ABSTRACT
Colonizations due to carbapenem-resistant Enterobacteriaceae (CRE) are a source of antimicrobial resistance transmission in health care settings. Eleven Citrobacter freundii strains producing KPC-3 carbapenemase were isolated from rectal swabs during a 3-year surveillance program. blaKPC-3-carrying plasmids were found to belong to the IncX3 group in 9 of the 11 strains, and complete nucleotide sequences were obtained for 2 of them. Our results highlight the possible role of C. freundii as reservoir of resistance genes.
KEYWORDS: KPC, IncX3, Citrobacter freundii, KPC-3, antibiotic resistance
TEXT
Carbapenem-resistant Enterobacteriaceae (CRE) species are a common cause of health care-associated infection, causing high morbidity and mortality rates. The most prevalent CRE species associated with nosocomial infection in Italy was shown to be Klebsiella pneumoniae, and KPC was found to be the most common carbapenemase (1). The extraordinary spread of carbapenem resistance observed worldwide (2) can be explained by the presence of blaKPC genes on mobile genetic elements that are horizontally transferred (3), not only among K. pneumoniae bacteria but also to other Enterobacteriaceae, including Citrobacter freundii (4, 5). In this study, we describe the emergence of carbapenem-resistant (CR) Citrobacter freundii during an active surveillance program designed to detect all CRE from rectal swabs of patients from the clinical and surgical wards of the L. Spallanzani National Institute for Infectious Diseases (INMI) in Rome, Italy.
Rectal swabs were taken from all patients upon admission to the hospital and then once weekly for the entire duration of their stay. Carbapenem-resistant strains, including 11 CR-C. freundii, were isolated using selective culture plates (chromID CARBA; bioMérieux, Marcy l'Etoile, France). Identification was performed by the use of a Vitek 2 system (bioMérieux, Marcy l'Etoile, France) and mass spectrometry (MS) (matrix-assisted laser desorption ionization–time of flight MS [MALDI-TOF MS]; Brucker Daltonics, Germany), and C. freundii isolates were confirmed using the specific recN biomarker (6, 7) (data not shown). Antimicrobial susceptibility was determined by reference broth microdilution (8), and MICs were interpreted according to the European Committee on Antimicrobial Susceptibility Testing recommendations (EUCAST breakpoint tables v6.0). Since the beginning of our surveillance program in 2013, the species of CRE most commonly isolated from rectal swabs was K. pneumoniae, as expected (data not shown). The first CR-C. freundii strain to be isolated was collected in March 2014, together with an Escherichia coli strain which was also carbapenem resistant and which was grown from the same swab from the same patient, who had been transferred from another hospital to our surgical unit. This E. coli strain was also included in this study and characterized. In the following months, our surveillance continued, with no CR-C. freundii strains detected until August 2015; between August 2015 and January 2017, a total of 10 nonduplicated CR-C. freundii strains were isolated in the surgery and postsurgery wards and in the intensive care unit (ICU) (Table 1).
TABLE 1.
