ABSTRACT
Despite recent advances in therapeutic strategies against hepatitis B virus (HBV) infection, chronic hepatitis B remains a major global health burden. Recent studies have shown that targeting host factors instead of viral factors can be an effective antiviral strategy with low risk of the development of resistance. Efforts to identify host factors affecting viral replication have identified p38 mitogen-activated protein kinase (MAPK) as a possible target for antiviral strategies against various viruses, including HBV. Here, a series of biphenyl amides were synthesized as novel p38 MAPK selective inhibitors and assessed for their anti-HBV activities. The suppression of HBV surface antigen (HBsAg) production by these compounds was positively correlated with p38 MAPK-inhibitory activity. The selected compound NJK14047 displayed significant anti-HBV activity, as determined by HBsAg production, HBeAg secretion, and HBV production. NJK14047 efficiently suppressed the secretion of HBV antigens and HBV particles from HBV genome-transfected cells and HBV-infected sodium taurocholate cotransporting polypeptide-expressing human hepatoma cells. Furthermore, NJK14047 treatment resulted in a significant decrease of pregenomic RNA and covalently closed circular DNA (cccDNA) of HBV in HBV-harboring cells, indicating its ability to inhibit HBV replication. Considering that suppression of HBsAg secretion and elimination of cccDNA of HBV are the major aims of anti-HBV therapeutic strategies, the results suggested the potential use of these compounds as a novel class of anti-HBV agents targeting host factors critical for viral infection.
KEYWORDS: hepatitis B, antiviral agents, hepatitis B virus, p38 MAPK
INTRODUCTION
Hepatitis B virus (HBV) infection is associated with a broad spectrum of liver diseases, including acute hepatitis, chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC), and is a leading cause of cirrhosis and HCC (1). Although vaccines and antiviral agents, including nucleoside/nucleotide analogues and recombinant type I interferons, are available, more than 350 million people suffer from chronic hepatitis B worldwide, and approximately 1 million deaths are caused by HBV-related diseases every year (2, 3).
HBV initiates infection by interaction between the pre-S1 region of the large surface protein (LHB) with a recently identified receptor, sodium taurocholate cotransporting polypeptide (NTCP), in hepatocytes (4). HBV virions contain partially double-stranded relaxed circular DNA (rcDNA) genomes. Once internalized by receptor mediated-endocytosis, released rcDNA enters the nucleus and is converted into covalently closed circular DNA (cccDNA), which acts as a template for RNA synthesis, including mRNAs and pregenomic RNAs (pgRNAs). Packaged pgRNAs are converted into partially double-stranded rcDNA in the capsid by reverse transcriptase (RT).
Although nucleoside RT inhibitors, such as entecavir and tenofovir, efficiently suppress HBV replication, none of them completely eliminates the virus; thus, patients require long-term treatment, which can lead to undesirable side effects, such as nephrotoxicity and drug resistance (5–9). Therefore, the development of different classes of antiviral agents targeting HBV is urgently needed.
Mitogen-activated protein kinases (MAPKs) are a class of cytoplasmic serine-threonine kinases ubiquitously expressed and activated by various upstream signaling pathways, such as growth factors, inflammatory cytokines, and G protein-coupled receptor signaling (10, 11). Among MAPKs, p38 MAPKs, comprising α, β, γ, and δ isoforms, play key roles in immune responses by regulating proinflammatory cytokines. Thus, the MAPKs have been recognized as attractive therapeutic targets for inflammatory autoimmune diseases, such as asthma, rheumatoid arthritis, multiple sclerosis, and psoriasis (12, 13).
Interestingly, several viruses, including Ebola virus, Epstein-Barr virus, influenza virus, and HBV, have been known to utilize p38 MAPK for their life cycles, and suppression of p38 MAPK results in suppression of viral replication (14–17). p38 MAPK plays a crucial role in HBV surface antigen (HBsAg)/hepatitis B e antigen (HBeAg) secretion, virus production, and the maintenance of HBV cccDNA in HBV-infected cells (18). Therefore, inhibiting p38 MAPK may be an effective approach for anti-HBV therapies. Within the past decade, several biphenyl amides have been developed as novel p38 MAPK inhibitors (19). In particular, we recently reported a class of novel biphenyl amide p38 MAPK inhibitors containing a benzophenone moiety and demonstrated that some of these inhibitors have potent p38 MAPK-inhibitory activities and kinase selectivities (14, 20). In the current study, we explored the antiviral activities of these p38 MAPK inhibitors against HBV.
RESULTS
Synthesis and evaluation of p38 MAPK-inhibitory activities of biphenyl amides.
