ABSTRACT
This large-scale retrospective analysis (n = 60,551) of the Premier inpatient database (1 January 2011 to 31 December 2014) found an overall prevalence of carbapenem-resistant Enterobacteriaceae strains of 2.3% (range, 0.9% to 5.8% by geographic region) among patients with infections due to Enterobacteriaceae. Ongoing monitoring and development of decision support tools/algorithms are needed for identification of high-risk patients.
KEYWORDS: bacterial drug resistance, anti-infective agents, carbapenems, bacterial infections, urinary tract infections, intra-abdominal infections, bacterial pneumonia, bacteremia
TEXT
Although the U.S. Centers for Disease Control and Prevention (CDC) identifies infections due to carbapenem-resistant Enterobacteriaceae (CRE) as an urgent public health threat (1), limited data exist regarding the true prevalence of these infections among adult hospitalized patients. Existing surveillance programs potentially underestimate prevalence owing to limited site participation and clinical specimen availability. This study sought to quantify the prevalence of carbapenem resistance in invasive infections due to Enterobacteriaceae among adult hospitalized patients within 9 geographic regions across the United States.
(Preliminary results from this study were presented at the Society for Healthcare Epidemiology of America Spring 2015 Conference, 14–17 May, 2015, Orlando, FL.)
To accomplish the study objectives, a retrospective observational study was performed using hospital discharge data from approximately 178 U.S. health care facilities in the Premier research database with accessible microbiology results (see Appendix A in the supplemental material). In total, the Premier hospital database includes data from approximately 80 million admissions (>5 million added per year since 2011). Admissions data are provided by >500 participating acute care hospitals from across the United States and account for approximately 20% of all inpatient discharges in the nation (Premier Healthcare Database: Data that Informs and Performs, Premier, Inc., Charlotte, NC). The infective episode for a patient was included if all of the following criteria were met: (i) patient ≥18 years of age; (ii) inpatient discharge between 1 January 2011 and 31 December 2014; (iii) primary or secondary diagnosis at discharge using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), codes of complicated urinary tract infection (cUTI), complicated intra-abdominal infection (cIAI), hospital-associated pneumonia (HAP), or bloodstream infection (BSI) (see Appendix B in the supplemental material); (iv) positive culture for an Enterobacteriaceae strain obtained from a site consistent with the infection type (see Appendix C in the supplemental material); (v) receipt of antibiotic treatment on day of Enterobacteriaceae culture collection or ≤3 days after index Enterobacteriaceae culture; and (vi) available susceptibility data for the index isolate.
Cohorts by infection type were not mutually exclusive; patients were allowed to contribute information to multiple cohorts. However, in any qualifying admission, a patient could contribute data to a given disease cohort only once. All infections observed in qualifying admissions were considered independent.
Because of changing susceptibility breakpoints during the study period, resistance was defined as nonsusceptibility to meropenem, imipenem, doripenem, or ertapenem. Carbapenem resistance rates were stratified by U.S. geographic region (9 CDC regions), hospital type and characteristics (teaching versus nonteaching, urban versus rural), hospital unit (intensive care unit [ICU] versus non-ICU), infection type (cUTI, cIAI, HAP, or BSI), and pathogen (Klebsiella spp., Enterobacter spp., Escherichia coli, or other [Citrobacter spp. and Serratia spp.]). All calculations were computed using SAS 9.3 (SAS Institute, Cary, NC).
