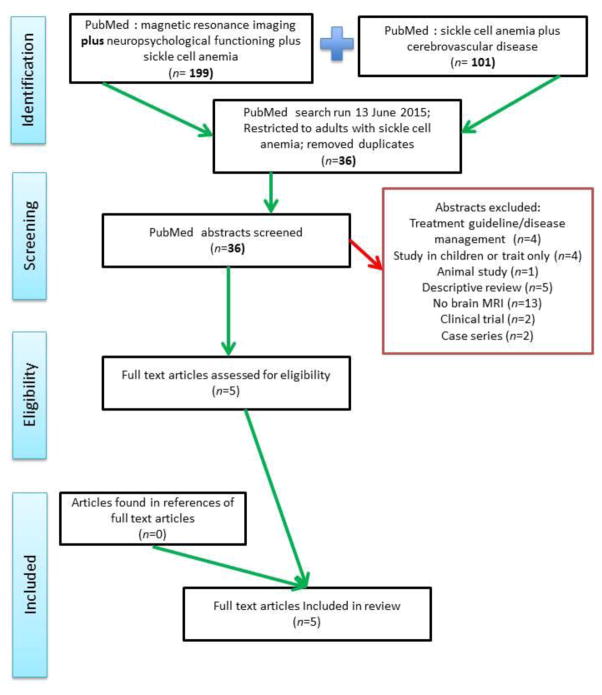
A total of 286 studies were identified electronically from two separate PubMed searches on June 13th, 2015. A list of search terms used in each search is available in the Appendix. Additional articles were identified using bibliographies or were referenced in the full text articles that were screened.
The 286 studies were restricted to adult populations only (using the filter tool in PubMed) and duplicates were removed, leaving 36 articles to screen. Articles were first screened for relevance of the title, followed by abstract content; full papers that passed these eligibility checks were then screened to match the inclusion and exclusion criteria listed above. Full text papers were expected to include a measure of relationship between physical structures on MRI and a cognitive measure. The search strategy utilized is outlined in Figure 1.
Figure 1.
Flow diagram: targeted literature search of the use of MRI to identify pathophysiology of cognitive dysfunction in adults with sickle cell anemia.
The type of study design, description of subjects included, magnitude of relationship between MRI abnormalities and cognition, magnetic field power and type of MRI sequences used, and the types of cognitive tests were extracted from these papers. The results were extracted by a single researcher from text and figures; no additional requests for data were made.
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (26) guidelines, the quality of each article included in this analysis was critically reviewed by population, study design, methodology and significance. Studies were grouped into subtypes based on MRI field power to determine and reduce any heterogeneity of findings between the relationship of cognition and abnormal MRI.
Results
Articles were identified using PubMed as a search engine on June 13th, 2015. Two different queries combined to ascertain eligible studies returned 300 papers, but after restricting the searches to adults with sickle cell anemia and removing duplicate papers, 36 papers remained to be screened for eligibility. In total, 31 articles were excluded for the following reasons: 1) being a treatment guide and not a study (n = 4), being conducted in children (n = 4), being an animal study (n = 1), being a descriptive review (n = 5), not including an MRI or mentioning MRI results (n = 13), being a clinical trial of a drug (n = 2) or being a case series (n = 2). This method of searching was repeated to identify any new publications on May 10th, 2016 but yielded no additional publications.
Of the five studies included, three used a 1.5T field, one had both 3T and 7T, and one had only 7T. These studies had very little overlap in the tests used to measure cognitive function; however, when grouping the tests by functional domain, three main domains were tested: global functioning and/or intelligence quotient (IQ), executive function and/or processing speed, and memory (for a full list of the tests used, see the Appendix). Ages were similar across three of the studies, Vichinsky et al. (7), Mackin et al. (27), Novelli et al. (28), with mean ages near 30 (31.6, 32.0, and 31.0, respectively); the Kulger et al. (29) and van der Land et al. (30) studies had younger groups of patients with sickle cell anemia with a mean age of near 20 (mean 19.9, range 11–28 and mean 23 years, range 19–25, respectively). Four of the five studies also had chosen a priori brain regions of interest. The MRI sequences collected varied across all of the studies (Table 1).