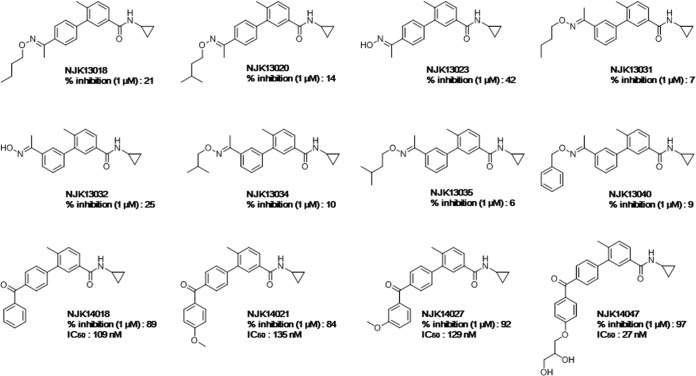
Based on the potential use of biphenyl amides as p38 MAPK inhibitors, we synthesized various biphenyl amides to identify novel anti-HBV agents (Fig. 1). Twelve biphenyl amides were efficiently synthesized, as previously reported by our group and other researchers (19, 20), and tested for their abilities to suppress in vitro p38 MAPK enzyme activity using the SelectScreen kinase-profiling service (Life Technologies). Inhibition of p38 MAPK with 1 μM biphenyl amide compound ranged from 6% to 97% (Fig. 1).
FIG 1.
Chemical structures and p38 MAPK-inhibitory activities of the tested compounds. In vitro p38 MAPK enzyme-inhibitory activities (percent inhibition) at 1 μM were measured.
p38 MAPK-inhibitory activities were positively correlated with the suppression of HBsAg secretion.
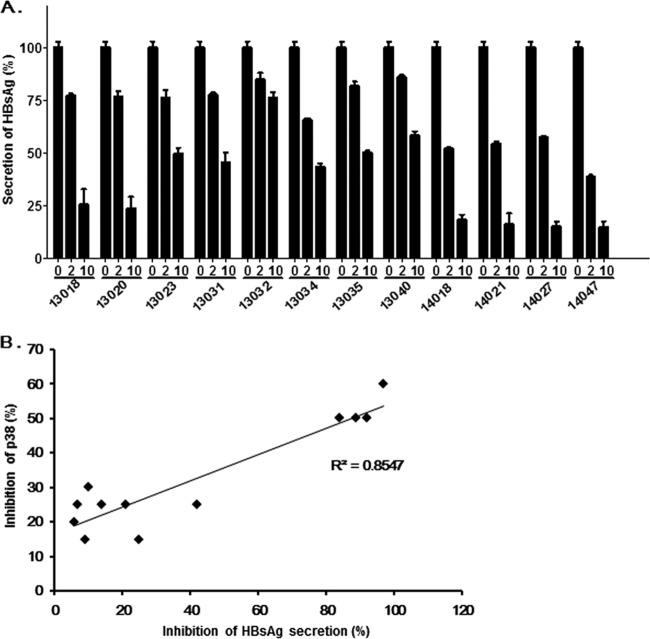
To examine the anti-HBV activities of the compounds, HepG2.2.15 cells harboring HBV genotype D were incubated with the compounds for 48 h. All the compounds except NJK13032 and NJK13040 suppressed HBsAg secretion more than 50% at 10 μM, as determined by HBsAg enzyme-linked immunosorbent assays (ELISAs) (Bio-Kit). NJK14021 and NJK14027 showed approximately 50% inhibition at 2 μM. NJK14047 showed the greatest inhibition of p38 MAPK and HBsAg secretion (Fig. 2A). As depicted in Fig. 2B, in vitro p38 MAPK enzyme inhibition and suppression of HBsAg secretion by the compounds showed high positive correlation (r2 = 0.8547), suggesting that the compounds suppressed HBsAg secretion via p38 MAPK inhibition. NJK14047, which exhibited the most potent HBsAg-suppressive effect, was selected for further evaluation.
FIG 2.
Positive correlation between p38 MAPK-inhibitory activities and HBsAg secretion-inhibitory effects of tested biphenyl amides. (A) Suppression of HBsAg secretion by the compounds. Culture supernatants from HepG2.2.15 cells were treated with the compounds (0, 2, or 10 μM) for 48 h, and HBsAg secretion was then analyzed by ELISA. The experiment was done in triplicate, and the error bars indicate standard deviations. (B) Correlation between p38 MAPK and HBsAg secretion-inhibitory activities of the compounds.
Characterization of NJK14047.