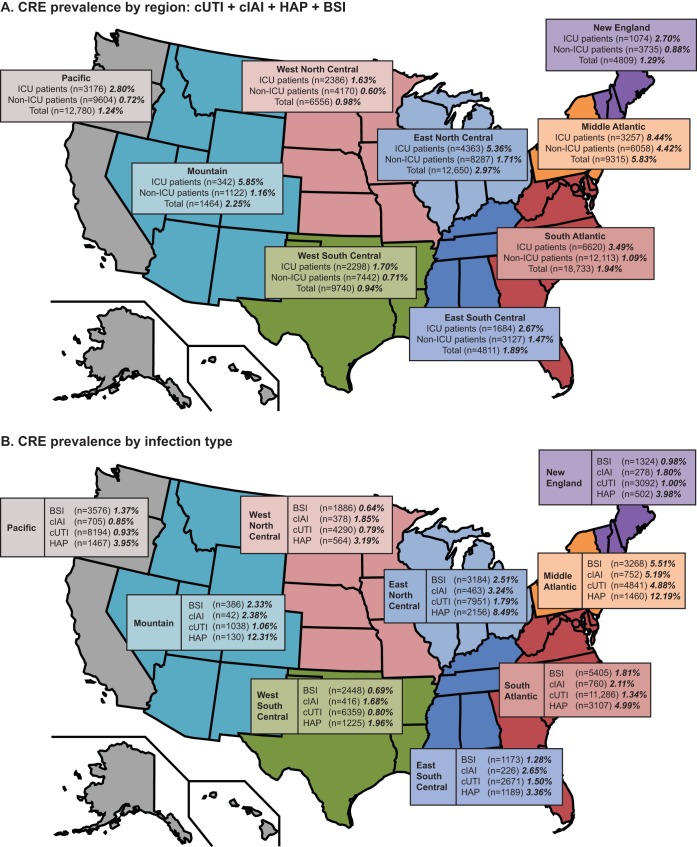
A total of 60,551 infective episodes met the study criteria. Overall resistance to carbapenem was 2.3% with substantial variation by geographic region (Fig. 1). The highest rates of CRE were observed in the Mid-Atlantic, East North Central, and Mountain areas, whereas the lowest rates were observed in the West North Central and West South Central areas. Prevalence of CRE was higher in ICUs than in non-ICUs across all geographic regions (Fig. 1A). Prevalence of CRE was also highest among patients with HAP relative to those with other infection types (Fig. 1B). Stratification by hospital type showed that resistance rates were higher in teaching than in nonteaching hospitals (3.2% versus 1.6%) and higher in urban than in rural hospitals (2.4% versus 0.8%). Rates also tended to be higher in larger hospitals, with rates of 1.0%, 2.3%, and 2.8%, respectively, for hospitals with 0 to 199, 200 to 499, and ≥500 beds.
FIG 1.
Prevalence of carbapenem resistance rates among hospitalized patients with infection due to Enterobacteriaceae, stratified by (A) geographic area (U.S. Centers for Disease Control and Prevention regions, combined cUTI, cIAI, HAP, and BSI) and hospital unit, 2011 to 2014, and (B) infection type. Rates are shown in bold italic type. BSI, bloodstream infection; cIAI, complicated intra-abdominal infection; CRE, carbapenem-resistant Enterobacteriaceae; cUTI, complicated urinary tract infection; HAP, hospital-associated pneumonia; ICU, intensive care unit.
Prevalence of CRE by geographic region, pathogen, and hospital unit are displayed in Table 1. In several regions, the prevalence of carbapenem resistance among patients with invasive infections due to Klebsiella spp. and Enterobacter spp. exceeded 5% and approached 10% (i.e., several with a prevalence of ≥8%). In contrast, rates of carbapenem resistance among infections due to E. coli were less than 1% for all geographic regions except in the ICUs in the East South Central, Mid-Atlantic, and Mountain regions. Prevalence of CRE by infection type, pathogen, and hospital unit are shown in Table 2. Irrespective of infection type, the highest rates of carbapenem resistance were observed in patients in the ICU with an infection due to Klebsiella spp. or Enterobacter spp.
TABLE 1.
