ABSTRACT
It has been known from previous studies that body fluids, such as cerebrospinal fluid, lung surfactant, and urine, have a strong impact on the bacterial killing of many anti-infective agents. However, the influence of human bile on the antimicrobial activity of antibiotics is widely unknown. Human bile was obtained and pooled from 11 patients undergoing cholecystectomy. After sterilization of the bile fluid by gamma irradiation, its effect on bacterial killing was investigated for linezolid (LZD) and tigecycline (TGC) against Enterococcus faecalis ATCC 29212. Further, ciprofloxacin (CIP), meropenem (MEM), and TGC were tested against Escherichia coli ATCC 25922. Time-kill curves were performed in pooled human bile and Mueller-Hinton broth (MHB) over 24 h. Bacterial counts (in CFU per milliliter after 24 h) of bile growth controls were approximately equal to MHB growth controls for E. coli and approximately 2-fold greater for E. faecalis, indicating a promotion of bacterial growth by bile for the latter strain. Bile reduced the antimicrobial activity of CIP, MEM, and TGC against E. coli as well as the activity of LZD and TGC against E. faecalis. This effect was strongest for TGC against the two strains. Degradation of TGC in bile was identified as the most likely explanation. These findings may have important implications for the treatment of bacterial infections of the gallbladder and biliary tract and should be explored in more detail.
KEYWORDS: biliary tract infections, human bile, antibiotics, time-kill curves
INTRODUCTION
Infections of the biliary tract, such as cholecystitis and cholangitis, may lead to life-threatening situations, including community-acquired bacteremia causing sepsis and shock with high mortality especially in elderly patients (>70 years) (1, 2).
A number of previous studies have shown a bacterial colonization in gallbladder bile from patients, most frequently with Escherichia coli and Enterococcus spp. (1, 3–6). Antibiotics, such as fluoroquinolones (e.g., ciprofloxacin [CIP]), carbapenems (e.g., meropenem [MEM]), and tigecycline (TGC), have been considered for treatment and are highly effective against E. coli (3, 7–9). Furthermore, TGC as well as linezolid (LZD), a protein synthesis inhibitor, is effective against other Gram-positive bacteria, namely, Enterococcus faecalis and Enterococcus faecium (9, 10). Even though the actual benefit of antimicrobials for the treatment of biliary tract infections has been heavily debated, the employed antibiotic substances have found widespread use due to their spectrum of activity and their ability to penetrate the gallbladder wall, thereby reaching the bile (3, 7, 8, 11).
It has been discussed that the target tissue itself may have a strong impact on antimicrobial activity and influence satisfactory bacterial killing (12). Studies have shown that various biological fluids, such as urine, cerebrospinal fluid (CSF), plasma, and lung surfactant, may hamper the activity of antibiotics due to chemical or enzymatic processes (13–15). Sauermann et al. were able to demonstrate decreased bactericidal activity of fosfomycin in CSF compared to regular culture medium, such as Mueller-Hinton broth (MHB) (13). Other studies disclosed a negative effect of lung surfactant on the activity of various antibiotics, such as tobramycin or daptomycin (14, 16).
So far, only few studies have been investigating the effect of bile fluid on antibiotics, indicating that bile fluid may either negatively or positively affect antibiotic activity, depending on the antibiotic agents and the bacterial strains used (17–21).
To investigate the effect of human bile on bacterial killing in more detail, we set out to test the activity of selected antibiotics, such as CIP and MEM (E. coli), TGC (E. coli and E. faecalis), and LZD (E. faecalis), over 24 h.
RESULTS
For the following descriptive in vitro study, 29 subjects were screened. Out of the 25 individuals undergoing cholecystectomy due to cholecystolithiasis and chronic cholecystitis, bile fluid collection was successful in 19 subjects. Ultimately, a total of approximately 120 ml human bile was pooled from 11 patients, including 6 female and 5 male, at an average age of 47.1 years (Table 1).
TABLE 1.
Demographic data
| Characteristic | Value |
|---|---|
| No. of screened subjects | 29 |
| No. of screening failures | 4 |
| No. of subjects with successful bile obtainment | 19 |
| Female | 12 |
| Male | 7 |
| Histological diagnosis | |
| No. cholecystolithiasis with chronic cholecystitis | 17 |
| No. gallbladder polyp with cholecystitis | 2 |
| Average age (yr) (min; max) | 45.9 (31; 71) |
| Total bile vol (ml) | 194 |
| Average bile vol (ml) (min; max) | 10.2 (0.4; 25) |
| No. bile samples selected for experiments | 11 |
| Female | 6 |
| Male | 5 |
| Histological diagnosis of selected samples | |
| No. cholecystolithiasis with chronic cholecystitis | 10 |
| No. gallbladder polyp with cholecystitis | 1 |
| Average age (yr) (min; max) | 47.1 (31; 61) |
| Total bile vol (ml) | 120.5 |
| Average bile vol (ml) (min; max) | 11.0 (2.5; 25) |
Growth of E. faecalis and E. coli in bile.