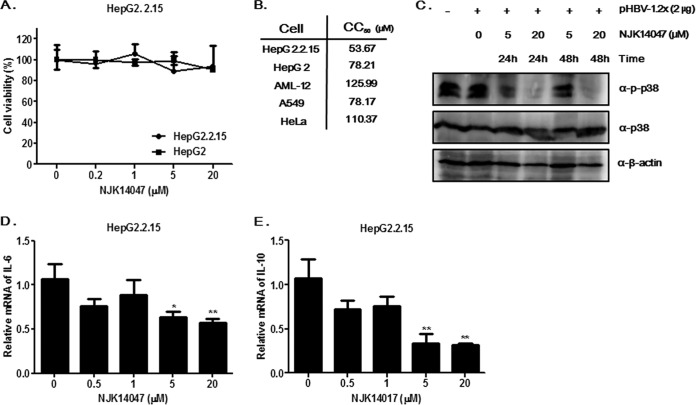
The cytotoxic effects of NJK14047 on HepG2 and HepG2.2.15 cells were analyzed to exclude the possibility that antiviral activity was caused by cytotoxicity. Treatment with NJK14047 for 48 h did not have any significant cytotoxic effects on HepG2.2.15 cells at concentrations of up to 20 μM; therefore, 20 μM NJK14047 was used as a maximum concentration for subsequent studies (Fig. 3A). The cytotoxic effects of NJK14047 on other cell lines were then further analyzed. As shown in Fig. 3B, 50% cytotoxic concentration (CC50) values ranged from 53.7 to 110.4 μM for different cancer cell lines, and the CC50 value of NJK14047 in normal mouse hepatocytes (AML-12 cells) was slightly higher than those in the other tested cell lines (125.99 μM).
FIG 3.
Suppression of HBV antigen secretion by NJK14047. (A) Effects of NJK14047 on the viability of HepG2 and HepG2.2.15 cells. HepG2 and HepG2.2.15 cells were treated with the indicated concentrations of NJK14047 for 48 h. Cell viability was determined by MTT assay. (B) Cytotoxicity of NJK14047 in various cell lines. The CC50 values were determined by MTT assays using the indicated cell lines. (C) Suppression of p38 MAPK activation by NJK14047. HepG2 cells transfected with the HBV genome (pHBV-1.2x) were treated with NJK14047 as indicated. Cell lysates were analyzed by immunoblotting using the indicated antibodies. (D and E) Effects of NJK14047 on synthesis of IL-6 (D) and IL-10 (E) mRNAs in HepG2.2.15 cells. The cells were treated with increasing concentrations of NJK14047 for 12 h. mRNA levels were determined by RT-qPCR. The experiments were done in triplicate. The data represent means and standard deviations. *, P < 0.05, and **, P < 0.01 versus the control.
In our previous study, NJK14047 was found to show dose-dependent inhibitory effects on p38 MAPK (IC50 = 27 nM) (20). To confirm p38 MAPK inhibition in hepatocytes, HepG2 cells transfected with a plasmid containing the HBV genome (pHBV-1.2x; GenBank accession no. AY641558) (21) were treated with 5 or 20 μM NJK14047 and analyzed by immunoblotting. Treatment with NJK14017 decreased p38 MAPK phosphorylation without affecting total protein levels, indicating that NJK14047 was capable of suppressing p38 MAPK activation in HBV-infected cells (Fig. 3C). In addition, NJK14047 treatment markedly suppressed the synthesis of mRNAs encoding interleukin 6 (IL-6) and IL-10 in HepG2.2.15 cells in a dose-dependent manner, further confirming that NJK14047 was capable of suppressing p38 MAPK-mediated inflammatory responses (Fig. 3D and E).
NJK14047 inhibited the secretion of HBV antigens and blocked viral replication.
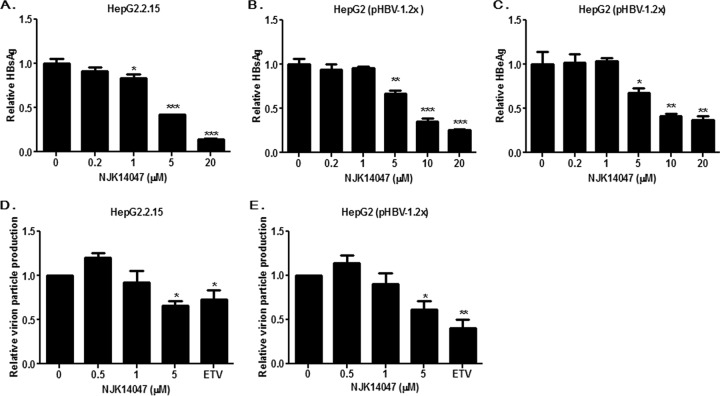
To further delineate the anti-HBV activity of NJK14047, HepG2.2.15 cells were treated with increasing concentrations of NJK14047, and the secretion of HBsAg was analyzed by ELISA. NJK14047 significantly suppressed HBsAg secretion from HepG2.2.15 cells in a dose-dependent manner (IC50 = 5.3 μM) (Fig. 4A). In the experimental setting using HepG2.2.15 cells, we could not detect any significant effect of NJK14047 on HBeAg secretion, which was also determined by ELISA (data not shown). This result suggests that NJK14047 is not capable of suppressing HBeAg production and secretion from HBV genomes stably integrated into chromosomes. The antiviral effects of NJK14047 were also evaluated using an HBV genome transfection model with the genotype C viral genome. HepG2 cells were transfected with pHBV-1.2x, as described previously (21). Twenty-four hours after transfection, the cells were treated with NJK14047 for 48 h, and the supernatants were analyzed by ELISA. Unlike the HepG2.2.15 cell system, NJK14047 treatment led to dose-dependent decreases in both HBsAg and HBeAg secretion (Fig. 4B and C).
