ABSTRACT
Patients with hematologic malignancies as well as allogeneic hematopoietic stem cell transplantation (HSCT) patients are at high risk for invasive aspergillosis. Here, we report a culture- and autopsy-proven fatal invasive aspergillosis in an allogeneic HSTC patient which he developed despite posaconazole prophylaxis. The agent was determined to be an azole-resistant Aspergillus fumigatus strain bearing the cyp51A mutation combination TR46 Y121F M172I T289A. At increasing frequency, the azole resistance of A. fumigatus is being reported globally, limiting treatment options and complicating regimens.
KEYWORDS: Aspergillus fumigatus, azole resistance, hematopoietic stem cell transplantation, invasive aspergillosis, cyp51A mutation, breakthrough aspergillosis
CASE PRESENTATION
In August 2014, a patient in his early 70s was referred to our hospital for treatment of progressive acute myeloid leukemia (AML).
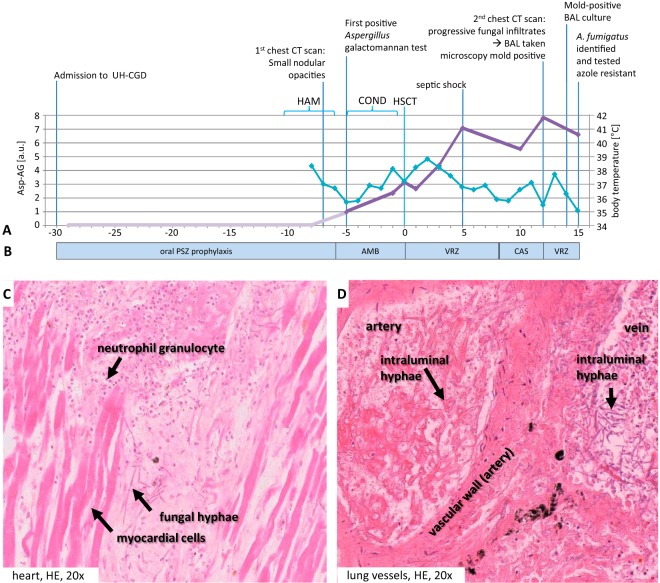
Three months prior, the patient had been treated in a general hospital for AML with adverse-risk cytogenetic features. There, the patient received induction chemotherapy (idarubicin at 12 mg/m2/day intravenously [i.v.], days 2, 4, and 6; cytarabine at 100 mg/m2/day i.v., days 1 to 7; etoposide at 100 mg/m2/day i.v., days 3 to 7) and posaconazole oral suspension (200 mg per os [p.o.] three times a day [t.i.d.]) for antifungal prophylaxis. Eventually, a new bone marrow aspirate revealed residual AML. Thus, decitabine therapy (20 mg/m2/day i.v., days 1 to 5) was initiated, and because of progressive AML, the patient was then transferred to the University Hospital Dresden (Fig. 1A). Here, the patient received salvage chemotherapy (mitoxantrone at 30 mg/m2/day i.v., days 1 and 5; cytarabine at 2,000 mg/m2/day i.v. b.i.d., days 1 and 5) (1), followed by reduced-intensity conditioning chemotherapy (anti-thymocyte globulin [ATG] at 10 mg/kg of body weight/day i.v., days −5 to −2 [Fresenius, Bad Homburg, Germany]; fludarabin at 30 mg/m2/day, days −6 to −2; and melphalan at 150 mg/m2 i.v. on day −2) and allogeneic hematopoietic stem cell transplantation (HSCT) (day 0) accompanied by antifungal prophylaxis with posaconazole (200 mg p.o. t.i.d.) (Fig. 1B).
FIG 1.