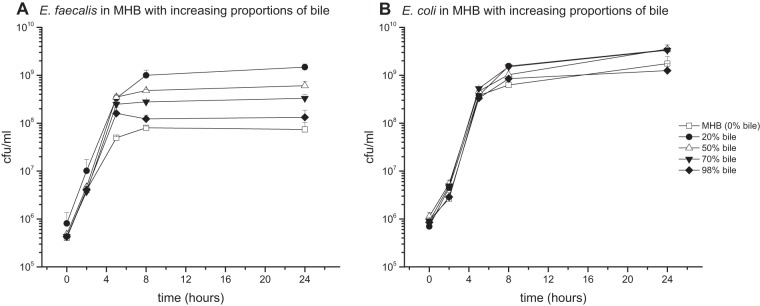
When bacterial cultures were grown in pure MHB growth medium, pure bile, or in mixed media containing MHB with various proportions of bile, for media containing both bile and MHB, a strong growth enhancement was detected for both strains within the first 8 h of culture and remained stable for up to 24 h (Fig. 1A and B). For example, in medium containing only 20% bile fluid, E. faecalis achieved bacterial counts more than 1 log step higher than pure MHB or bile; additionally, for 96% bile, a growth enhancement from 7.89 log10 CFU/ml in MHB to 8.19 log10 CFU/ml was observed after 24 h (Fig. 1A and B). The effect on growth of E. coli cultures was similar but less pronounced.
FIG 1.
Mean (± standard deviation) time-growth curves of E. faecalis (A) and E. coli (B) in pure Mueller-Hinton broth (98% MHB, 2% NaCl), pure bile (98% bile, 2% NaCl), and different alloys of both.
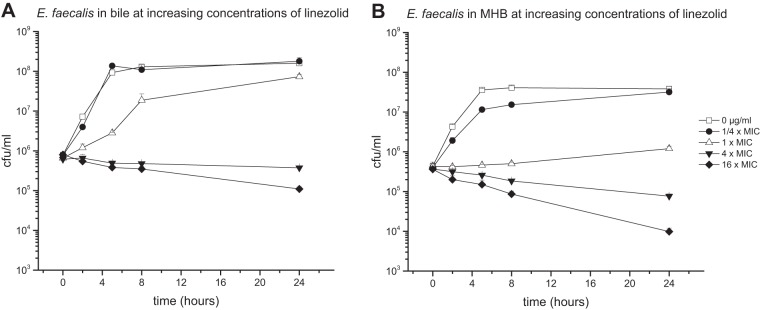
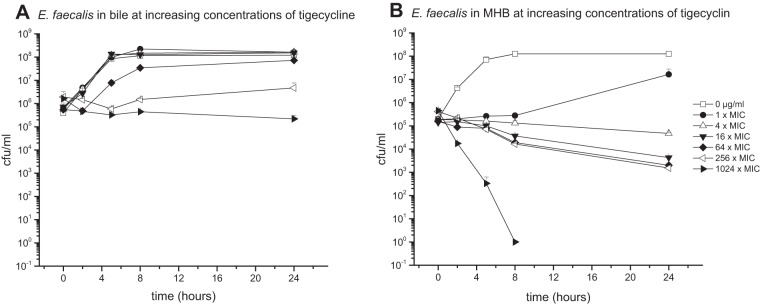
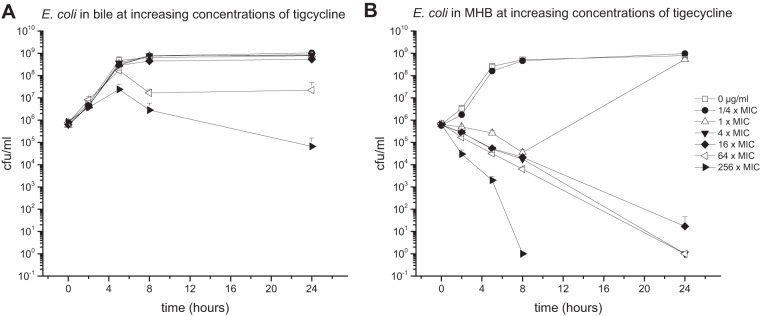
Antibiotic activity against E. faecalis in bile.
The E. faecalis bacterial strain cultured in pure bile or MHB medium was introduced to various concentrations of LZD (1-fold MIC, 2 μg/ml) (Fig. 2) or TGC (1-fold MIC, 0.0625 μg/ml) (Fig. 3).