FIG 4.
Antiviral activity of NJK14047 against HBV. (A and B) Suppression of HBsAg secretion by NJK14047. HepG2.2.15 cells (A) and HepG2 cells transfected with pHBV-1.2x (B) were treated with increasing amounts of NJK14047. HBsAg secretion was analyzed by ELISA. (C) Suppression of HBeAg secretion by NJK14047. HepG2 cells were transfected with pHBV-1.2x and treated with increasing concentrations of NJK14047 for 48 h. The amount of secreted HBeAg was determined by ELISA. (D and E) Suppression of HBV particle production by NJK14047. HepG2.15 (D) and HepG2 cells transfected with the HBV genome (pHBV-1.2x) (E) were treated with NJK14047 for 48 h. Virus production was determined by measuring extracellular viral DNA using quantitative PCR. All the experiments were done in triplicate. Statistical significance was tested by Student's t test. The data are presented as means and standard deviations. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus the control.
The effects of NJK14047 on HBV virion production were analyzed to examine its effect on HBV replication. HBV particles were precipitated from culture supernatants of HepG2.2.15 cells treated with NJK14047, and viral DNA was purified and quantified by quantitative PCR (qPCR), as described previously (22). HBV production was significantly reduced by NJK14047 treatment, which indicated that NJK14047 was capable of suppressing not only viral antigen production, but also viral replication and virion particle production (Fig. 4D). A similar result was obtained from the experiment using HepG2 cells transfected with pHBV-1.2x (Fig. 4E).
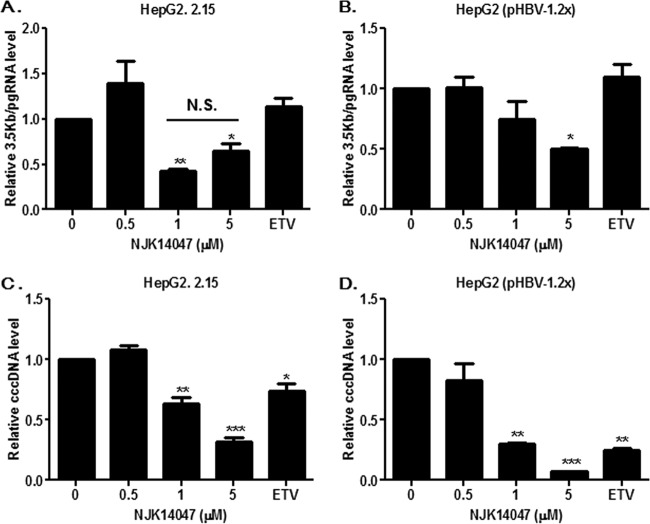
NJK14047 downregulated HBV pgRNA and cccDNA levels.
In order to further confirm the effects of NJK14047 on HBV replication, its effects on HBV pgRNA levels were analyzed. A significant decrease of 3.5-kb pgRNA was observed in NJK14047-treated cells compared with that in vehicle-treated cells, similar to experiments using HepG2.2.15 cells (Fig. 5A) or HepG2 cells transfected with pHBV-1.2x (Fig. 5B). To examine whether HBV pgRNA reduction by NJK14047 was due to reduced levels of cccDNA, which acts as a template for pgRNA, the level of cccDNA was determined in HepG2.2.15 cells and HepG2 cells transfected with linearized pHBV1.2-x. As shown in Fig. 5C and D, treatment with NJK14047 resulted in significant reductions of cccDNA in both experimental settings. Treatment with 5 μM NJK14047 resulted in 70% and 90% reductions of cccDNA in HepG2.2.15 cells and pHBV-1.2x-transfected cells, respectively. These results suggested that p38 MAPK inhibition by NJK14047 could suppress cccDNA establishment and subsequent pgRNA synthesis.
FIG 5.
Suppression of HBV pgRNA synthesis and cccDNA establishment by NJK14047. (A and B) HepG2.2.15 cells (A) and pHBV-1.2x-transfected HepG2 cells (B) were treated with increasing amounts of NJK14047 or entecavir (ETV) (30 nM) for 48 h. The levels of pgRNA (3.5 kb) were determined by quantitative RT-PCR. (C and D) HepG2.2.15 cells (C) and pHBV-1.2x-transfected HepG2 cells (D) were treated with increasing amounts of NJK14047 or ETV (30 nM) for 48 h. cccDNA levels were determined by quantitative PCR. Statistical significance was tested by Student's t test. The data are presented as means and standard deviations. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus the control. N.S., not significant.