Molecular typing and antibiotic susceptibility profiles of strains characterized in this study
| Code | Date of first screeninga | Date of isolation of CRE | Wardb | Species | MLST | β-Lactamase genes | Replicon(s)c | Antibiotic MIC value (mg/liter)d |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | AMC | FEP | CTX | CAZ | CIP | CST | ERT | FOF | GEN | IPM | MEM | TZP | TGC | SXT | ||||||||
| 30 | 15 March 2014 | 15 March 2014 | S | C. freundii | ST91 | KPC-3, SHV-11 | X3A, FIB | ≤2 | ≥32 | ≥64 | ≥64 | ≥64 | ≥4 | ≤0.5 | ≥8 | ≥256 | ≥16 | ≥16 | ≥16 | ≥128 | ≤0.5 | ≥320 |
| 31 | 15 March 2014 | 15 March 2014 | S | E. coli | ST5 | KPC-3, SHV-11 | X3A, FIB, colE | 2 | ≥32 | 2 | ≥64 | ≥64 | ≤0.25 | ≤0.5 | 2 | ≥16 | ≤1 | 8 | ≥16 | ≥128 | ≤0.5 | ≤20 |
| 365 | 26 July 2015 | 03 August 2015 | S | C. freundii | ST91 | KPC-3, SHV-11 | X3A | ≤2 | ≥32 | I–2 | 8 | ≥64 | ≥4 | 1 | 4 | ≥256 | ≥16 | ≥16 | ≥16 | ≥128 | ≤0.5 | ≥320 |
| 138 | 02 November 2015 | 11 November 2015 | PS | C. freundii | ST96 | KPC-3, VIM-2, SHV-11, TEM-1, CTX-M-9 | X3A, HI2 | 16 | ≥32 | ≥64 | ≥64 | ≥64 | ≥4 | ≤0.5 | ≥8 | ≤16 | ≥16 | ≥16 | ≥16 | ≥128 | 1 | ≥320 |
| 145 | 06 January 2016 | 13 January 2016 | PS | C. freundii | ST119 | KPC-3, SHV-11, TEM-1 | X3B | ≤2 | ≥32 | ≥64 | ≥64 | ≥64 | ≥4 | ≤0.5 | ≥8 | ≤16 | 8 | ≥16 | ≥16 | ≥128 | 1 | ≥320 |
| 19 | 21 March 2016 | 28 March 2016 | S | C. freundii | ST91 | KPC-3, SHV-11 | X3A | ≤2 | ≥32 | 8 | 8 | ≥64 | ≥4 | ≤0.5 | ≥8 | ≥256 | ≥16 | ≥16 | ≥16 | ≥128 | ≤0.5 | ≥320 |
| 167 | 21 March 2016 | 23 April 2016 | PS | C. freundii | ST118 | KPC-3, TEM-1 | HI1, N | ≤2 | ≥32 | 16 | ≥64 | 32 | ≥4 | ≤0.5 | ≥8 | ≤16 | ≥16 | ≥16 | ≥16 | ≥128 | 1.5 | ≥320 |
| 134 | 06 May 2016 | 13 May 2016 | ICU | C. freundii | ST22 | KPC-3, SHV-11 | X3 | ≤2 | ≥32 | 16 | ≥64 | 32 | ≥4 | ≤0.5 | ≥8 | ≤16 | ≤1 | ≥16 | ≥16 | ≥128 | 1.5 | ≥320 |
| 124 | 22 June 2016 | 28 June 2016 | PS | C. freundii | ST96 | KPC-3, VIM-2, SHV-11, TEM-1, CTX-M-9 | X3A, HI2, N | 16 | ≥32 | ≥64 | ≥64 | ≥64 | ≥4 | ≤0.5 | ≥8 | ≤16 | ≥16 | ≥16 | ≥16 | ≥128 | ≤0.5 | ≥320 |
| 80 | 30 June 2016 | 06 July 2016 | PS | C. freundii | ST118 | KPC-3, TEM-1 | HI1 | ≤2 | ≥32 | ≥64 | ≥64 | ≥64 | ≥4 | ≤0.5 | ≥8 | ≤16 | 8 | ≥16 | ≥16 | ≥128 | 1.5 | ≥320 |
| 87 | 29 July 2016 | 06 September 2016 | S | C. freundii | ST91 | KPC-3, SHV-11 | X3A | ≤2 | ≥32 | 8 | 8 | ≥64 | ≥4 | ≤0.5 | ≥8 | ≥256 | ≥16 | ≥16 | ≥16 | ≥128 | ≤0.5 | ≥320 |
| 162 | 27 December 2016 | 11 January 2017 | PS | C. freundii | ST22 | KPC-3, SHV-11, TEM-1 | X3A, A/C, FIIS | ≤2 | ≥32 | 16 | ≥64 | 32 | ≥4 | ≤0.5 | ≥8 | ≤16 | ≤1 | ≥16 | ≥16 | ≥128 | 1.5 | ≥320 |
Date of rectal swab performed upon admission to the ward.
