ABSTRACT
Fosfomycin is widely used for the treatment of uncomplicated urinary tract infection (UTI), and it has recently been recommended that fosfomycin be used to treat infections caused by multidrug-resistant (MDR) Gram-negative bacilli. Whether urine acidification can improve bacterial susceptibility to fosfomycin oral dosing regimens has not been analyzed. The MIC of fosfomycin for 245 Gram-negative bacterial isolates, consisting of 158 Escherichia coli isolates and 87 Klebsiella isolates which were collected from patients with urinary tract infections, were determined at pH 6.0 and 7.0 using the agar dilution method. Monte Carlo simulation of the urinary fosfomycin area under the concentration-time curve (AUC) after a single oral dose of 3,000 mg fosfomycin and the MIC distribution were used to determine the probability of target attainment (PTA). Fosfomycin was effective against E. coli (MIC90 ≤ 16 μg/ml) but not against Klebsiella spp. (MIC90 > 512 μg/ml). Acidification of the environment increased the susceptibility of 71% of the bacterial isolates and resulted in a statistically significant decrease in bacterial survival. The use of a regimen consisting of a single oral dose of fosfomycin against an E. coli isolate with an MIC of ≤64 mg/liter was able to achieve a PTA of ≥90% for a target pharmacodynamic index (AUC/MIC) of 23 in urine; PTA was not achieved when the MIC was higher than 64 mg/liter. The cumulative fractions of the bacterial responses (CFR) were 99% and 55% against E. coli and Klebsiella spp., respectively, based on simulated drug exposure in urine with an acidic pH of 6.0. A decrease of the pH from 7.0 to 6.0 improved the PTA and CFR of the target pharmacodynamic index in both E. coli and Klebsiella isolates.
KEYWORDS: Enterobacteriaceae, fosfomycin, Monte Carlo simulation, acidic pH, pharmacodynamics, urinary tract infection
INTRODUCTION
Urinary tract infections (UTIs) are the most common infections worldwide, and members of the family Enterobacteriaceae are the main pathogens responsible for UTIs (1). The rise in the rate of antibiotic resistance over the last several years has resulted in limited treatment options currently available for the treatment of infections caused by multidrug-resistant (MDR) bacteria. Fosfomycin is an old antibiotic agent frequently used to treat uncomplicated UTIs and has been reevaluated as a potential option for the treatment of infections caused by MDR Gram-negative bacteria (2). The dosage currently approved for the treatment of an uncomplicated UTI is a single 3,000-mg dose. However, for more complicated UTI cases (for which fosfomycin is not approved), multiple doses have been used. In parts of the world, including Brazil, where intravenous fosfomycin is not available, interest in regimens with multiple doses of oral fosfomycin for treating complicated UTIs and related infections has been renewed (3, 4).
Fosfomycin is a phosphonic acid derivative (cis-1,2-epoxypropyl phosphonic acid) isolated from a Streptomyces species (5). The molecule acts by inhibiting the first step in the biosynthesis of the bacterial cell wall and shows broad-spectrum bactericidal activity against both Gram-positive and Gram-negative pathogens (6). The parenteral formulation, fosfomycin disodium, is commercially available only in some European countries and Japan (7), whereas the oral formulation, fosfomycin tromethamine, is approved for use in Brazil and in other countries for the treatment of uncomplicated UTIs (8). Ninety-five percent of the absorbed drug is excreted by the kidney, which results in high drug concentrations in urine. These pharmacokinetic properties are favorable for the treatment of UTIs (9, 10).
The therapeutic response to antibacterial agents can be affected by the pH of body fluids (11). Previous studies have shown that fosfomycin exhibits optimal antimicrobial activity in acidic urine at pHs ranging from 5.0 to 6.0, whereas an acidic pH has an opposite effect on the MICs of fluoroquinolones, including ciprofloxacin (12–17). However, there is a lack of information related to whether an acidic environment can enhance bacterial susceptibility to the current commercial fosfomycin oral dosing regimen for the treatment of uncomplicated UTIs. This study utilized simulations of fosfomycin pharmacokinetics (PK) and a target pharmacodynamic (PD) index of fosfomycin to investigate whether the pH environment has a significant effect on the susceptibility of Escherichia coli and Klebsiella isolates collected from patients with uncomplicated UTIs to the fosfomycin oral dosing regimen and to determine the extent of bacterial killing due to fosfomycin that was achieved in urine acidified to pH 6.0. The current analyses are aimed at the evaluation of fosfomycin only for the treatment of uncomplicated UTIs.