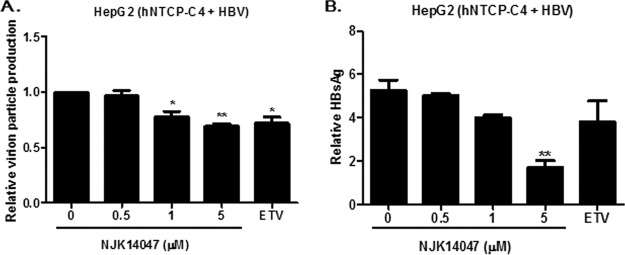
NJK14047 exhibited antiviral activity in an in vitro HBV infection model.
The antiviral activity of NJK14047 was assessed using an in vitro infection model system with HepG2 cells stably expressing the HBV receptor. NTCP-expressing HepG2-NTCP-C4 cells were infected with HBV, and production of HBV particles and HBsAg was monitored with or without NJK14047 treatment. As expected, NJK14047 treatment resulted in significant decreases in both HBV virion particle production (Fig. 6A) and HBsAg secretion (Fig. 6B) in a dose-dependent manner.
FIG 6.
Suppression of viral replication and HBsAg production by NJK14047 in an in vitro HBV infection model. HBV was obtained from HepG2.2.15 cell culture supernatants by ultracentrifugation. HepG2 cells stably expressing NTCP (NTCP-HepG2-C4) were infected with HBV and treated with the indicated concentrations of NJK14047 or ETV (30 nM) for 48 h. Extracellular viral-DNA levels (A) and secreted HBsAg levels (B) were determined by quantitative PCR and ELISA, respectively. Statistical significance was tested by Student's t test. The data are presented as means and standard deviations. *, P < 0.05, and **, P < 0.01 versus the control.
DISCUSSION
Restriction of viral replication by targeting host factors critical for viral replication can be an attractive strategy, since it may confer a higher genetic barrier for the development of resistance than antiviral agents targeting direct viral factors (23, 24). Current anti-HBV therapeutic agents, such as type I interferon and nucleoside/nucleotide analogues, successfully suppress viral replication and reduce the risk of HBV-related liver complications, including cirrhosis and HCC. However, current nucleoside/nucleotide regimens require long-term treatment, and the stability of cccDNA in nuclei is a main obstacle to curing chronic HBV infections. Long-term antiviral therapy has several disadvantages, including high cost and cumulative toxicity, resulting in bone diseases and renal tubular injury, and is also limited to patients with significant viremia and either elevated alanine aminotransferase (ALT) or significant fibrosis (25). Therefore, there is an urgent unmet need for efficacious therapies to treat chronic hepatitis B.
p38 MAPK is a promising therapeutic target for various inflammatory diseases and cancers. Despite extensive efforts to develop specific p38 MAPK inhibitors, no therapeutically approved p38 MAPK inhibitors have been established. In an effort to develop novel p38 MAPK-inhibiting chemical agents, we have reported on several novel biphenyl amides and showed that some of them specifically inhibit p38 MAPK activity (20).
NJK14047 displayed significant p38 MAPK-inhibitory activity and subsequent antiviral activity against different genotypes of HBV. Significantly higher concentrations of NJK14047 were required for antiviral activity at the cellular level than for its in vitro p38 MAPK enzyme inhibition (IC50 = 27 nM). This might be due to its low cellular penetrating activity or properties interacting with serum components in culture medium. p38 MAPK activity is known to be required for efficient HBV replication and the maintenance of cccDNA. Thus, inhibiting p38 MAPK activity by using selective inhibitors can be a potential therapeutic strategy to suppress HBV replication and to decrease the amount of HBV cccDNA that cannot be eliminated by the currently approved RT inhibitors. In addition, strategies that target host factors, such as p38 MAPK, would be expected to lower the risk of generating drug-resistant mutants. Considering the crucial role of p38 MAPK in the inflammatory process and related pathological conditions, p38 MAPK inhibitors may have additional advantages of alleviating HBV-related hepatic inflammation. In our previous studies, NJK14047 was shown to be specific to p38 MAPKα/β, showing no or little effect when tested on 92 other kinases. Moreover, the drug showed the robust ability to suppress the replication of other viruses, such as respiratory syncytial virus (RSV) and influenza A virus, which require p38 MAPK for their life cycles.