As shown in Table 2, four of the five studies reported abnormalities consistent with the presence of CSVD while the Mackin et al. study did not find any associations with CVSD. Specifically, the four studies reporting CSVD documented the presence of white matter hyperintensities (WMH), subcortical lesions, or differences in small vessel structure in a region of interest as markers of CSVD on MRI images, and three out of four studies comparing sickle cell anemia patients to controls reported that patients with sickle cell anemia had significantly higher CSVD. The 7T MRI study found more WMH in patients with sickle cell anemia than the van der Land et al. (30), study conducted with 3T MRI. Also, using a 7T MRI, Novelli et al. (28) found that patients with sickle cell anemia had lower total venular density and a higher proportion of short vessels when compared to African-American controls.
Among the other neuroradiological markers, the studies of Vichinsky et al. (7) and Mackin et al. (27) found differences in brain volumes; with the Mackin et al. (27) study finding that patients with sickle cell anemia had lower cortical volume, smaller basal ganglia and thalamic structures as compared to controls and the Vichinsky et al. (7) study finding a smaller intracranial volume in patients with sickle cell anemia. Only one article, by Vichinsky et al. (7) found that patients with sickle cell anemia had significantly more lacunae, defined as regions of at least 5 mm in diameter that were hyperintense on the T2-weighted images as well as the proton-density weighted images, with corresponding hypointensity on T1-weighted images. Lacunae were seen in 13.0% of patients and 2.0% of controls [difference: 11.0%; 95% confidence interval (CI): 4.1% to 18.4%; p = 0.05] and were mainly in the frontal lobe, parietal lobe and basal ganglia. Kugler et al. (29) also observed that the number of subcortical lesions tended to be higher in the group with sickle cell anemia with an abnormal MRI at baseline, but this finding was not significant. van der Land et al. (30) observed more lacunae (five vs. four) in the group with sickle cell anemia than in Caucasian controls but reported no statistical values with this finding.
Two of the five studies found slower processing speed, worse memory, and lower global functioning in patients with sickle cell anemia compared to controls. Kugler et al. (29) was the only other paper to compare cognitive scores independent of MRI markers. Kugler et al. (29) found there were no significant differences between group 1 (sickle cell anemia with abnormal MRI at baseline) and group 2 (sickle cell anemia with normal MRI at baseline), although group 1 tended to perform worse using a summary global performance rating score (combining measures of memory, visuospatial function, attention, language, general IQ, and executive and motor functions) than group 2. When patients were compared with normative data adjusted for age and education, 83.0% of the group 1 patients and 88.0% of the group 2 patients had defective scores in one or more areas of cognitive functioning. Furthermore, one patient in group 1 and two patients in group 2 actually met cognitive criteria for dementia.
Four of the five studies examined the relationship between neuroradiological findings and cognitive tests and only two [Novelli et al. (28) and Mackin et al. (27)] found a significant difference. Novelli et al. (28) found that a higher number of short vessels in a region of interest between the frontal and occipital cornu on each cerebral hemisphere was associated with poorer memory. Mackin et al. (27) found that smaller volumes of the basal ganglia and thalamus were associated with lower IQ, poorer executive functioning, and worse memory. Kugler et al. (29), found that the relationships tended towards significance. Vichinsky et al. (7) found an initial relationship between lacunae and IQ, but this relationship became non-insignificant after adjusting for age.
While four of the studies were limited to cross-sectional findings, Kugler et al. (29) explored the association between MRI findings at baseline and cognitive functioning longitudinally. This study found no association between MRI abnormalities at baseline and cognitive tests administered on average 3.6 years later.