Time course of diagnostic and therapeutic events. (A) Diagnostic parameters. On the primary y axis, the Bio-Rad Platelia Aspergillus galactomannan antigen test results (Asp-AG; light and dark purple lines) are shown. On the secondary axis, body temperatures (light blue) are shown. UH-CGD, University Hospital Carl Gustav at the Technische Universität Dresden, Dresden, Germany; HAM, high-dose cytosine arabinoside and mitoxantrone salvage therapy (mitoxantrone at 30 mg/m2/day i.v. q.d. and high-dose cytarabine at 2,000 mg/m2/day i.v. b.i.d., days 1 and 5 before conditioning chemotherapy, corresponding to days −11 and −7 with respect to HSCT); COND, conditioning chemotherapy with ATG (10 mg/kg/day i.v. q.d., days −5 to −2; Fresenius, Bad Homburg, Germany), fludarabin (30 mg/m2/day i.v. q.d., days −6 to −2), and melphalan (150 mg/m2 i.v. q.d., day −2); HSCT, day of human stem cell transplantation (day 0); a.u., arbitrary units. (B) Antifungal management. Posaconazole (PSZ) prophylaxis was at 200 mg p.o. b.i.d. Liposomal amphotericin B (AMB) was at 3 mg/kg/day i.v. q.d. Voriconazole (VZR) was at 4 mg/kg/day i.v. b.i.d., with a loading dose of 6 mg/kg/day i.v. b.i.d. (day 0 and day 12, respectively). Caspofungin (CAS) was at 50 mg i.v. q.d., with a loading dose of 70 mg i.v. q.d. (day +9). (C, D) Postmortem analyses. (C) Fungal abscess in the anterior cell wall of the heart with surrounding granulocytic reaction; (D) vascular invasive growth and detection of dichotomously branched and septate fungi in the lumens of lung vessels. HE, hematoxylin and eosin.
Because of neutropenic fever and increasingly elevated C-reactive protein (CRP), a computed tomography (CT) scan was performed on day −7, revealing very small nodular opacities suspected of being fungal infiltrates. Antifungal treatment was initiated with liposomal amphotericin B (day −6; 3 mg/kg/day i.v.) (2). Monitoring of serum Aspergillus galactomannan (GM) (Platelia Aspergillus enzyme immunoassay [EIA]; Bio-Rad, France) resulted in a first low-positive test on day −5 (GM index, 0.9 [normal, <0.5]). The treatment course since initial diagnosis of AML, however, suggested chemotherapy refractoriness, with only a short-lived response to chemotherapeutic agents. The chance that the patient would stay neutropenic and would have an increment in his white blood cells only with his AML blasts was therefore considered too high to postpone HSCT (day 0).
Despite an initial response with declining fever after HSCT, both temperature and inflammatory parameters increased again. Therefore, the central venous catheter was replaced and, in the absence of apparent improvement, the antifungal regimen was switched to voriconazole (day 0; 4 mg/kg/day i.v. twice a day [b.i.d.], with a loading dose of 6 mg/kg/day i.v. b.i.d.) (2).
The patient went into septic shock on day +5. Despite third-party granulocyte transfusions and descending inflammatory markers, his general condition declined. The patient had to be intubated and mechanically ventilated on day +9. Because of increasing liver function test results (alanine transaminase [ALAT] at 3.65 μmol/s/liter, aspartate aminotransferase [ASAT] at 16.41 μmol/s/liter [normal, 0.85 μmol/s/liter], and total bilirubin at 121 μmol/liter [normal, <21 μmol/liter]), the antifungal therapy was switched to caspofungin (50 mg i.v. four times a day [q.d.]; loading dose, 70 mg i.v. q.d.). The GM titer rose continuously and eventually peaked on day +12 (GM index, 8.0). A follow-up chest CT scan revealed progressive fungal infiltrates. A bronchoalveolar lavage (BAL) specimen obtained on day +12 presented small amounts of dichotomously branched and septate fungal hyphae but no evidence of a relevant inflammatory reaction. Therefore, administration of voriconazole was chosen after microscopy results led to a suspicion of Aspergillus spp.
Subsequently, Aspergillus fumigatus was cultured from the BAL fluid on day +14. Routinely implemented susceptibility testing results revealed elevated MICs of posaconazole and voriconazole on day +15. Azole susceptibility results were retrospectively confirmed in a reference laboratory by EUCAST broth microdilution (3, 4) (Table 1).
TABLE 1.
MIC valuesa
| Antifungal agent | Etest MIC | Microdilution MIC | EUCAST breakpoints | Interpretation |
|---|---|---|---|---|
| Posaconazole | 0.5 | 0.5 | S ≤ 0.12, R > 0.25 | Resistant |
| Itraconazole | 2 | 1 | S ≤ 1, R > 2 | Sensitive |
| Voriconazole | >32 | >32 | S ≤ 1, R > 2 | Resistant |
| Amphotericin B | 0.25 | Not tested | S ≤ 1, R > 2 | Sensitive |
| Caspofungin | <0.02 (trailing) | >4 | NA | Inconclusive |
MICs are in micrograms per milliliter. EUCAST, European Committee on Antimicrobial Susceptibility Testing; S, sensitivity; R, resistance; NA, not available (insufficient evidence).