S, surgery; PS, postsurgery; ICU, intensive care unit.
blaKPC-3 genes carrying replicon plasmids are underlined. X3A, IncX3 plasmid presenting the insertion carrying blaKPC-3 in the umuD gene; X3B, IncX3 plasmid presenting the insertion carrying blaKPC-3 in the tnpA Tn3.
AMK, amikacin; AMC, amoxicillin-clavulanic acid; FEP, cefepime; CTX, cefotaxime; CAZ, ceftazidime; CIP, ciprofloxacin; CST, colistin; ERT, ertapenem; FOF, fosfomycin, GEN; gentamicin; IPM, imipenem; MEM, meropenem; TZP, piperacillin-tazobactam; TGC, tigecyclin, SXT, trimethoprim-sulfamethoxazole.
All strains were analyzed by multilocus sequence typing (MLST) as described previously (9, 10), and the resulting sequence types (ST) were designated using the Pasteur database (http://pubmlst.org/cfreundii) and the scheme of the University of Warwick, United Kingdom (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli) for C. freundii isolates and E. coli isolates, respectively. Bacterial genotyping revealed that the CR-C. freundii strains belonged to 5 different STs: ST91 (4/11), ST96 (2/11), ST22 (2/11), ST118 (2/11), and ST119 (1/11) (Table 1). None of the observed STs have been previously reported in association with a specific resistance mechanism. The E. coli strain that was coisolated with C. freundii belonged to ST5, which has been found in association with OXA, NDM, and IMP carbapenemases (11–13); to the best of our knowledge, this is the first report of a KPC-3-producing ST5 E. coli strain.
Beta-lactamase genes (blaKPC, blaNDM, blaOXA-48, blaVIM, blaSHV, blaTEM, and blaCTX-M-GROUP) were screened by PCR followed by sequencing for the identification of variants (14, 15).
Our results showed the presence of the blaKPC-3 gene in all CR-C. freundii isolates; the two ST96 strains also carried blaVIM-2 and extended-spectrum-beta-lactamase (ESBL) blaCTX-M-9 genes. The blaSHV-11 gene was identified in 9/11 C. freundii strains, while blaTEM-1 was detected in 6/11 strains. The E. coli strain also carried blaKPC-3 and blaSHV-11 genes (Table 1).
Plasmid replicons were typed using a PCR-based replicon typing (PBRT) kit (Diatheva) (16): all isolates, with the exception of two ST118 isolates, carried IncX3 replicons (Table 1). To verify whether blaKPC-3 was located on IncX3, the blaKPC-3-carrying plasmid DNAs from these strains were purified by the use of a PureYield Plasmid Midiprep kit (Promega, USA) and transformed into competent E. coli DH5α cells (Invitrogen, USA). Transformants were selected on Luria-Bertani agar plates containing ampicillin (Sigma; 50 μg/ml) and were screened for the presence of the blaKPC-3 gene. The PBRT kit was used to assign the replicons on the transferred plasmids. Our KPC-3 transformation experiments conducted on the blaKPC-3-positive transformants obtained from all IncX3-carrying CR-C. freundii strains showed that the blaKPC-3 gene was located on IncX3 and that the blaVIM-2 gene was located on a different plasmid only in the two isolates belonging to ST96.