FIG 2.
Mean (± standard deviation) time-kill curves of linezolid against E. faecalis in pure bile (96% bile fluid, 4% NaCl) (A) and MHB (96% MHB, 4% NaCl) (B).
FIG 3.
Mean (± standard deviation) time-kill curves of tigecycline against E. faecalis in pure bile (96% bile fluid, 4% NaCl) (A) and MHB (96% MHB, 4% NaCl) (B).
Results expressed as time-kill curves showed bile to reduce the effect of LZD against E. faecalis, especially at antibiotic concentrations at and below the MIC.
Furthermore, the bactericidal effect in bile was decreased in particular when TGC was introduced to the culture. While the reduction of CFU per milliliter of E. faecalis in MHB medium was identified already at antibiotic concentrations as low as 0.0625 μg/ml (1-fold MIC), none of the investigated concentrations of TGC up to levels corresponding to 1,024-fold MIC could achieve bacterial killing.
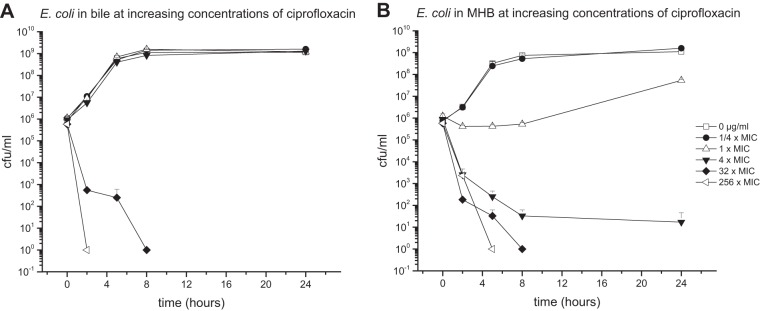
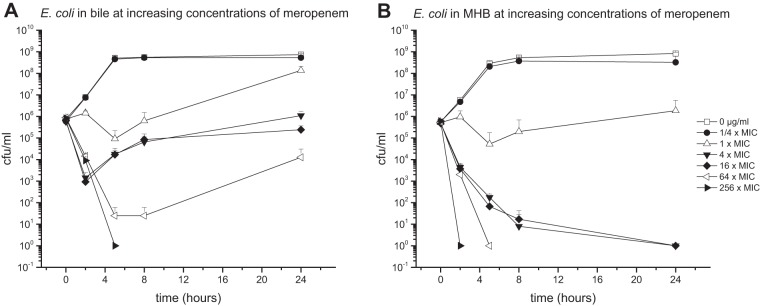
Antibiotic activity against E. coli in bile.
E. coli bacteria cultured in pure bile or MHB media were introduced to different concentrations of CIP (1-fold MIC, 0.004 μg/ml) (Fig. 4), MEM (1-fold MIC, 0.03125 μg/ml) (Fig. 5), or TGC (1-fold MIC, 0.125 μg/ml) (Fig. 6).
FIG 4.
Mean (± standard deviation) time-kill curves of ciprofloxacin against E. coli in pure bile (96% bile fluid, 4% NaCl) (A) and MHB (96% MHB, 4% NaCl) (B).
FIG 5.
Mean (± standard deviation) time-kill curves of meropenem against E. coli in pure bile (96% bile fluid, 4% NaCl) (A) and MHB (96% MHB, 4% NaCl) (B).
FIG 6.
Mean (± standard deviation) time-kill curves of tigecycline against E. coli in pure bile (96% bile fluid, 4% NaCl) (A) and MHB (96% MHB, 4% NaCl) (B).
Compared to MHB medium, pure bile was found to reduce the killing potential of all tested antibiotics. However, the most prominent differences between the two culture conditions were detected at antibiotic concentrations corresponding to 4-fold MIC, for which differences between 6.04 log steps for MEM, 7.89 log steps for CIP, and 8.91 log steps higher for TGC were found.
Similar to E. faecalis, the most prominent impact of bile on antimicrobial activity was detected for TGC. Antibiotic concentrations as high as 32 μg/ml (256-fold MIC) did not show strong bactericidal effects in bile cultures throughout the experimental time course. In contrast, TGC enfolds bactericidal properties in MHB cultures already at 4-fold MIC.
Stability of tigecycline in bile.