Discussion
There is only a very limited number of studies examining the relationship between neuroimaging markers of CSVD and CI in adult patients with sickle cell anemia. Taken as a whole, these studies found a higher prevalence of CSVD and CI in people with sickle cell anemia compared to healthy individuals. This consistent finding is remarkable, considering the variability across studies in patient inclusion criteria, neuropsychological tests and neuroimaging methods.
By contrast, the relationship between CSVD and cognition in sickle cell anemia was less consistent. This association appears to be dependent on the MRI field strength, as it tended to be less evident at lower field strengths; only one out of three studies using a 1.5T MRI found a significant relationship, while another found a non-significant trend. As for the two studies using 7T MRI, one found a significant relationship between CVSD and cognition, but the other did not report any associations with cognition. Discrepancies across studies may have stemmed from high variability in cognitive measures, making it impossible to conclude whether a null association with cognition is a real finding or, likely, an artifact from unstandardized measures and protocols (31).
This review supports CSVD as a possible contributor to CI in people with sickle cell anemia. Two of the four studies looking at lacunae found an increase in the number of lacunae in persons with sickle cell anemia compared to controls, and one found more on a 7T image compared to a 3T image. White matter hyperintensities were more frequent in those with an abnormal MRI in the Kugler et al. (29) study. Moreover, WMH were more visible on the 7T MRI than a 3T MRI of the same subject. No significant differences in WMH were found in the other two studies using 1.5T MRI. Detection of WMH is particularly sensitive to MRI field strength, so future studies using higher-powered magnetic field MRI are needed to determine the contribution of WMH to CI.
Generalized brain atrophy (e.g. loss of volume) is thought to be the result of CSVD and has been associated with steeper declines in cognitive function in older adults (32). Both Vichinsky et al. (7) and Mackin et al. (27) examined volume differences. Vichinsky et al. (7) only found differences in the total intracranial volume, which reflects skull size and not cortical volume, limiting the interpretation of this finding. Lower intracranial volume can also result from nutritional deficiencies during the development of the skull, genetic factors and decreased blood flow (33,34). Mackin et al. (27) found cortical volumes to be significantly lower in the group with sickle cell anemia and Vichinsky et al. (7) found that patients with sickle cell anemia had lower mean intracranial volume compared to controls. While only Mackin et al. (27), found intracranial volume to be related to cognition, these findings are strongly suggestive of CSVD pathology underpinning CI in sickle cell anemia; due to the energy intensive processes that occur in the brain, any loss of blood flow is detrimental to neurons and could lead to neuronal cell death and atrophy of cortical regions (12). Mackin et al. (27) reported a significant relationship with cognitive measures that persisted even after controlling for Hb levels, suggesting pathology beyond anemia. Furthermore, in support of a vascular mechanism, Novelli et al. (28) found differences in venular structure of patients with sickle cell anemia on a 7T MRI. However, this study did not have a volumetric analysis that would have helped to solidify a link between pathological vascular changes in the brain, brain atrophy, and CI. The Novelli et al. (28) study was further limited by the use of only one cognitive test measuring function in a single domain.
The longitudinal design of the Kugler et al. (29) study sets it apart from the other studies in this review. While no significant differences in cognition between two groups of people with sickle cell anemia who had either a normal or an abnormal MRI at baseline were reported, the study showed a higher burden of CSVD markers over a short follow-up period (<5 years) in the group with an abnormal baseline MRI. This finding suggests that similar to other vascular complications (12), CSVD may have a rapidly progressive course in patients with sickle cell anemia. The finding that two of eight patients with a normal MRI at baseline met the criteria for dementia is intriguing, as it suggests that 1.5T MRI may not be sufficiently sensitive to detect CSVD lesions associated with cognitive function in this population.