Prevalence of carbapenem resistance rates among hospitalized patients with infection due to Enterobacteriaceae, stratified by geographic area (U.S. Centers for Disease Control and Prevention regions), pathogen, and hospital unit, 2011–2014
| Geographic division | Hospital unit |
Klebsiella spp. |
Enterobacter spp. |
Escherichia coli |
Citrobacter spp. |
Serratia spp. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | % with CREa | No. of patients | % with CRE | No. of patients | % with CRE | No. of patients | % with CRE | No. of patients | % with CRE | ||
| East North Central | ICUb | 1,503 | 12.18 | 579 | 6.04 | 2,346 | 0.34 | 193 | 2.07 | 311 | 1.61 |
| Non-ICU | 1,742 | 5.97 | 484 | 3.72 | 5,980 | 0.15 | 267 | 1.50 | 193 | 4.15 | |
| Total | 3,245 | 8.84 | 1063 | 4.99 | 8,326 | 0.20 | 460 | 1.74 | 504 | 2.58 | |
| East South Central | ICU | 554 | 3.97 | 242 | 4.55 | 935 | 1.18 | 59 | 5.08 | 126 | 2.38 |
| Non-ICU | 701 | 3.99 | 254 | 3.94 | 2,123 | 0.19 | 82 | 1.22 | 120 | 2.50 | |
| Total | 1,255 | 3.98 | 496 | 4.23 | 3,058 | 0.49 | 141 | 2.84 | 246 | 2.44 | |
| Mid-Atlantic | ICU | 1,250 | 14.96 | 502 | 14.34 | 1,656 | 1.15 | 185 | 3.24 | 238 | 4.62 |
| Non-ICU | 1,395 | 14.48 | 434 | 8.53 | 4,183 | 0.65 | 218 | 0.92 | 160 | 1.88 | |
| Total | 2,645 | 14.71 | 936 | 11.65 | 5,839 | 0.79 | 403 | 1.99 | 398 | 3.52 | |
| Mountain | ICU | 114 | 7.89 | 37 | 8.11 | 186 | 1.61 | 10 | 0.00 | 18 | 27.78 |
| Non-ICU | 137 | 1.46 | 53 | 7.55 | 925 | 0.54 | 25 | 0.00 | 8 | 25.00 | |
| Total | 251 | 4.38 | 90 | 7.78 | 1,111 | 0.72 | 35 | 0.00 | 26 | 26.92 | |
| New England | ICU | 312 | 1.92 | 161 | 6.83 | 568 | 0.88 | 66 | 1.52 | 109 | 5.50 |
| Non-ICU | 726 | 1.24 | 317 | 3.15 | 2,583 | 0.39 | 175 | 1.14 | 95 | 3.16 | |
| Total | 1,038 | 1.45 | 478 | 4.39 | 3,151 | 0.48 | 241 | 1.24 | 204 | 4.41 | |
| Pacific | ICU | 1,018 | 3.54 | 370 | 5.14 | 1,931 | 0.88 | 128 | 2.34 | 156 | 8.97 |
| Non-ICU | 1,572 | 1.84 | 429 | 1.86 | 7,638 | 0.26 | 268 | 0.37 | 146 | 8.22 | |
| Total | 2,590 | 2.51 | 799 | 3.38 | 9,569 | 0.39 | 396 | 1.01 | 302 | 8.61 | |
| South Atlantic | ICU | 2,410 | 4.73 | 930 | 8.82 | 3,412 | 0.59 | 263 | 3.04 | 411 | 2.68 |
| Non-ICU | 2,489 | 2.61 | 732 | 4.10 | 8,701 | 0.29 | 357 | 0.56 | 302 | 3.64 | |
| Total | 4,899 | 3.65 | 1662 | 6.74 | 12,113 | 0.37 | 620 | 1.61 | 713 | 3.09 | |
| West North Central | ICU | 679 | 3.98 | 259 | 3.09 | 1,473 | 0.27 | 100 | 0.00 | 89 | 0.00 |
| Non-ICU | 756 | 2.51 | 266 | 1.50 | 3,106 | 0.03 | 129 | 0.00 | 81 | 1.23 | |
| Total | 1,435 | 3.21 | 525 | 2.29 | 4,579 | 0.11 | 229 | 0.00 | 170 | 0.59 | |
| West South Central | ICU | 702 | 2.56 | 250 | 3.20 | 1,431 | 0.42 | 103 | 2.91 | 108 | 4.63 |
| Non-ICU | 1,202 | 1.41 | 360 | 2.50 | 5,844 | 0.29 | 199 | 3.52 | 140 | 2.86 | |
| Total | 1,904 | 1.84 | 610 | 2.79 | 7,275 | 0.32 | 302 | 3.31 | 248 | 3.63 | |
CRE, carbapenem-resistant Enterobacteriaceae.
ICU, intensive care unit.
TABLE 2.