The condition of the patient declined further, and despite all efforts, the patient died on day +15 in the state of multiorgan failure after allogeneic, unrelated peripheral blood stem cell transplantation.
At the time of death, the patient had only a low mixed-donor chimerism. Neutrophil engraftment could not be observed due to artificial increments with third-party granulocyte transfusions. Engraftment of platelets could not be observed either. This is accordance with administration of ATG in the conditioning regimen, where engraftments occur rarely until day +15 after transplantation (5). Autopsy revealed invasive pulmonary aspergillosis with multiple fungal abscesses and invasive vascular growth (Fig. 1D), as well as dissemination to the heart (Fig. 1C) and the brain (not shown). No bacteremia or alternative reason for septic shock was identified. We conclude that the patient died of invasive aspergillosis (IA), despite extensive antifungal prophylaxis and treatment.
CHALLENGE QUESTION
In patients with febrile neutropenia undergoing allogeneic hematopoietic stem cell transplantation and on azole prophylaxis, which of the following statements is not valid?
A. Repeated positive galactomannan serum tests despite azole prophylaxis are suspicious for a breakthrough invasive aspergillosis.
B. Since azole resistance has not yet been shown for Aspergillus fumigatus, breakthrough invasive aspergillosis is generally caused by low serum azole levels.
C. An alternative antifungal agent to azoles should be considered in cases in which an azole-resistant invasive aspergillosis is suspected.
D. In areas where azole resistance is known to be elevated in the environment, initial therapy of invasive aspergillosis should be started with a combination therapy.
TREATMENT AND OUTCOME
Voriconazole is the first-line drug for IA in hemato-oncological patients (2, 6). The management of patients suspected of having IA as a result of a rarely occurring breakthrough infection despite azole prophylaxis is individualized, since no guidelines exist. Recommendations comprise a change of antifungal class to another mold-active agent (6); in the present case, liposomal amphotericin B was chosen. Antifungal combination therapy should be considered but was not initiated due to the lack of prospective data in favor of combination therapy over monotherapy, to the best of our knowledge. Other options are therapeutic drug monitoring of posaconazole prophylaxis to control serum levels or a change from oral posaconazole suspension to the retarded/extended release posaconazole tablet with substantial improved bioavailability. Mycological culturing and azole susceptibility testing of isolates obtained were performed as recommended, but cultivation often remains unsuccessful for pretreated patients.
For early detection of IA in the absence of a culture isolate, repeated positive or rising serum GM detection has a good positive predictive value for carefully preselected subpopulations at high risk (e.g., allogeneic HSCT patients) (7, 8). Positive results should lead to a further diagnostic approach, including lung CT and BAL for fungal culture, since dissemination of Aspergillus often follows lung infection. False reactivity of GM is possible and can be due to an exogenous dietary intake (9) or non-Aspergillus fungal infections (10), whereas cross-reactivity between GM and piperacillin-tazobactam preparations seems to diminish (11). False-negative GM results are possible, too, depending on different factors, like the infection site, the presence of GM antibodies, pretreatment with antifungals, and other factors underlining systematic monitoring with repeated testing (8). Recent studies show strong inverse correlation between GM indices and patient survival (12), suggesting GM decay as a surrogate marker for all-cause mortality (13). However, GM decreasing to a normal level as the single criterion is not sufficient for discontinuation of antifungal therapy (8). Our patient reached a GM plateau on day +5, which was maintained at a high level (GM indices, 5.6 to 8.0) (Fig. 1A).
Development of invasive aspergillosis despite azole antifungal prophylaxis was previously attributed to mutation of the A. fumigatus cyp51A gene under treatment, e.g., at the G54 or M220 codon (14, 15). cyp51A encodes 14-alpha sterol demethylase, the target enzyme of azole antifungal compounds. Refractory invasive aspergillosis is, however, becoming increasingly more common due to exogenous infection with resistant strains from the environment bearing the TR34 L98H or TR46 Y121F T289A polymorphisms. Such strains were first described in The Netherlands (16) but were retrospectively also detected in other countries (17) and have now been described to occur worldwide. The current hypothesis is that they have arisen from the extensive use of agricultural azole antifungals with a structural resemblance to clinically used compounds (18).