Using both 7T and 3T, van der Land et al. (30) had the unique opportunity to compare intra-person imaging differences, however, this study only included patients with a National Institute of Health Stroke Scale (NIHSS) score of zero and no differences in cognition were reported. We posit that the failure to detect associations with cognitive function in this study originated from the choice of a suboptimal neurocognitive test; the NIHSS is designed to score the severity of stroke and is not apt at assessing cognitive functioning beyond this purpose (35). However, this study did illustrate that a 7T MRI is more sensitive to CSVD than a 3T MRI.
In conclusion, this review shows that patients with sickle cell anemia have a higher burden of markers of CSVD than healthy controls, supporting CSVD as a pathway to CI in sickle cell anemia. This review also highlights the need to standardize the neurocognitive measures, sequences, and markers of CSVD used to investigate CI in sickle cell anemia; a core neurocognitive battery focusing on the domains of memory and processing speed should be used and MRI sequences should be optimized towards brain areas involved in these functions. Finally, future studies may be aided by higher field MRI to enhance detection of subtler abnormalities.
Acknowledgments
Funding
Neurovascular Determinants of Cognitive Function in Adults with Sickle Cell Disease (PI: Enrico Novelli) NHLBI [2016-2021R01HL127107]; Cardiovascular Epidemiology Training Program Grant NHLBI [#T32: HL083825], Program Director: Dr. Trevor Orchard, MBBCh, MMedSci, FAHA.
Appendix
Table 1.
List of terms used in the search.
| MeSh | Magnetic resonance imaging |
| Anemia, sickle cell | |
| Cognition disorders | |
| Cognition | |
| Memory | |
| Cognitive functioning | |
|
| |
| Specific | IQ |
| Executive functioning | |
| Neuropsychological functioning | |
| Brain | |
| Brain health | |
| Cognitive deficits | |
| Cognitive decline | |
|
| |
| Excluded | Child |
This table shows the terms specified for the systematic literature review.
Table 2.
List of tests used to assess cognitive performance.
| Refs. | Measure of Cognitive Function |
|---|---|
| 1 | The Selective Reminding Test; Visual Reproductions from Wechsler Memory Scale, third edition (WMS-III); Target Cancellation Test; Digit Symbol; the Trail Making Test; the Odd Man Out; the Purdue Pegboard; the Boston Naming Test; Verbal Fluency; Animal Naming from Boston Diagnostic Aphasia Exam; Rosen Drawing Test; Digit span, Block Design and Similarities from Wechsler Adult Intelligence Scale-Revised (WAIS-R); and the Ravens Standard Matrices short form. |
| 2 | California Verbal Learning Test, second edition (CVLT-II); Delis-Kaplan Executive Function System (D-KEFS); Tests of Everyday Attention (TEA); Wechsler Adult Intelligence Scale, third edition (WAIS-III); Wisconsin Card Sorting Test, computer version 4 (WCST-CV4); Wechsler Memory Scale, third edition (WMS-III). |
| 3 | Wechsler Memory Scale, third edition (WMS-III); Performance IQ (PIQ), Processing Speed Index (PSI), Perceptual Organization Index (POI), Working Memory Index (WMI), and Full Scale IQ (FSIQ). Secondary outcome variables included the Verbal IQ and Verbal Comprehension Index from WAIS-III. |
| 4 | National Institutes of Health Stroke Scale (NIHSS). |
| 5 | Hopkins Verbal Learning Test, Revised (HVLT-R). |
This table lists the exact cognitive tests each article reported using for neuropsychological testing. These tests were grouped by cognitive domains tested for this review.
References
Kugler S, Anderson B, Cross D, et al. Abnormal cranial magnetic resonance imaging scans in sickle-cell disease: neurological correlates and clinical implications. Arch Neurol. 1993;50(6):629–635.
Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 2010;303(18):1823–1831.
Mackin RS, Insel P, Truran D, et al. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology. 2014;28(10):835–841.
van der Land V, Zwanenburg JJ, Fijnvandraat K, et al. Cerebral lesions on 7 Tesla MRI in patients with sickle cell anemia. Cerebrovasc Dis. 2015;39(3–4):181–189.