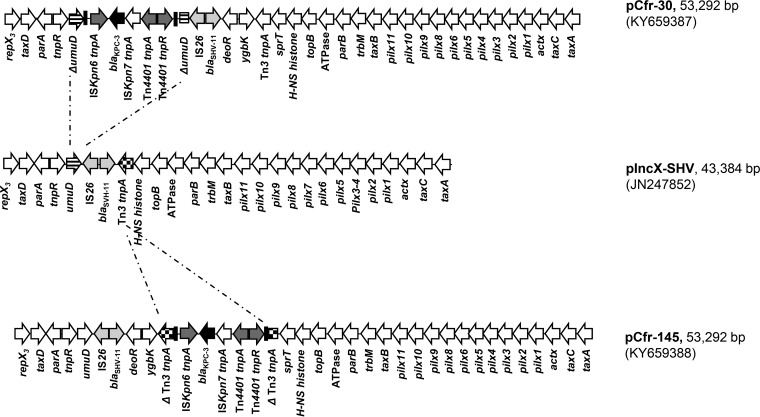
Complete plasmid sequences were obtained for the C. freundii Cfr-30 and Cfr-145 prototypic transformants using an Illumina MiSeq next-generation sequencer with 2x300PE (Illumina Inc., CA, USA) according to the manufacturer's instructions. De novo assembly was performed using Galaxy version 20150522 of the A5 pipeline through the ARIES public Galaxy server (https://w3.iss.it/site/aries/) (17), and open reading frames (ORFs) were annotated using the Sequin server (http://www.ncbi.nlm.nih.gov/Sequin/). GenBank files of the pCfr-30 and pCfr-145 plasmid sequences were deposited at the NCBI GenBank database (see below). As shown in Fig. 1, the pCfr-30 and pCfr-145 plasmids (both 53,292 bp in size) showed 100% nucleotide identity and a typical IncX3 scaffold compared with the DNA sequence of the pIncX-SHV plasmid (JN247852) (18), with which they showed 99% nucleotide identity. The insertion of the Tn4401a transposon (3) carrying blaKPC-3 occurred in the umuD gene for the pCfr-30 plasmid and in the tnpA transposase gene of the Tn3 transposon for pCfr-145 (Fig. 1). The same interruption of the umuD gene found in pCfr-30 had been previously identified in pKPC-Ny79 (JX104759) (19). However, the pKPC-Ny79 plasmid carried a blaKPC-2 gene variant and showed a deletion involving the ATPase and hns-topB genes. Interestingly, both plasmids showed 100% identity with a blaKPC-3-carrying IncX3 plasmid (KU934011) of a Serratia marcescens strain recently isolated in Italy from a kidney-liver-transplanted patient, in which the Tn4401a transposon was also inserted in the tnpA of Tn3-like pCfr-145, although in the opposite orientation. In both plasmids, the blaSHV-11 gene was found to be associated with an IS26 element. These findings are further evidence of the circulation of these plasmids in hospital settings and in Enterobacteriaceae other than K. pneumoniae. Specific PCR assays were devised to detect the integration regions of Tn4401a, discerning the umuD or tnpA integration sites, respectively (KPC-Fw [5′-GCTACACCTAGCTCCACCTTC-3′] [14]; umuD Rv [5′-TTTTCCATCGCCAGCAACC-3′]; and tnpA Rv [5′-GTCGAGTGCCCGGTATTTAG-3′]). The KPC-3-IncX3 plasmids were then classified on the basis of the integration sites as IncX3-type A and IncX3-type B, respectively (Table 1). PCR results showed that all the ST91 and ST96 strains and one of the two ST22 C. freundii strains isolated in 2017, as well as the E. coli strain, contained an IncX3-type A plasmid with the integration of blaKPC-3 in the umuD gene (Table 1). These results were confirmed by PstI restriction fragment length polymorphism (RFLP) analysis performed on plasmids from transformants Cfr-30, Cfr-138, and Cfr-145 obtained from C. freundii and E. coli 31 (Ec-31) from E. coli (data not shown). Plasmid DNAs of transformants Cfr-30, Ec-31, and Cfr-138 showed identical profiles (IncX3-type A), differing in only one band from the profile of Cfr-145 (IncX3-type B); this additional band was due to the integration of the blaKPC-3 gene in the Tn3 transposase. It is noteworthy that all of the IncX3-carrying CR-C. freundii strains described above were from the surgical and postsurgical wards, except for one of the two ST22 strains, which was isolated in 2016 from the ICU (Table 1). This strain was also the only one that was negative in both IncX3-integration-site PCRs, suggesting a possible Tn4401a insertion in other regions of the plasmid scaffold.