Given that most reported p38 MAPK inhibitors, including SB203580, show several off-target effects and low efficacy, partly due to the lack of kinase selectivity, NJK14047 may be a promising drug candidate, possessing potent p38 MAPK-inhibitory activity and high kinase selectivity. In addition, NJK14047 meets the Lipinski rule of five (molecular weight, 445.51; calculated logarithm of the partition coefficient (cLogP), 3.120; number of hydrogen bonding donors, 3; number of hydrogen bonding acceptors, 6), which suggests that it may possess suitable physicochemical properties for the development of an orally active drug. Although the inhibition of p38 MAPK has been considered a potential therapeutic strategy for various pathological conditions, a concern regarding toxicity when using p38 MAPK inhibitors has been addressed. Although NJK14047 displayed significant anti-HBV effects at concentrations that did not affect cell viability, its therapeutic indexes (TI) (the CC50 divided by the 50% effective concentration [EC50]), determined by HBsAg secretion from HBV-harboring cells, were around 10. Therefore, the toxicity and efficacy of NJK14047 should be carefully monitored through in vivo studies to determine the optimal therapeutic window.
Taken together, these results suggested that NJK-14047 might be a potential candidate for further evaluation as an antiviral agent against HBV and other viruses by suppressing viral replication and infection-mediated inflammatory responses (20).
MATERIALS AND METHODS
Synthesis and analysis of biphenylamide compounds.
Synthetic procedures for NJK14018, NJK14021, NJK14027, and NJK14047 were previously reported (20). Synthetic procedures for the other compounds (NJK13018, NJK13020, NJK13023, NJK13031, NJK13032, NJK13034, NJK13035, and NJK13040) are described in the supplemental material (supplementary synthesis procedures). All the structures and purities of the compounds synthesized in this study were confirmed by analyzing spectral data, such as 1H nuclear magnetic resonance, low-resolution mass spectrometry, and high-resolution mass spectrometry (see the supplemental material).
Cells and viral-genome transfection.
HepG2, HepG2.2.15, A549, and HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (100 U/ml). AML12 cells were maintained in AML12 medium (DMEM/F-12 [1:1] medium supplemented with GlutaMax, insulin [0.005 mg/ml], transferrin [0.005 mg/ml], selenium [5 ng/ml], dexamethasone [40 ng/ml], and 10% FBS). Human NTCP (hNTCP)-HepG2-C4 cells (kindly provided by Koichi Watashi) were maintained in AML12 medium containing G418 (500 μg/ml). For HBV genome (pHBV-1.2x) plasmid transfection, HepG2 cells were seeded into 6-well plates (4 × 105 cells/2 ml). After incubation for 16 h, the cells were transfected with DNA (4 μg) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. To assess cccDNA establishment from the transfected cells, pHBV-1.2x was linearized by SmaI enzyme treatment (25°C; 12 h), and DNA purification was carried out using a Mega Quick spin kit (Intron, Republic of Korea) before transfection.
Cell viability assay.
The cytotoxic effects of NJK14047 on various cells were assessed by 3-(4,5-dimenthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, as described previously (26). Briefly, HepG2, HepG2.2.15, AML12, A549, or HeLa cells in 96-well plates were incubated with different concentrations of NJK14047 for 48 h. Next, the cell culture media were replaced with fresh medium containing MTT (40 μg/ml) and further incubated for 4 h. After aspiration, dimethyl sulfoxide (DMSO) was added to lyse the cells and to solubilize water-insoluble formazan. Absorbance at 570 nm was determined with a microplate reader.
Analysis of HBsAg and HBeAg secretion.
Cells were treated with the indicated compounds, and the cell culture supernatants were collected and subjected to ELISAs. HBsAg ELISA (Biokit, Barcelona, Spain) and HBeAg ELISA (DIAsource, Louvain-la-Neuve, Belgium) were conducted according to the manufacturers' instructions.
Viral-particle production analysis.
HepG2.2.15 and HepG2 cells transfected with the HBV genome were treated with the indicated concentrations of NJK14047 for 48 h. Viral particles in the collected culture supernatants were precipitated using polyethylene glycol 6000 (PEG 6000), as described previously (27). Viral pellets acquired from HBV genome-transfected cells were treated with RQ1 DNase I (Promega, WI, USA) according to the manufacturer's instructions to remove the remaining DNA used for transfection. The viral genome levels were quantified using quantitative real-time PCR with the following primers: forward primer, 5′-TTAACAAGAATCCTCACAATACC-3′; reverse primer, 5′-GGAGGTTGGGGACTGCGAAT-3′.
Determination of pgRNA levels.
HBV pgRNA levels in the cells were determined as described previously, with slight modifications (28). Total RNA was isolated from HepG2.2.15 or HepG2 cells transfected with the HBV genome using TRIzol and treated with RQ1 DNase I (Promega). RNA was purified by conventional phenol-chloroform-isoamyl alcohol extraction and ethanol precipitation, and RNA samples (1 μg) were subjected to cDNA synthesis. The amount of pgRNA was determined by TaqMan real-time RT-qPCR using the following primers and probe: forward primer, 5′-GGTCCCCTAGAAGAAGAACTCCCT-3′; reverse primer, 5′-CATTGAGATTCCCGAGATTGAGAT-3′; TaqMan probe, 5′-6-carboxyfluorescein (FAM)-TCTCAATCGCCGCGTCGCAGA-6-carboxytetramethylrhodamine (TAMRA)-3′. All values were normalized to those of GAPDH as a control.