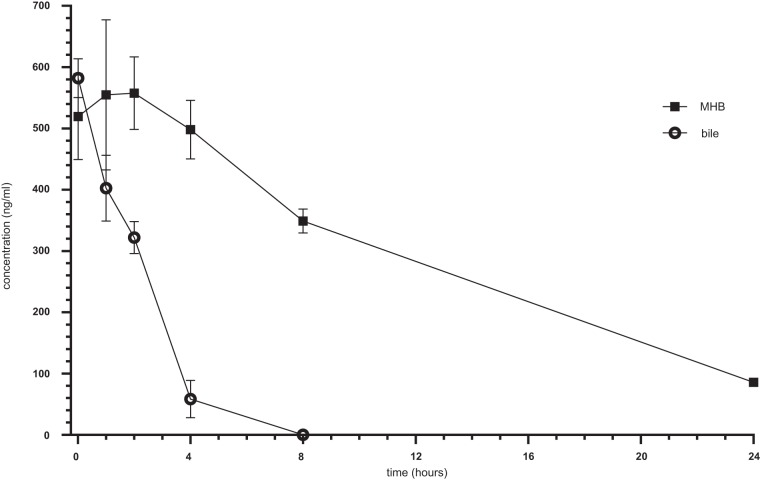
Due to the strong impact of bile on bactericidal activity of TGC, the question was raised whether this effect was due to a decreased stability of TGC in bile. Thus, stability testing of TGC was performed for a defined time course of 24 h.
TGC incubated in bile showed a decrease in the antibiotic concentration within the first 4 h and ultimately fell below the detection limit 8 h after incubation (Fig. 7).
FIG 7.
Stability of tigecycline in MHB and bile. Error bars indicate means of 3 independent experiments.
Moderate TGC stability was also observed in MHB media, demonstrating a decrease of the antibiotic concentration starting at 0.6 μg/ml over time. However, detectable concentrations were maintained throughout the period of measurement, revealing levels of 0.350 μg/ml after 8 h and 0.1 μg/ml after 24 h (Fig. 7).
DISCUSSION
Various body fluids, such as CSF, pulmonary surfactant, or urine, have been demonstrated to have an impact on the bacterial killing of anti-infective agents. However, even though infections of the biliary tract system are highly prevalent and are often accompanied by life-threatening complications (e.g., sepsis), very little is known about the effect of bile fluid on antibiotics.
In the present study, we investigated the influence of pure human bile fluid on the bactericidal activity of various antibiotics in vitro. To our knowledge, we present the first time-kill studies in pure human bile against multiple concentrations of antibiotics.
Overall, the present data indicate that bile has an inhibitory effect on the antimicrobial activity of CIP, MEM, TGC, and LZD against E. coli and E. faecalis, respectively. This finding was particularly emphasized in cultures treated with TGC (Fig. 3 and 6), where bactericidal activity could not be achieved for all investigated concentrations (up to 256- and 1,024-fold the MIC for E. coli and E. faecalis, respectively).
The influence of bile fluid on the bactericidal activity of antibiotics was discussed in some early studies. Rees et al. (17) found pronounced differences in the MICs of various antibiotics in media broth, human bile, and synthetic bile against Staphylococcus epidermidis, E. faecium, and Enterobacter cloacae, which were isolated from human bile. Whereas in bile S. epidermidis was resistant to vancomycin, bile did not show significant effects on the MICs of ciprofloxacin. Additionally, for E. cloacae strains, the MICs of ciprofloxacin were mostly unaffected by the presence of bile. In contrast, for S. epidermidis and E. faecium, 8-fold smaller MICs of co-amoxiclav were detected in bile compared to broth. Reverse results were discovered for E. cloacae (17).
In 1959, Glaz described for Salmonella typhi a reduction of the minimal bacterial concentration (MBC) of penicillin G and racemic chloramphenicol but a strong increase in the MBC of oxytetracycline in unmodified gallbladder bile obtained from cholecystectomy in comparison to an unspecified bouillon (19).
Helm et al. demonstrated good growth of Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Salmonella enterica serovar Typhimurium, and Pseudomonas aeruginosa in bile (pH 8.2) collected from T-tubes (21).
However, selected bacterial strains and antibiotic agents as well as the employed method to investigate pharmacodynamic behavior were different from those introduced in our experiments.
Surprisingly, bile turned out to be a very good growth medium for the cultivation of E. coli and E. faecalis and proved to be even superior to standard growth medium like MHB (Fig. 1). From an evolutionary perspective, increased growth in media containing both bile and MHB may be ascribed to the fact that both E. coli and E. faecalis are part of intestinal flora and, therefore, are selected by evolution to tolerate bile mixed to chymus (22).
Bile, produced continuously by the liver, stored in the gallbladder, and secreted into the duodenum, is comprised of 97% water and further of bile salts, fats, bilirubin, and 200 meq/liter inorganic salts (23, 24). Bile is contributing significantly to digestive and absorptive processes and also acts, to a certain degree, as a surfactant for the lipids in human nutrition (24).