FIG 1.
Linear maps of plasmids pCfr-30, pCfr-145, and pIncX-SHV. White arrows represent predicted open reading frames (ORFs); the functions of the plasmids are indicated below the arrows in the linear maps. The ORFs of pCfr-30 and pCfr-145 were identified in this study; ORFs of pIncX-SHV were deduced from the data available under GenBank accession no. JN247852. Transposase genes of the Tn4401a transposons are indicated by gray arrows; the blaKPC-3 genes inside Tn4401a are indicated by black arrows. The umuD and Tn3 tnpA genes targeted by the Tn4401 transposition events are indicated by striped and dotted arrows, respectively.
Taken together, the data presented in our report confirm the finding of an IncX3-type A plasmid in different C. freundii clones circulating in surgical wards and in an E. coli strain coisolated from the index patient. The fact that this patient had been colonized prior to admission to the surgical ward of our hospital in 2014, while all our other subjects had negative swabs upon arrival, supports the hypothesis that an IncX3–KPC-3 plasmid was maintained within the ward.
Further, the presence of the same IncX3 plasmid in the E. coli and C. freundii strains in the index patient suggests that the blaKPC-3-carrying IncX3 plasmid was probably transferred in vivo between the two species in the gut of the patient. We cannot exclude the possibility that E. coli may have been the original donor; however, IncX3 seems highly prevalent in C. freundii, having been previously identified not only in association with the blaKPC-3 gene but also in association with blaNDM (20, 21).
In conclusion, the observation of several cases of CR-C. freundii isolates carrying IncX3–KPC-3 plasmids suggests their ability to persist in hospital wards; further, the fact that the same ST22 clone circulating in different wards acquired two different KPC-3–IncX3 plasmids in the course of several months (Table 1) suggests that these plasmids can be effectively transferred and maintained within Enterobacteriaceae strains of different origins and sources.
All our CR-C. freundii isolates were colonizers since all C. freundii strains obtained from clinically relevant sites of patients treated in the surgical wards were also analyzed by MLST, revealing a heterogeneity of STs and, more importantly, the absence of the IncX3 plasmid and resistance determinants (data not shown). No other CRE were isolated from these patients, and no CR-C. freundii strains were isolated in the entire hospital, except for those described in this study; our results therefore reinforce the importance of maintaining an active surveillance of all CR CRE, together with effective infection control practices.
Accession number(s).
Sequence data have been submitted to GenBank under the following accession numbers: for pCfr-30, KY659387; for pCfr-145, KY659388.
REFERENCES
- 1.Giani T, Pini B, Arena F, Conte V, Bracco S, Migliavacca R; AMCLI-CRE Survey Participants, Pantosti A, Pagani L, Luzzaro F, Rossolini GM. 2013. Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: results of the first countrywide survey, 15 May to 30 June 2011. Euro Surveill 30:18(22) http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20489. [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. 2008. Genetic structures at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob Agents Chemother 52:1257–1263. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Yang L, Cai JC, Zhou HW, Chen GX. 2008. High-level carbapenem resistance in a Citrobacter freundii clinical isolate is due to a combination of KPC-2 production and decreased porin expression. J Med Microbiol 57(Pt 3):332–337. doi: 10.1099/jmm.0.47576-0. [DOI] [PubMed] [Google Scholar]