Novelli EM, Elizabeth Sarles C, Jay Aizenstein H, et al. Brain venular pattern by 7T MRI correlates with memory and haemoglobin in sickle cell anaemia. Psychiatry Res. 2015;233(1):18–22.
Table 3.
PRISMA 2009 checklist.
| Section/Topic | # | Checklist Item | ✓ (N/A)a |
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | ✓ |
| Abstract: Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | ✓ (page 7) |
| Introduction: Rationale | 3 | Describe the rationale for the review in the context of what is already known. | ✓ (abstract and page 3) |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study (PICOS) design. | ✓ (methods) |
| Methods: Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. Web address), and, if available, provide registration information including registration number. | ✓ |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale. | ✓ |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | ✓ |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | ✓ (page 6) |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | ✓ |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | ✓ |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made. | ✓ |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | ✓ (N/A – not enough articles to statistically test for bias) |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means). | ✓ |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis. | ✓ |
N/A stands for not applicable and may be a reasonable choice depending on the type of study performed.
Adapted from: Moher D, Liberati A, Tetzlaff J, Altman DG; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed1000097. For more information, visit: www.prisma-statement.org. (http://journals.plos.org/plosmedicine/article?id=10.1371/journal/pmed.1000097).
Provenance: Not commissioned; externally peer reviewed. In order to encourage dissemination of the PRISMA Statement, this article is freely accessible on th PloS Medicine website (http://medicine.plosjournals.org) and will also be published in the Annals of Internal Medicine, British Medical Journal, Journal of Clinical Epidemiology and Open Medicine. The authors jointly hold the copyright of this article. For details on further use, see the PRISMA website (http://www.prisma-statement.org).
Footnotes
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Serjeant GR. One hundred years of sickle cell disease. Br J Haematol. 2010;151(5):425–429. doi: 10.1111/j.1365-2141.2010.08419.x. [DOI] [PubMed] [Google Scholar]
- 2.Odievre MH, Verger E, Silva-Pinto AC, Elion J. Pathophysiological insights in sickle cell disease. Indian J Med Res. 2011;134(4):532–537. [PMC free article] [PubMed] [Google Scholar]
- 3.Mohandas N, Evans E. Adherence of sickle erythrocytes to vascular endothelial cells: requirement for both cell membrane changes and plasma factors. Blood. 1984;64(1):282–287. [PubMed] [Google Scholar]
- 4.Fasano RM, Meier ER, Hulbert ML. Cerebral vasculopathy in children with sickle cell anemia. Blood Cells Mol Dis. 2015;54(1):17–25. doi: 10.1016/j.bcmd.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 6.Dowling MM, Quinn CT, Rogers ZR, Buchanan GR. Acute silent cerebral infarction in children with sickle cell anemia. Pediatr Blood Cancer. 2010;54(3):461–464. doi: 10.1002/pbc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 2010;303(18):1823–1831. doi: 10.1001/jama.2010.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia Diagnostic criteria for research studies: report of the NINDS AIREN International Workshop. Neurology. 1993;43(2):250–250. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien JT, Firbank MJ, Krishnan MS, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14(10):834–841. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 11.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 13.Manolio TA, Olson J, Longstreth W., Jr Hypertension and cognitive function: pathophysiologic effects of hypertension on the brain. Cur Hypertens Rep. 2003;5(3):255–261. doi: 10.1007/s11906-003-0029-6. [DOI] [PubMed] [Google Scholar]