Quantification of HBV nuclear cccDNA.
HBV cccDNA in HepG2.2.15 or HepG2 cells transfected with the linearized HBV genome was quantified as described previously with some modifications (29). Collected cells were washed with cold phosphate-buffered saline and lysed by incubation in lysis buffer A (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1% NP-40) for 10 min on ice. After brief centrifugation (10,000 × g; 1 min), the pelleted nuclei were resuspended in lysis buffer B (10 mM Tris-HCl, 10 mM EDTA, 150 mM NaCl, 0.5% sodium dodecyl sulfate, and 0.5 mg/ml protein K) and incubated overnight at 37°C. DNAs extracted by phenol-chloroform extraction and ethanol extraction were treated with 10 U Plasmid-Safe ATP-dependent DNase I (10 U; Epicentre, WI, USA) for 45 min, followed by DNase inactivation at 70°C. cccDNAs were purified using a Mega Quick spin kit (Intron) and quantified by real-time quantitative PCR using the following primers and probe: cccF1, 5′-CCGTCTGTGCCTTCTCAT-3′; cccR1, 5′-CACAGCTTGGAGGCTTGAAC-3′; cccProbe, 5′-FAM-CGTGTGCACTTCGCTTCACCTCTGC-TAMRA-3′.
In vitro HBV infection assay.
Culture supernatants were collected every 2 days for 14 days from HepG2.2.15 cells (4 × 107 cells/150-mm dish). The supernatants were briefly centrifuged and filtered (0.45 μm) to remove cellular debris. Virus particles were concentrated by ultracentrifugation (100,000 × g; 3 h; 4°C) and resuspended with AML12 medium containing DMSO (2%) and PEG 8000 (4%). NTCP-HepG2-C4 cells in AML12 medium containing DMSO (2%) were infected with HBV (6 × 103 genome equivalents/cell), followed by centrifugation (1,300 rpm/1 h). After incubation for 24 h, the cells were washed and maintained in AML12 medium containing NJK14047 for 7 days. Extracellular viral DNA and HBsAg levels were determined by quantitative PCR and ELISA as described above.
Supplementary Material
ACKNOWLEDGMENTS
The hNTCP-HepG2-C4 cell line was kindly provided by Koichi Watashi (National Institute of Infectious Diseases, Japan). The pHBV-1.2x plasmid was kindly provided by G. Jung (Seoul National University). AML-12 cells were kindly provided by S.-G. Kim (Seoul National University).
This research was supported by Basic Science Research Program grants through the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT and Future Planning (NRF-2014R1A1A2058039 and NRF-2016R1A2B4015169).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00214-17.
REFERENCES
- 1.Chen DS. 1993. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science 262:369–370. doi: 10.1126/science.8211155. [DOI] [PubMed] [Google Scholar]
- 2.Kao JH, Chen PJ, Lai MY, Chen DS. 2002. Genotypes and clinical phenotypes of hepatitis B virus in patients with chronic hepatitis B virus infection. J Clin Microbiol 40:1207–1209. doi: 10.1128/JCM.40.4.1207-1209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. 2012. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 13 November 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. doi: 10.7554/eLife.00049. [DOI] [PubMed] [Google Scholar]
- 5.Zoulim F, Locarnini S. 2009. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 137:1593–1608. doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 6.Hongthanakorn C, Chotiyaputta W, Oberhelman K, Fontana RJ, Marrero JA, Licari T, Lok AS. 2011. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. Hepatology 53:1854–1863. doi: 10.1002/hep.24318. [DOI] [PubMed] [Google Scholar]
- 7.Clark DN, Hu J. 2015. Hepatitis B virus reverse transcriptase: target of current antiviral therapy and future drug development. Antiviral Res 123:132–137. doi: 10.1016/j.antiviral.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revill P, Testoni B, Locarnini S, Zoulim F. 2016. Global strategies are required to cure and eliminate HBV infection. Nat Rev Gastroenterol Hepatol 13:239–248. doi: 10.1038/nrgastro.2016.7. [DOI] [PubMed] [Google Scholar]
- 9.Gish R, Jia JD, Locarnini S, Zoulim F. 2012. Selection of chronic hepatitis B therapy with high barrier to resistance. Lancet Infect Dis 12:341–353. doi: 10.1016/S1473-3099(11)70314-0. [DOI] [PubMed] [Google Scholar]
- 10.Davis RJ. 1993. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 268:14553–14556. [PubMed] [Google Scholar]