Prevalence of carbapenem resistance rates among hospitalized patients with infection due to Enterobacteriaceae, stratified by infection type, pathogen, and hospital unit, 2011–2014
| Pathogen | Setting | No. of Patients | % with CREa | |
|---|---|---|---|---|
| Complicated urinary tract infection | E. coli | ICUb | 5,835 | 0.51 |
| E. coli | Non-ICU | 31,874 | 0.29 | |
| E. coli | Total | 37,709 | 0.32 | |
| Enterobacter spp. | ICU | 607 | 6.75 | |
| Enterobacter spp. | Non-ICU | 1,962 | 4.08 | |
| Enterobacter spp. | Total | 2,569 | 4.71 | |
| Klebsiella spp. | ICU | 2,292 | 7.20 | |
| Klebsiella spp. | Non-ICU | 6,737 | 4.72 | |
| Klebsiella spp. | Total | 9,029 | 5.35 | |
| Serratia spp. | ICU | 209 | 4.31 | |
| Serratia spp. | Non-ICU | 544 | 3.86 | |
| Serratia spp. | Total | 753 | 3.98 | |
| Citrobacter spp. | ICU | 394 | 2.54 | |
| Citrobacter spp. | Non-ICU | 1,296 | 1.00 | |
| Citrobacter spp. | Total | 1,690 | 1.36 | |
| Complicated intra-abdominal infection | E. coli | ICU | 1,396 | 1.07 |
| E. coli | Non-ICU | 1,589 | 0.19 | |
| E. coli | Total | 2,985 | 0.60 | |
| Enterobacter spp. | ICU | 315 | 10.16 | |
| Enterobacter spp. | Non-ICU | 155 | 4.52 | |
| Enterobacter spp. | Total | 470 | 8.30 | |
| Klebsiella spp. | ICU | 654 | 5.50 | |
| Klebsiella spp. | Non-ICU | 406 | 1.97 | |
| Klebsiella spp. | Total | 1,060 | 4.15 | |
| Serratia spp. | ICU | 41 | 2.44 | |
| Serratia spp. | Non-ICU | 21 | 4.76 | |
| Serratia spp. | Total | 62 | 3.23 | |
| Citrobacter spp. | ICU | 150 | 3.33 | |
| Citrobacter spp. | Non-ICU | 109 | 0.00 | |
| Citrobacter spp. | Total | 259 | 1.93 | |
| Hospital-associated pneumonia | E. coli | ICU | 3,058 | 0.88 |
| E. coli | Non-ICU | 1,223 | 0.74 | |
| E. coli | Total | 4,281 | 0.84 | |
| Enterobacter spp. | ICU | 1,868 | 8.40 | |
| Enterobacter spp. | Non-ICU | 458 | 4.37 | |
| Enterobacter spp. | Total | 2,326 | 7.61 | |
| Klebsiella spp. | ICU | 3,803 | 8.73 | |
| Klebsiella spp. | Non-ICU | 1,291 | 6.51 | |
| Klebsiella spp. | Total | 5,094 | 8.17 | |
| Serratia spp. | ICU | 1,067 | 4.22 | |
| Serratia spp. | Non-ICU | 389 | 5.40 | |
| Serratia spp. | Total | 1,456 | 4.53 | |
| Citrobacter spp. | ICU | 429 | 3.03 | |
| Citrobacter spp. | Non-ICU | 114 | 3.51 | |
| Citrobacter spp. | Total | 543 | 3.13 | |
| Bloodstream infection | E. coli | ICU | 5,949 | 0.79 |
| E. coli | Non-ICU | 9,426 | 0.27 | |
| E. coli | Total | 15,375 | 0.47 | |
| Enterobacter spp. | ICU | 934 | 7.17 | |
| Enterobacter spp. | Non-ICU | 909 | 3.19 | |
| Enterobacter spp. | Total | 1,843 | 5.21 | |
| Klebsiella spp. | ICU | 2,963 | 6.38 | |
| Klebsiella spp. | Non-ICU | 3,035 | 3.43 | |
| Klebsiella spp. | Total | 5,998 | 4.68 | |
| Serratia spp. | ICU | 434 | 4.15 | |
| Serratia spp. | Non-ICU | 364 | 1.92 | |
| Serratia spp. | Total | 798 | 3.13 | |
| Citrobacter spp. | ICU | 274 | 1.09 | |
| Citrobacter spp. | Non-ICU | 277 | 1.44 | |
| Citrobacter spp. | Total | 551 | 1.27 |
CRE, carbapenem-resistant Enterobacteriaceae.