- 14.Baker JG, Williams AJ, Ionita CC, et al. Cerebral small vessel disease: cognition, mood, daily functioning, and imaging findings from a small pilot sample. Dement Geriatr Cogn Dis Extra. 2012;2(1):169–179. doi: 10.1159/000333482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Sullivan M. Imaging small vessel disease: lesion topography, networks, and cognitive deficits investigated with MRI. Stroke. 2010;41(10 suppl 1):S154–S158. doi: 10.1161/STROKEAHA.110.595314. [DOI] [PubMed] [Google Scholar]
- 16.Araki Y, Nomura M, Tanaka H, et al. MRI of the brain in diabetes mellitus. Neuroradiology. 1994;36(2):101–103. doi: 10.1007/BF00588069. [DOI] [PubMed] [Google Scholar]
- 17.Manschot SM, Brands AM, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55(4):1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SC, Blane A, Wardlaw J, et al. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care. 2005;28(6):1431–1437. doi: 10.2337/diacare.28.6.1431. [DOI] [PubMed] [Google Scholar]
- 19.Lunetta M, Damanti AR, Fabbri G, et al. Evidence by magnetic resonance imaging of cerebral alterations of atrophy type in young insulin-dependent diabetic patients. J Endocrinol Invest. 1994;17(4):241–245. doi: 10.1007/BF03348967. [DOI] [PubMed] [Google Scholar]
- 20.Nomura K, Hamamoto Y, Takahara S, et al. Relationship between CarotidIntima-media thickness and silent cerebral infarction in Japanese subjects with type 2 diabetes. Diabetes Care. 2010;33(1):168–170. doi: 10.2337/dc09-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wootton-Gorges SL, Glaser NS. Imaging of the brain in children with type I diabetes mellitus. Pediatr Radiol. 2007;37(9):863–869. doi: 10.1007/s00247-007-0536-8. [DOI] [PubMed] [Google Scholar]
- 22.Balkaran B, Char G, Morris JS, et al. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr. 1992;120(3):360–366. doi: 10.1016/s0022-3476(05)80897-2. [DOI] [PubMed] [Google Scholar]
- 23.Moser FG, Miller ST, Bello JA, et al. The spectrum of brain MR abnormalities in sickle-cell disease: a report from the Cooperative Study of Sickle Cell Disease. Am J Neuroradiol. 1996;17(5):965–972. [PMC free article] [PubMed] [Google Scholar]
- 24.Solomou E, Kraniotis P, Kourakli A, Petsas T. Extent of silent cerebral infarcts in adult sickle-cell disease patients on magnetic resonance imaging: is there a correlation with the clinical severity of disease? Hematol Rep. 2013;5(1):8–12. doi: 10.4081/hr.2013.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merkel K, Ginsberg PL, Parker JC, Post MJ. Cerebrovascular disease in sickle cell anemia: a clinical, pathological and radiological correlation. Stroke. 1978;9(1):45–52. doi: 10.1161/01.str.9.1.45. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 27.Mackin RS, Insel P, Truran D, et al. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology. 2014;28(10):835–841. doi: 10.1212/WNL.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novelli EM, Elizabeth Sarles C, Jay Aizenstein H, et al. Brain venular pattern by 7T MRI correlates with memory and haemoglobin in sickle cell anaemia. Psychiatry Res. 2015;233(1):18–22. doi: 10.1016/j.pscychresns.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kugler S, Anderson B, Cross D, et al. Abnormal cranial magnetic resonance imaging scans in sickle-cell disease: neurological correlates and clinical implications. Arch Neurol. 1993;50(6):629–635. doi: 10.1001/archneur.1993.00540060059019. [DOI] [PubMed] [Google Scholar]
- 30.van der Land V, Zwanenburg JJ, Fijnvandraat K, et al. Cerebral lesions on 7 Tesla MRI in patients with sickle cell anemia. Cerebrovasc Dis. 2015;39(3–4):181–189. doi: 10.1159/000373917. [DOI] [PubMed] [Google Scholar]
- 31.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prins ND, van Dijk EJ, de Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128(9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 33.Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50(2):121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- 34.Barnes J, Ridgway GR, Bartlett J, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53(4):1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 35.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010;9(9):895–905. doi: 10.1016/S1474-4422(10)70164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]