RESULTS
In vitro susceptibility and effect of pH.
Table 1 presents the antimicrobial susceptibility profiles of 245 E. coli and Klebsiella urinary isolates at pH 6.0 and 7.0. E. coli isolates from patients with urinary tract infection were highly susceptible to fosfomycin at pH 7.0 with an MIC90 of ≤16 μg/ml. In contrast, fosfomycin was not active against Klebsiella spp. A high fosfomycin MIC90 of ≥512 μg/ml was observed against these isolates.
TABLE 1.
In vitro susceptibilities to fosfomycin of E. coli and Klebsiella spp. clinical isolates from patients with UTIs at pH 6.0 and 7.0a
| Microorganism | No. of strains | pH of test | No. of isolates with the following MIC (μg/ml): |
MIC (mg/liter) |
% of isolates |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
||||||||||||||||||||||
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | >512 | 50% | 90% | Range | S | I | R | S | R | |||
| Escherichia coli | 158 | 7.0 | − | − | 3 | 61 | 54 | 19 | 6 | 10 | 3 | 1 | − | − | 1 | 4 | 16 | 1 to >512 | 98 | 1 | 1 | 97 | 3 |
| 6.0 | − | 9 | 63 | 52 | 12 | 13 | 5 | 2 | 1 | − | − | − | 1 | 2 | 8 | 0.5 to >512 | 99 | 1 | 98 | 2 | |||
| Klebsiella spp.b | 87 | 7.0 | − | − | − | − | − | − | 2 | 2 | − | 23 | 21 | 22 | 17 | 256 | >512 | 16 to >512 | 5 | 26 | 69 | 5 | 95 |
| 6.0 | − | − | − | − | − | 2 | 3 | 6 | 15 | 26 | 19 | 7 | 9 | 128 | 512 | 8 to >512 | 30 | 30 | 40 | 13 | 87 | ||
−, no isolate; S, susceptible; I, intermediate susceptibility; R, resistant; CLSI, Clinical and Laboratory Standards Institute interpretative criteria (susceptible, MIC of ≤64 μg/ml; intermediate, MIC of 128 μg/ml; resistant, MIC of ≥256 μg/ml; EUCAST, European Committee on Antimicrobial Susceptibility Testing interpretative criteria (susceptible, MIC of ≤32 μg/ml; resistant, MIC of ≥32 μg/ml).
The species isolated included Klebsiella pneumoniae (n = 81) and K. oxytoca (n = 6).
The in vitro activity of fosfomycin against the two bacterial species tested was improved by acidification of the growth medium (Table 1). The fosfomycin MIC against E. coli and Klebsiella spp. was reduced for 71% (175/245) of the isolates. The MIC90 against E. coli and Klebsiella spp. was 2-fold lower in the lower-pH environment. Several strains that were previously resistant to fosfomycin at pH 7.0 became susceptible at pH 6.0, with the greatest effects being observed for the Klebsiella spp., given the Clinical and Laboratory Standards Institute (CLSI) breakpoint value of ≥64 μg/ml.
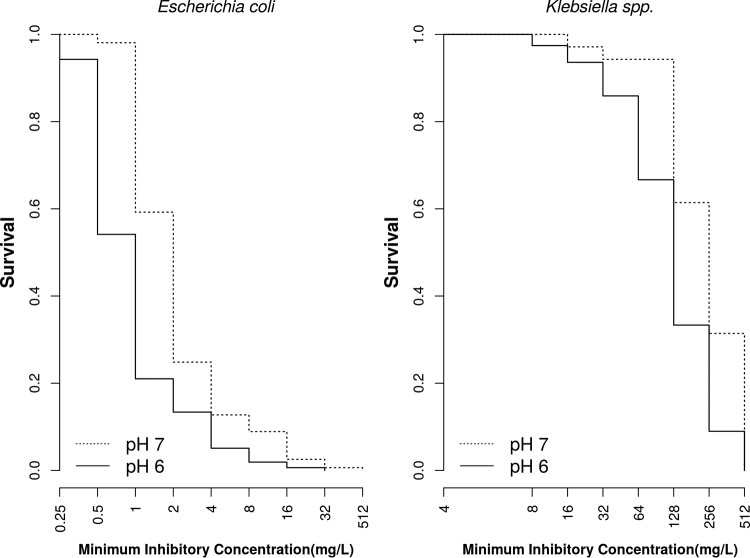
To evaluate whether the decrease in MIC values was statistically significant, we utilized a survival analysis approach, replacing the time component with MIC values. Figure 1 shows the survival curves at pH 6.0 and 7.0 for E. coli and Klebsiella spp. isolated from patients with UTIs. Applying the log-rank test to compare the two curves, we rejected the hypothesis that the survival curves were equal (P = 0.0001 for both, log-rank test).