Indeed, the city of Dresden lies within what has previously been identified as a corridor of increased environmental prevalence of TR34 L98H and TR46 Y121F T289A strains (19) across northern Germany. Sequencing of the cyp51A gene and its promoter region revealed the presence of the voriconazole resistance-conferring allele TR46 Y121F T289A along with an M172I polymorphism in both the cultured BAL fluid isolate and fixated tissue from the lung (20, 21). Genetic strain typing (22) identified this isolate as of the csp1 type t01 group. Since the first description of azole-resistant A. fumigatus bearing the cyp51A TR46 Y121F T289A mutation in 2009 (23), that allele has been detected in environmental and clinical strains, including two from allogeneic HSCT patients in Germany (24).
The additional cyp51A M172I polymorphism found in the isolate described here is present in the wild A. fumigatus population and is described throughout the literature in conjunction with several resistance mutations, as well as in susceptible isolates. It is not thought to have an impact on reduced drug susceptibility but rather appears to be a marker for phylogenetic spread of the resistance mutations. Independently of this case, an environmental isolate with exactly the same cyp51A and csp1 alleles, including the M172I polymorphism, has been found in the corridor described above (19). We therefore presume a high likelihood of exogenous origin of the infection.
A progressive dispersion of azole resistance is of particular clinical concern, since azole-resistant strains are associated with high case fatality rates (24, 25). In the absence of management recommendations for A. fumigatus drug resistance in current clinical guidelines, an international expert opinion on the antifungal management of azole-resistant pulmonary IA (26) has recently suggested that the initial therapy regimen should be based on the local environmental azole resistance rate; in areas with azole resistance of ≤5%, the current guidelines for a voriconazole regimen should be followed, but in areas with resistance rates of >10%, initial treatment with voriconazole plus an echinocandin or liposomal amphotericin B (26) should be considered.
Azole resistance in A. fumigatus is an increasingly important phenomenon, leading to breakthrough infections despite prophylaxis with mold-active, azole-based antifungals (for the challenge question above, answer “B” is the statement that is not valid). The consequence may be an inappropriate or delayed therapy with a higher rate of therapy failure/higher fatality rate. Especially in regions with an increased environmental prevalence of resistant strains, mycological culturing and azole susceptibility testing of isolates obtained should be performed in high-risk populations at the earliest time point possible to detect elevated MICs of azoles. Single itraconazole testing as an “indicator substance,” as previously proposed for the detection of the TR34 L98H mutation, must be considered inadequate for the detection of derivatives of the TR46 Y121F T289A mutations, since low itraconazole MICs are described here and elsewhere (24) for isolates with these mutations. Diagnostic PCR assays for directly identifying cyp51A alterations from clinical samples have been developed (21). These can be implemented to facilitate earlier detection of frequent resistance traits independently of a culture isolate.
COMMENTARY
Rößler et al. present a very instructive case of a patient with acute myeloid leukemia (AML) and hematopoietic stem cell transplantation (HSCT) infected with an azole-resistant strain of Aspergillus fumigatus.
The case clearly showcases this patient population as at high risk for invasive fungal infection and exemplifies the complexity encountered in contemporary clinical settings with cutting-edge diagnostic tools. Per international guidelines, the patient received prophylaxis with posaconazole (27) when he experienced breakthrough invasive pulmonary aspergillosis identified through a combination of computed tomography (CT) and serum galactomannan (GM) level. The patient was then treated with liposomal amphotericin B followed by voriconazole. After an initial response, the patient deteriorated, and then salvage maneuvers, such as granulocyte transfusion and caspofungin therapy, were attempted. The patient eventually grew A. fumigatus for which the MICs of both posaconazole and voriconazole were elevated. The strain was eventually genetically characterized and found to have a well-described mutation that confers azole resistance. Unfortunately, the patient died, and the autopsy showed invasive pulmonary aspergillosis with multiple fungal abscesses and the classic vascular invasion by these fungi.