- 11.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Boehm J, Lee JC. 2003. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 13.Khorasanizadeh M, Eskian M, Gelfand EW, Rezaei N. 2017. Mitogen-activated protein kinases as therapeutic targets for asthma. Pharmacol Ther 174:112–126. doi: 10.1016/j.pharmthera.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Choi MS, Heo J, Yi CM, Ban J, Lee NJ, Lee NR, Kim SW, Kim NJ, Inn KS. 2016. A novel p38 mitogen activated protein kinase (MAPK) specific inhibitor suppresses respiratory syncytial virus and influenza A virus replication by inhibiting virus-induced p38 MAPK activation. Biochem Biophys Res Commun 477:311–316. doi: 10.1016/j.bbrc.2016.06.111. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Cohen JI. 2015. Epstein-Barr Virus (EBV) tegument protein BGLF2 promotes EBV reactivation through activation of the p38 mitogen-activated protein kinase. J Virol 90:1129–1138. doi: 10.1128/JVI.01410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JC, Martinez O, Honko AN, Hensley LE, Olinger GG, Basler CF. 2014. Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells. Antiviral Res 107:102–109. doi: 10.1016/j.antiviral.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchant D, Singhera GK, Utokaparch S, Hackett TL, Boyd JH, Luo Z, Si X, Dorscheid DR, McManus BM, Hegele RG. 2010. Toll-like receptor 4-mediated activation of p38 mitogen-activated protein kinase is a determinant of respiratory virus entry and tropism. J Virol 84:11359–11373. doi: 10.1128/JVI.00804-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang WW, Su IJ, Chang WT, Huang W, Lei HY. 2008. Suppression of p38 mitogen-activated protein kinase inhibits hepatitis B virus replication in human hepatoma cell: the antiviral role of nitric oxide. J Viral Hepat 15:490–497. doi: 10.1111/j.1365-2893.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 19.Angell RM, Bamborough P, Cleasby A, Cockerill SG, Jones KL, Mooney CJ, Somers DO, Walker AL. 2008. Biphenyl amide p38 kinase inhibitors 1: discovery and binding mode. Bioorg Med Chem Lett 18:318–323. doi: 10.1016/j.bmcl.2007.10.076. [DOI] [PubMed] [Google Scholar]
- 20.Heo J, Shin H, Lee J, Kim T, Inn KS, Kim NJ. 2015. Synthesis and biological evaluation of N-cyclopropylbenzamide-benzophenone hybrids as novel and selective p38 mitogen activated protein kinase (MAPK) inhibitors. Bioorg Med Chem Lett 25:3694–3698. doi: 10.1016/j.bmcl.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Gong JR, Lee SA, Kim BJ. 2015. Discovery of a novel mutation (X8Del) resulting in an 8-bp deletion in the hepatitis B virus X gene associated with occult infection in Korean vaccinated individuals. PLoS One 10:e0139551. doi: 10.1371/journal.pone.0139551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Lee SA, Won YS, Lee H, Kim BJ. 2015. Occult infection related hepatitis B surface antigen variants showing lowered secretion capacity. World J Gastroenterol 21:1794–1803. doi: 10.3748/wjg.v21.i6.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khattab MA. 2009. Targeting host factors: a novel rationale for the management of hepatitis C virus. World J Gastroenterol 15:3472–3479. doi: 10.3748/wjg.15.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arhel N, Kirchhoff F. 2010. Host proteins involved in HIV infection: new therapeutic targets. Biochim Biophys Acta 1802:313–321. doi: 10.1016/j.bbadis.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Gane EJ. 2017. Future anti-HBV strategies. Liver Int 37(Suppl 1):S40–S44. doi: 10.1111/liv.13304. [DOI] [PubMed] [Google Scholar]
- 26.Shin HB, Choi MS, Yi CM, Lee J, Kim NJ, Inn KS. 2015. Inhibition of respiratory syncytial virus replication and virus-induced p38 kinase activity by berberine. Int Immunopharmacol 27:65–68. doi: 10.1016/j.intimp.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 27.Wei Y, Tavis JE, Ganem D. 1996. Relationship between viral DNA synthesis and virion envelopment in hepatitis B viruses. J Virol 70:6455–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verrier ER, Colpitts CC, Bach C, Heydmann L, Weiss A, Renaud M, Durand SC, Habersetzer F, Durantel D, Abou-Jaoude G, Lopez Ledesma MM, Felmlee DJ, Soumillon M, Croonenborghs T, Pochet N, Nassal M, Schuster C, Brino L, Sureau C, Zeisel MB, Baumert TF. 2016. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology 63:35–48. doi: 10.1002/hep.28013. [DOI] [PubMed] [Google Scholar]
- 29.Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. 2012. IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest 122:529–537. doi: 10.1172/JCI58847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.