In a study by Sutor and Wooley (25), bile was collected from patients undergoing cholecystectomy and showed a pH in the common bile duct between 7.50 and 8.05 and in the gallbladder itself between 6.80 and 7.65. The latter changed depending on the physical activity status of the patient (25). In the present study, the pH value of pure pooled bile fluid and bile growth medium (96% bile fluid and 4% isotonic sodium chloride solution) was 8.6.
Previous studies have already addressed the pH in body fluids (e.g., CSF and urine) as playing a crucial role in antibiotic efficacy (13, 15, 26, 27). Sauermann et al. showed that in addition to the presence of CSF the pH value also affected the bactericidal activity of certain antibiotics (13, 15). Similar effects were found in human urine. Acidification of urine resulted in a significant decrease in bactericidal activity of many antibiotics, including ciprofloxacin, which was shown by a MIC increase of up to 40-fold compared to MHB (26, 27).
Based on those previous findings, we therefore hypothesized that differences in the pH value and therewith associated degradation of the antibiotics may be a possible explanation for the impact of bile fluid on the antibiotic efficacy of TGC observed in the present study. Whether this observation was only due to pH or if enzymatic degradation also took place was not investigated. Upon TGC stability testing, a drop in antibiotic concentration was observed for both bile and MHB medium; however, the degradation of TGC was much faster in bile, resulting in concentrations below the detection limit after 8 h of incubation.
Due to the limited availability of human bile fluid, the number and type of experiments that were to be performed in the present study were limited. In general, patient inclusion was, in particular, difficult, as often times volunteers did not meet inclusion criteria due to preoperative antibiotic treatment. Furthermore, in many cases, patients' gallbladders were filled with calculi instead of bile fluid. Additionally, if bile was present, fluids were often highly viscous or contained antibiotic remnants, which made the bile samples unsuitable for further experimental use.
The present study demonstrated that bacteria are expected to grow well in bile, while its presence has a pronounced impact on the activity of some antibiotics. Standard pharmacokinetic/pharmacodynamic (PK/PD) indices for gallbladder and bile duct infections thereby may not hold true. Despite the limited access to bile fluid, further investigations testing a larger spectrum of antibiotics and bacteria are warranted.
MATERIALS AND METHODS
The following study was performed in accordance with the actual International Conference on Harmonization—Good Clinical Practice guidelines and the Declaration of Helsinki at the Department of Clinical Pharmacology at the Medical University of Vienna (Austria). Prior to study initiation, approval was obtained by the Ethics Committee (EC) of the Medical University of Vienna, EC number 1247/2013. All subjects participating in this study gave oral and written informed consent before inclusion.
Twenty-nine patients between 31 and 71 years of age (mean, 47.3 years) suffering from cholecystitis or cholecystolithiasis and scheduled for cholecystectomy were screened and included between October 2013 and January 2015. Subjects receiving antibiotic treatment within 1 week prior to cholecystectomy were excluded from participation.
Human bile was successfully collected from 19 resected gallbladders immediately after cholecystectomy and was stored immediately at −80°C until use.
Preparation of bile.
In order to trace antibiotic residues, a bioassay with Bacillus subtilis DSM 618 was performed for each sample prior to further in vitro experiments. For inhibition testing, agar plates were prepared with test agar pH 6 (catalog no. 110663; Merck, Darmstadt, Germany) and Bacillus subtilis spore suspension (catalog no. 110649; Merck, Darmstadt, Germany). Therefore, three 6-mm filter paper discs were placed on each agar plate and inoculated with 10 μl of bile fluid, kept at 37°C, and evaluated 24 h later. Remaining bile samples were kept at −80°C. Upon Bacillus subtilis inhibition testing, a total of eight samples had to be excluded due to various reasons. Four samples exhibited a marked zone of inhibition as defined by ≥2 mm from the disc (n = 4). One sample was acholic, another sample contained less than 1 ml (n = 1), and two samples contained bile of high viscosity (n = 2) and therewith were not used for further experiments. No further analysis was performed for the discarded samples.
Ultimately, bile fluid from 11 patients was pooled to a total volume of 120.5 ml and centrifuged to separate bile from solid impurities, such as small concrements. Supernatant bile was then collected, split in 5-ml aliquots, and kept in borosilicate glass reagent bottles. Sterilization was performed by Mediscan GmbH & Co KG (Seibersdorf, Austria) applying gamma irradiation (approximately 25 kGy) for 24 h. Prior to further experimental use, sterility testing was performed for each bile aliquot by plating 20 μl on Columbia agar (bioMérieux, Marcy-l'Etoile, France) and incubating for 24 h at 37°C.
Bacterial strains.