This case is instructive because it highlights the increasing problem of azole resistance in patients with invasive aspergillosis (28, 29), which is thought to be related to the use of agricultural pesticides and the selection of these strains in the environment as well as increasing azole use in clinical settings. It also highlights invaluable diagnostic tools, such as CT and both serum and bronchio-alveolar lavage fluid GM (30, 31), as part of the contemporary management of this disease, both in terms of diagnosis and monitoring for progression or resolution of the disease (32). Finally, it also reminds us of the importance of postmortem studies, a disappearing trend, in determining the cause of death and quantifying the burden of disease that the patient experienced. The message is loud and clear: azole resistance is on the rise (28, 33), and centers that routinely deal with these complex hosts should be screening for these organisms.
ACKNOWLEDGMENTS
The case authors thank Susann Kolewa and Agnieszka Goretzki for expert technical assistance.
Characterization of the isolate was supported by Pfizer Pharma Germany through grant no. WI186302 (to O.B.). O.B. receives research grants from Pfizer and Gilead. D.B. serves in the speaker's bureaus of Astellas, Gilead Sciences, Merck/MSD, and Pfizer; receives research grants from Gilead Sciences and Pfizer and travel grants from Astellas, Merck/MSD, and Pfizer; and is a consultant to Basilea and Gilead Sciences. F.S. received travel grants from Astellas. All other case authors have nothing to declare. L.O.-Z. has received grants and/or honoraria from Merck, Astellas, Pfizer, Scynexis, Cidara, Meiji, Johnson & Johnson, and T2 Biosystems.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. An expert clinician then provides a commentary on the case.
REFERENCES
- 1.Stolzel F, Platzbecker U, Mohr B, Rollig C, Middeke JM, Thiede C, Fussel M, Hanel M, Schaich M, Ehninger G, Schetelig J, Bornhauser M. 2013. Early intervention with allogeneic hematopoietic cell transplantation during chemotherapy-induced aplasia in patients with high-risk acute myeloid leukemia. Leukemia 27:2068–2072. doi: 10.1038/leu.2013.142. [DOI] [PubMed] [Google Scholar]
- 2.Mousset S, Buchheidt D, Heinz W, Ruhnke M, Cornely OA, Egerer G, Kruger W, Link H, Neumann S, Ostermann H, Panse J, Penack O, Rieger C, Schmidt-Hieber M, Silling G, Sudhoff T, Ullmann AJ, Wolf HH, Maschmeyer G, Bohme A. 2014. Treatment of invasive fungal infections in cancer patients—updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 93:13–32. doi: 10.1007/s00277-013-1867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2008. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 14:982–984. doi: 10.1111/j.1469-0691.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 4.EUCAST. 12 August 2014, posting date Antifungal agents—breakpoints table for interpretation of MICs. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Antifungal_breakpoints_v_7.0.pdf.
- 5.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, Kolbe K, Mayer J, Maertens JA, Linkesch W, Holler E, Koza V, Bornhauser M, Einsele H, Kolb HJ, Bertz H, Egger M, Grishina O, Socie G, ATG-Fresenius Trial Group. 2009. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 6.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lombardi G, Farina C, Andreoni S, D'Antonio D, Faggi E, Manso E, Mazzoni A. 2002. Multicenter evaluation of an enzyme immunoassay (Platelia Aspergillus) for the detection of Aspergillus antigen in serum. Mycopathologia 155:129–133. doi: 10.1023/A:1020416308399. [DOI] [PubMed] [Google Scholar]
- 8.Mennink-Kersten MA, Donnelly JP, Verweij PE. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis 4:349–357. doi: 10.1016/S1473-3099(04)01045-X. [DOI] [PubMed] [Google Scholar]
- 9.Guigue N, Menotti J, Ribaud P. 2013. False positive galactomannan test after ice-pop ingestion. N Engl J Med 369:97–98. doi: 10.1056/NEJMc1210430. [DOI] [PubMed] [Google Scholar]
- 10.Wheat LJ, Hackett E, Durkin M, Connolly P, Petraitiene R, Walsh TJ, Knox K, Hage C. 2007. Histoplasmosis-associated cross-reactivity in the Bio-Rad Platelia Aspergillus enzyme immunoassay. Clin Vaccine Immunol 14:638–640. doi: 10.1128/CVI.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikulska M, Furfaro E, Del Bono V, Raiola AM, Ratto S, Bacigalupo A, Viscoli C. 2012. Piperacillin/tazobactam (Tazocin) seems to be no longer responsible for false-positive results of the galactomannan assay. J Antimicrob Chemother 67:1746–1748. doi: 10.1093/jac/dks111. [DOI] [PubMed] [Google Scholar]