ICU, intensive care unit.
In light of our findings, the use of empirical carbapenems should be revisited in some hospitals located in areas of high CRE prevalence in patients with suspected or documented invasive infection due to Klebsiella spp. and Enterobacter spp., especially among those patients in the ICU and those with HAP. Although there are no definitive guidelines regarding empirical antibiotic selection for treatment of a suspected or documented infection due to Enterobacteriaceae, it is typically not advisable to select an antibiotic as empirical treatment once rates of resistance to that antibiotic exceed 20% (2). The rapid spread of CRE over the past 10 years across the United States also highlights the need for ongoing monitoring at the regional and hospital levels. Furthermore, the results suggest that institutions should proactively identify patient populations at greatest risk for carbapenem resistance and develop decision support tools/algorithms for identifying patients who are at high risk for CRE. This information will be important for clinicians to support selection of the most appropriate empirical treatment for patients within the first 48 to 72 h of infection onset before culture results are available.
Several limitations affect the interpretation of these findings. Because data were extracted from an electronic database, our findings may be limited by the accuracy of the coding of those data, including the accuracy of the microbiological data. However, Premier does link patient keys (unique identifiers) to microbiological specimens and specimen information, including isolated organisms and susceptibility data, to ensure accuracy within the database. In addition, Premier conducts a separate validation process to ensure that the data are entered into the database correctly. In addition to relying on ICD-9-CM coding, we also required culture data from sites appropriate for the ICD-9-CM code to minimize the impact of any coding errors. Nonetheless, data abstraction from medical records or prospective data collection would provide more accurate and comprehensive clinical data. Although resistance rates were stratified by region and hospital characteristics, these findings may not apply to every institution in a given region. In addition, the variations in resistance rates may in part be due to the small number of patients with CRE in certain regions. As such, local antibiograms and patient and disease characteristics should always drive empirical antibiotic selection. However, the data clearly highlight carbapenem resistance concerns among hospitalized patients with an invasive infection due to Enterobacteriaceae. Carbapenem resistance was defined as nonsusceptibility to ≥1 carbapenem only, as opposed to resistance to ≥1 carbapenem and nonsusceptibility to ≥1 relevant third-generation cephalosporin. Due to the changing Clinical and Laboratory Standards Institute and U.S. Food and Drug Administration definitions of carbapenem susceptibility over the study period, this study relied solely on nonsusceptibility to carbapenem. This study did not evaluate antibiotic resistance factors at a patient-specific level. Despite these limitations, the characterization of CRE trends at the regional level and by hospital characteristics provides information to support clinicians in empirical antibiotic selection.
The findings from this study underscore the urgent public health threat from infections caused by CRE, especially among patients with an invasive infection due to Klebsiella spp. or Enterobacter spp. in the Mid-Atlantic and surrounding regions. Rising rates of CRE necessitate ongoing monitoring at regional and hospital levels. Given the importance of initiating early appropriate therapy, the findings highlight the need for developing decision support tools to identify patients at high risk for CRE and should be considered alongside patient and hospital characteristics when selecting empirical treatment for patients before availability of culture results.
Supplementary Material
ACKNOWLEDGMENTS
Writing and editorial assistance was provided by Bret Fulton and Kevin Ryder of Complete Healthcare Communications, LLC (Chadds Ford, PA) and was funded by Allergan plc (Dublin, Ireland).
Thomas Lodise has received consulting fees or honoraria and support for travel to meetings for this study or other purposes from Allergan. He has also been a consultant for Merck and The Medicines Company and has received payment for lectures, including service on speakers bureaus for Allergan for work not associated with the current study. Michael J. Ye is an employee of Allergan. Qi Zhao was an employee of Allergan at the time of study conduct and analysis.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00228-17.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of America, European Society for Microbiology and Infectious Diseases. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.