Escherichia coli ATCC 25922 (Gram-negative) and Enterococcus faecalis ATCC 29212 (Gram-positive) were obtained from the American Type Culture Collection (ATCC, The Global Bioresource Center, Manassas, Virginia, USA). Individual strains were plated on Columbia agar plates and precultured overnight under regular culture conditions (37°C).
Time-growth curves.
To evaluate the effect of bile fluid on bacterial growth and for selection of adequate growth media for time-kill studies, time-growth curves were generated for both bacterial strains in pure and mixed media containing different concentrations of bile fluid (0, 20, 50, 70, and 98%) added to cation-adjusted Mueller-Hinton Broth (MHB; Fluka Analytical from Sigma-Aldrich, Saint Louis, MO, USA). Isotonic sodium chloride solution as strain inoculum accounted for 2% in each medium.
Broth microdilution/MICs.
To predict the efficacy of the antibiotic agents against the two bacterial strains, the respective MICs were determined for MHB only by applying the CLSI method (initial inoculum of ∼5 × 105 CFU/ml). Due to the small available volume of bile fluid and expected difficulties in the definition of visible bacterial growth in brownish colored bile fluid, the microdilution method could not be applied for bile samples.
Time-kill curves.
To evaluate the effect of bile fluid on antibiotic activity, bacterial time-kill curves (TKC) were performed in triplicate in 1 ml MHB and in duplicate in 1 ml bile fluid at static concentrations 4-fold below, at, and 4-fold above the respective antibiotic MIC. Antibiotic-free controls were included for each experiment.
In detail, conical 15-ml polypropylene (PP) centrifuge tubes were filled with 1,028.6 μl medium and inoculated with 21.4 μl test strain inoculum (manufactured in 0.9% sodium chloride solution) to achieve a final concentration of ∼106 CFU/ml. For bacterial counts at baseline (0 h), 70 μl was removed again. The addition of 20 μl of antibiotic solution (antibiotic stock diluted in 0.9% sodium chloride solution) resulted in 1,000 μl growth medium, consisting of 96% pooled human bile fluid or cation-adjusted MHB. The remaining 4% contained 0.9% sodium chloride solution, bacterial strains, and the antibiotics of interest. Inoculated medium was incubated in a shaking water bath at 37°C for up to 24 h. Two, 5, 8, and 24 h after inoculation, bacterial counts were determined. Therefore, a serial sample dilution was performed by plating the series on Columbia agar plates supplemented with 5% sheep blood (bioMérieux, Marcy-I'Etoile, France). Each tube was vortex mixed for 10 to 15 s, and samples of 70 μl were retrieved. Samples were then further diluted with 0.9% sodium chloride solution in seven log10 steps, and 20 μl of each dilution was applied onto the surface of the prepared agar plates. After 18 to 24 h of incubation at 37°C, bacterial colonies (CFU/ml) were counted, back extrapolated to the original volume, and means (± standard deviations [SD]) plotted as a function of time for each strain and study substance.
Time-kill curves were repeated at increasing antibiotic concentrations as described to achieve at least bacteriostasis, which was defined as an absence of increase or even a reduction of bacterial counts of less than 3 log steps over a time course of 24 h (28).
Bacterial strains and antibiotic concentrations.
E. faecalis bacterial cultures were incubated with LZD (Zyvoxid; Fresenius Kabi Norge AS, Halden, Norway) or TGC (Tygacil; Wyeth Pharmaceuticals, Havant, United Kingdom), and E. coli cultures were incubated with ciprofloxacin (CIP) (Ciprofloxacin Kabi; Fresenius Kabi Norge AS, Halden, Norway), MEM (M2574 Sigma, Meropenem trihydrate, dissolved powder; Sigma-Aldrich, Vienna, Austria), or TGC at concentrations below, at, and above MIC.
Tigecycline stability assay.
To evaluate the stability of TGC, bile and pure MHB were spiked with TGC to achieve a final concentration of 0.6 μg/ml (accounting to 10× MIC of E. faecalis) and kept in the incubator at 37°C. Test solutions were then retrieved 30 s and 1, 2, 4, 8, and 24 h after incubation and stored at −80°C until use. All experiments were prepared in triplicate.