- 12.Maertens J, Buve K, Theunissen K, Meersseman W, Verbeken E, Verhoef G, Van Eldere J, Lagrou K. 2009. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer 115:355–362. doi: 10.1002/cncr.24022. [DOI] [PubMed] [Google Scholar]
- 13.Koo S, Bryar JM, Baden LR, Marty FM. 2010. Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J Clin Microbiol 48:1255–1260. doi: 10.1128/JCM.02281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL. 2003. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 47:1120–1124. doi: 10.1128/AAC.47.3.1120-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2004. Substitutions at methionine 220 in the 14alpha-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob Agents Chemother 48:2747–2750. doi: 10.1128/AAC.48.7.2747-2750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozmerdiven GE, Ak S, Ener B, Agca H, Cilo BD, Tunca B, Akalin H. 2015. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J Infect Chemother 21:581–586. doi: 10.1016/j.jiac.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Snelders E, Huis In 't Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bader O, Tunnermann J, Dudakova A, Tangwattanachuleeporn M, Weig M, Gross U. 2015. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob Agents Chemother 59:4356–4359. doi: 10.1128/AAC.00100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiess B, Postina P, Reinwald M, Cornely OA, Hamprecht A, Hoenigl M, Lass-Flörl C, Rath PM, Steinmann J, Miethke T, Lauten M, Will S, Merker N, Hofmann WK, Buchheidt D. 2014. Incidence of cyp51A key mutations in Aspergillus fumigatus—a study on primary clinical samples of immunocompromised patients in the period of 1995–2013. PLoS One 9:e103113. doi: 10.1371/journal.pone.0103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiess B, Seifarth W, Merker N, Howard SJ, Reinwald M, Dietz A, Hofmann WK, Buchheidt D. 2012. Development of novel PCR assays to detect azole resistance-mediating mutations of the Aspergillus fumigatus cyp51A gene in primary clinical samples from neutropenic patients. Antimicrob Agents Chemother 56:3905–3910. doi: 10.1128/AAC.05902-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klaassen CH, de Valk HA, Balajee SA, Meis JF. 2009. Utility of CSP typing to sub-type clinical Aspergillus fumigatus isolates and proposal for a new CSP type nomenclature. J Microbiol Methods 77:292–296. doi: 10.1016/j.mimet.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 23.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 24.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 25.van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, Van Tiel FH, Melchers WJ, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis 17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Bruggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21–22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Tacke D, Buchheidt D, Karthaus M, Krause SW, Maschmeyer G, Neumann S, Ostermann H, Penack O, Rieger C, Ruhnke M, Sandherr M, Schweer KE, Ullmann AJ, Cornely OA. 2014. Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol 93:1449–1456. doi: 10.1007/s00277-014-2108-y. [DOI] [PubMed] [Google Scholar]
- 28.Meis JF, Chowdhary A, Rhodes JL, Fisher MC, Verweij PE. 2016. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond B Biol Sci 371:20150460. doi: 10.1098/rstb.2015.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiederhold NP, Patterson TF. 2015. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med 36:673–680. doi: 10.1055/s-0035-1562894. [DOI] [PubMed] [Google Scholar]
- 30.Park SY, Lee SO, Choi SH, Sung H, Kim MN, Choi CM, Hong SB, Oh YM, Shim TS, Koh Y, Kim YS, Woo JH, Kim SH. 2010. Aspergillus galactomannan antigen assay in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Infect 61:492–498. doi: 10.1016/j.jinf.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Penack O, Rempf P, Graf B, Blau IW, Thiel E. 2008. Aspergillus galactomannan testing in patients with long-term neutropenia: implications for clinical management. Ann Oncol 19:984–989. doi: 10.1093/annonc/mdm571. [DOI] [PubMed] [Google Scholar]
- 32.Marr KA. 2008. Aspergillus galactomannan index: a surrogate end point to assess outcome of therapy? Clin Infect Dis 46:1423–1425. doi: 10.1086/528715. [DOI] [PubMed] [Google Scholar]
- 33.Verweij PE, Lestrade PP, Melchers WJ, Meis JF. 2016. Azole resistance surveillance in Aspergillus fumigatus: beneficial or biased? J Antimicrob Chemother 71:2079–2082. doi: 10.1093/jac/dkw259. [DOI] [PubMed] [Google Scholar]