- 5.Adler A, Khabra E, Paikin S, Carmeli Y. 2016. Dissemination of the blaKPC gene by clonal spread and horizontal gene transfer: comparative study of incidence and molecular mechanisms. J Antimicrob Chemother 71:2143–2146. doi: 10.1093/jac/dkw106. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro TG, Novais Â, Branquinho R, Machado E, Peixe L. 2015. Phylogeny and comparative genomics unveil independent diversification trajectories of qnrB and genetic platforms within particular Citrobacter species. Antimicrob Agents Chemother 59:5951–5958. doi: 10.1128/AAC.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro TG, Clermont D, Branquinho R, Machado E, Peixe L, Brisse S. 2017. Citrobacter europaeus sp. nov., isolated from water and human faecal samples. Int J Syst Evol Microbiol 67:170–173. doi: 10.1099/ijsem.0.001606. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI). 2015. M07-A10. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard, 10th ed CLSI, Wayne, PA. [Google Scholar]
- 9.Bai L, Xia S, Lan R, Liu L, Ye C, Wang Y, Jin D, Cui Z, Jing H, Xiong Y, Bai X, Sun H, Zhang J, Wang L, Xu J. 2012. Isolation and characterization of cytotoxic, aggregative Citrobacter freundii. PLoS One 7:e33054. doi: 10.1371/journal.pone.0033054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunha K, Chanawong A, Lulitanond A, Wilailuckana C, Charoensri N, Wonglakorn L, Saenjamla P, Chaimanee P, Angkititrakul S, Chetchotisakd P. 2016. High-level carbapenem-resistant OXA-48-producing Klebsiella pneumoniae with a novel OmpK36 variant and low-level, carbapenem-resistant, non-porin-deficient, OXA-181-producing Escherichia coli from Thailand. Diagn Microbiol Infect Dis 85:221–226. doi: 10.1016/j.diagmicrobio.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Dortet L, Poirel L, Anguel N, Nordmann P. 2012. New Delhi metallo-β-lactamase 4-producing Escherichia coli in Cameroon. Emerg Infect Dis 18:1540–1542. doi: 10.3201/eid1809.120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peirano G, Lascols C, Hackel M, Hoban DJ, Pitout JD. 2014. Molecular epidemiology of Enterobacteriaceae that produce VIMs and IMPs from the SMART surveillance program. Diagn Microbiol Infect Dis 78:277–281. doi: 10.1016/j.diagmicrobio.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70(Suppl 1):S119–S123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Mabilat C, Goussard S, Sougakoff W, Spencer RC, Courvalin P. 1990. Direct sequencing of the amplified structural gene and promoter for the extended-broad-spectrum beta-lactamase TEM-9 (RHH-1) of Klebsiella pneumoniae. Plasmid 23(Suppl 1):S27–S34. doi: 10.1016/0147-619X(90)90041-A. [DOI] [PubMed] [Google Scholar]
- 16.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63(Suppl 3):S219–S228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Tritt A, Eisen JA, Facciotti MT, Darling AE. 2012. An integrated pipeline for de novo assembly of microbial genomes. PLoS One 7:e42304. doi: 10.1371/journal.pone.0042304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Fernández A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, Mancini C, Carattoli A. 2012. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob Agents Chemother 56:2143–2145. doi: 10.1128/AAC.05308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho PL, Cheung YY, Lo WU, Li Z, Chow KH, Lin CH, Chan JF, Cheng VC. 2013. Molecular characterization of an atypical IncX3 plasmid pKPC-NY79 carrying blaKPC-2 in a Klebsiella pneumoniae. Curr Microbiol 67:493–498. doi: 10.1007/s00284-013-0398-2. [DOI] [PubMed] [Google Scholar]
- 20.Ho PL, Li Z, Lo WU, Cheung YY, Lin CH, Sham PC, Cheng VC, Ng TK, Que TL, Chow KH. 2012. Identification and characterization of a novel incompatibility group X3 plasmid carrying blaNDM-1 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. Emerg Microbes Infect 1:e39. doi: 10.1038/emi.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng J, Qiu Y, Yin Z, Chen W, Yang H, Yang W, Wang J, Gao Y, Zhou D. 2015. Coexistence of a novel KPC-2-encoding MDR plasmid and an NDM-1-encoding pNDM-HN380-like plasmid in a clinical isolate of Citrobacter freundii. J Antimicrob Chemother 70:2987–2991. doi: 10.1093/jac/dkv232. [DOI] [PubMed] [Google Scholar]