The concentration of TGC in MHB and human bile was determined by high-pressure liquid chromatography (HPLC). Frozen samples were thawed at room temperature. Briefly, after the addition of 600 μl of ice-cold methanol to 200 μl of MHB medium or bile, the samples were centrifuged (13,000 g for 5 min) and 100 μl of the clear supernatant was injected onto the HPLC column. Determination of TGC was performed using a Dionex UltiMate 3000 system (Dionex Corp., Sunnyvale, CA, USA) with UV detection at 348 nm. Chromatographic separation was carried out at 45°C on a Hypersil BDS C18 column (5 μm, 250 by 4.6 mm inside diameter [i.d.], Thermo Fisher Scientific, Inc., Waltham, MA, USA), preceded by a Hypersil BDS C18 precolumn (5 μm, 10 by 4.6 mm i.d.) at a flow rate of 1.0 ml/min. The mobile phase A consisted of potassium phosphate (50 mM, pH 3.0, with phosphoric acid) and heptanesulfonic acid (5 mM), and the mobile phase B consisted of methanol. The gradient ranged from 0% B (0 min) to 60% B at 15 min, kept constant at 60% until 20 min, and decreased linearly to 0% B again at 22 min. The columns were allowed to reequilibrate for 8 min between the runs. Linear calibration curves were performed from the peak areas of TGC to the external standard by spiking drug-free MHB and human bile with standard solutions of TGC to obtain a concentration range of 0.02 to 2 μg/ml (average correlation coefficients, >0.999). For this method, the limit of quantification for TGC was determined to be 20 ng/ml in MHB and bile (coefficients of accuracy and precision were <9%).
pH values.
pH values of pure bile fluid (100%) and bile growth media (96% bile fluid) were measured by using a micro pH meter at room temperature, revealing a value of 8.6. According to the manufacturer, the pH of MHB was 7.3.
ACKNOWLEDGMENTS
This project was funded by the Department for Clinical Pharmacology at the Medical University of Vienna. All mentioned supplies, including bile fluids, were provided by the Department for Clinical Pharmacology and in part by the Department for General Surgery at the Medical University of Vienna.
We thank the Department for General Surgery at the Medical University of Vienna, including its staff members, for its support and cooperation.
We declare no conflict of interest.
All authors participated in the interpretation of data, reviewed the final manuscript, and approved its publication.
REFERENCES
- 1.Ortega M, Marco F, Soriano A, Almela M, Martinez JA, Lopez J, Pitart C, Mensa J. 2012. Epidemiology and prognostic determinants of bacteraemic biliary tract infection. J Antimicrob Chemother 67:1508–1513. doi: 10.1093/jac/dks062. [DOI] [PubMed] [Google Scholar]
- 2.Kimura Y, Takada T, Kawarada Y, Nimura Y, Hirata K, Sekimoto M, Yoshida M, Mayumi T, Wada K, Miura F, Yasuda H, Yamashita Y, Nagino M, Hirota M, Tanaka A, Tsuyuguchi T, Strasberg SM, Gadacz TR. 2007. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 14:15–26. doi: 10.1007/s00534-006-1152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida M, Takada T, Kawarada Y, Tanaka A, Nimura Y, Gomi H, Hirota M, Miura F, Wada K, Mayumi T, Solomkin JS, Strasberg S, Pitt HA, Belghiti J, de Santibanes E, Fan ST, Chen MF, Belli G, Hilvano SC, Kim SW, Ker CG. 2007. Antimicrobial therapy for acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 14:83–90. doi: 10.1007/s00534-006-1160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maluenda F, Csendes A, Burdiles P, Diaz J. 1989. Bacteriological study of choledochal bile in patients with common bile duct stones, with or without acute suppurative cholangitis. Hepatogastroenterology 36:132–135. [PubMed] [Google Scholar]
- 5.Keighley MR, Lister DM, Jacobs SI, Giles GR. 1974. Hazards of surgical treatment due to microorganisms in the bile. Surgery 75:578–583. [PubMed] [Google Scholar]
- 6.Ballal M, Jyothi KN, Antony B, Arun C, Prabhu T, Shivananda PG. 2001. Bacteriological spectrum of cholecystitis and its antibiogram. Indian J Med Microbiol 19:212–214. [PubMed] [Google Scholar]
- 7.Leung JW, Ling TK, Chan RC, Cheung SW, Lai CW, Sung JJ, Chung SC, Cheng AF. 1994. Antibiotics, biliary sepsis, and bile duct stones. Gastrointest Endosc 40:716–721. [PubMed] [Google Scholar]
- 8.Orda R, Berger SA, Levy Y, Shnaker A, Gorea A. 1992. Penetration of ceftriaxone and cefoperazone into bile and gallbladder tissue in patients with acute cholecystitis. Dig Dis Sci 37:1691–1693. doi: 10.1007/BF01299860. [DOI] [PubMed] [Google Scholar]
- 9.Rose WE, Rybak MJ. 2006. Tigecycline: first of a new class of antimicrobial agents. Pharmacotherapy 26:1099–1110. doi: 10.1592/phco.26.8.1099. [DOI] [PubMed] [Google Scholar]
- 10.Moellering RC. 2003. Linezolid: the first oxazolidinone antimicrobial. Ann Intern Med 138:135–142. doi: 10.7326/0003-4819-138-2-200301210-00015. [DOI] [PubMed] [Google Scholar]
- 11.van Dijk AH, de Reuver PR, Tasma TN, van Dieren S, Hugh TJ, Boermeester MA. 2016. Systematic review of antibiotic treatment for acute calculous cholecystitis. Br J Surg 103:797–811. doi: 10.1002/bjs.10146. [DOI] [PubMed] [Google Scholar]
- 12.Schwameis R, Zeitlinger M. 2013. Methods to measure target site penetration of antibiotics in critically ill patients. Curr Clin Pharmacol 8:46–58. doi: 10.2174/1574884711308010007. [DOI] [PubMed] [Google Scholar]
- 13.Sauermann R, Schwameis R, Fille M, Ligios ML, Zeitlinger M. 2009. Cerebrospinal fluid impairs antimicrobial activity of fosfomycin in vitro. J Antimicrob Chemother 64:821–823. doi: 10.1093/jac/dkp261. [DOI] [PubMed] [Google Scholar]
- 14.van 't Veen A, Mouton JW, Gommers D, Kluytmans JA, Dekkers P, Lachmann B. 1995. Influence of pulmonary surfactant on in vitro bactericidal activities of amoxicillin, ceftazidime, and tobramycin. Antimicrob Agents Chemother 39:329–333. doi: 10.1128/AAC.39.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauermann R, Schwameis R, Fille M, Ligios ML, Zeitlinger M. 2008. Antimicrobial activity of cefepime and rifampicin in cerebrospinal fluid in vitro. J Antimicrob Chemother 62:1057–1060. doi: 10.1093/jac/dkn312. [DOI] [PubMed] [Google Scholar]
- 16.Schwameis R, Erdogan-Yildirim Z, Manafi M, Zeitlinger MA, Strommer S, Sauermann R. 2013. Effect of pulmonary surfactant on antimicrobial activity in vitro. Antimicrob Agents Chemother 57:5151–5154. doi: 10.1128/AAC.00778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rees EN, Elliott TS. 1998. The influence of bile on antimicrobial activity in vitro. J Antimicrob Chemother 41:659–660. doi: 10.1093/jac/41.6.659. [DOI] [PubMed] [Google Scholar]
- 18.Holton J. 1985. Effect of radiographic contrast medium and bile on antimicrobial activity. Eur J Clin Microbiol 4:517. doi: 10.1007/BF02014441. [DOI] [PubMed] [Google Scholar]
- 19.Glaz ET. 1959. Effect of antibiotics on Salmonella typhi in human bile. Acta Microbiol Acad Sci Hung 6:103–111. (In German.) [PubMed] [Google Scholar]
- 20.Hyacinta M, Hana KS, Andrea B, Barbora C. 2015. Bile tolerance and its effect on antibiotic susceptibility of probiotic Lactobacillus candidates. Folia Microbiol (Praha) 60:253–257. doi: 10.1007/s12223-014-0365-8. [DOI] [PubMed] [Google Scholar]
- 21.Helm EB, Paulus I, Shah PM, Stille W. 1976. Antibacterial activity of antibiotics in human bile (author's transl). Infection 4:94–101. (In German.) doi: 10.1007/BF01638724. [DOI] [PubMed] [Google Scholar]
- 22.Thadepalli H, Lou MA, Bach VT, Matsui TK, Mandal AK. 1979. Microflora of the human small intestine. Am J Surg 138:845–850. doi: 10.1016/0002-9610(79)90309-X. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann AF. 1999. Bile acids: the good, the bad, and the ugly. News Physiol Sci 14:24–29. [DOI] [PubMed] [Google Scholar]
- 24.Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. 2009. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol 15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutor DJ, Wooley SE. 1974. The sequential deposition of crystalline material in gallstones: evidence for changing gallbladder bile composition during the growth of some stones. Gut 15:130–131. doi: 10.1136/gut.15.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burian A, Erdogan Z, Jandrisits C, Zeitlinger M. 2012. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology 90:281–287. doi: 10.1159/000342423. [DOI] [PubMed] [Google Scholar]
- 27.Erdogan-Yildirim Z, Burian A, Manafi M, Zeitlinger M. 2011. Impact of pH on bacterial growth and activity of recent fluoroquinolones in pooled urine. Res Microbiol 162:249–252. doi: 10.1016/j.resmic.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Zurenko GE, Yagi BH, Schaadt RD, Allison JW, Kilburn JO, Glickman SE, Hutchinson DK, Barbachyn MR, Brickner SJ. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother 40:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]