FIG 1.
Survival-type antimicrobial susceptibility curves for Escherichia coli isolates (n = 158) and Klebsiella isolates (n = 87) from patients with urinary tract infections stratified on the basis of pHs of 6.0 (solid line) and 7.0 (dotted line).
Pharmacodynamic analyses.
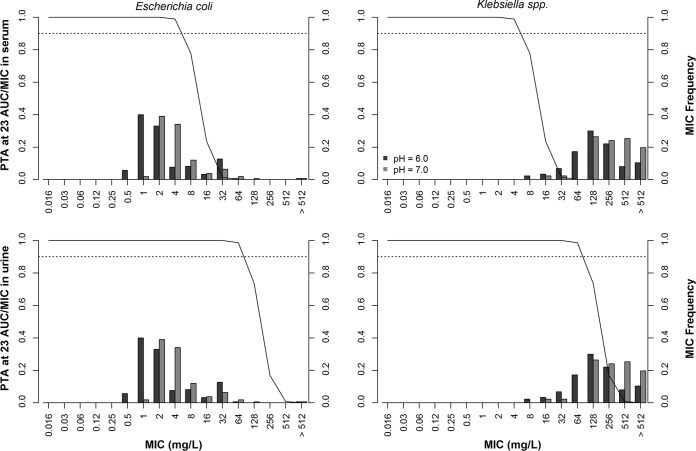
Figure 2 shows the probability of target attainment (PTA) for an area under the concentration-time curve (AUC)/MIC of 23 for a fosfomycin dosing regimen of a single dose of 3,000 mg and the MIC frequency of fosfomycin by microorganism type at pH 6.0 and 7.0. Oral fosfomycin achieved a PTA of ≥90% for the target of an AUC/MIC of 23 at an MIC of ≤4 μg/ml in serum and an MIC of ≤64 μg/ml in urine, indicating that the antimicrobial coverage was sufficient to achieve the MIC90 against E. coli isolates at both pH 6.0 and 7.0. The oral dosing regimen was not able to achieve a PTA of ≥90% at the MIC50/MIC90 against Klebsiella isolates at pH 6.0 or 7.0. The breakpoints for susceptibility of both European Committee on Antimicrobial Susceptibility Testing (EUCAST) (MIC ≤ 32 μg/ml) and CLSI (MIC = 64 μg/ml) were achieved in urine using the criterion of a PTA of ≥90% for a target of an AUC/MIC ratio of 23. An acidic pH resulted in a higher PTA at the MIC50 and MIC90. However, this condition was not sufficient for the fosfomycin regimen against bacteria harboring fosfomycin resistance with an MIC of >64 μg/ml on the basis of the AUC/MIC PD index.
FIG 2.
Fosfomycin MIC frequency in 158 Escherichia coli and 87 Klebsiella clinical isolates at pH 6.0 and 7.0 and probability of target attainment of an AUC/MIC of 23 against E. coli (right) and Klebsiella spp. (left) in serum (top) and urine (bottom) matrices for a fosfomycin dosing regimen consisting of a single dose of 3,000 mg in 10,000 virtual patients. PTA, probability of target attainment; AUC, area under the concentration-time curve.
A summary of the cumulative fractions of the bacterial responses (CFR) by fosfomycin dosing regimen at pH 6.0 and 7.0 in serum and urine is shown in Table 2. For the Klebsiella spp., the CFR improved from 28% to 55% when the urine pH was changed from 7.0 to 6.0. There was excellent coverage against E. coli regardless of the urine pH.
TABLE 2.
CFR at AUC/MIC of 23 for a single 3,000-mg oral dose of fosfomycin against a collection of clinical isolates by bacterial type
| Bacterial type | % of isolates |
|||
|---|---|---|---|---|
| pH 6 |
pH 7 |
|||
| Serum | Urine | Serum | Urine | |
| E. coli | 93.5 | 99 | 85 | 99 |
| Klebsiella spp. | 2.7 | 55 | 0.6 | 28 |
DISCUSSION
MDR Gram-negative bacterial infections have prompted the revival of fosfomycin (2, 18). Our study showed that an oral fosfomycin dosing regimen that is commonly used in clinical practice for the treatment of uncomplicated UTIs was more likely to achieve the PTA at the MIC90 against E. coli. Fosfomycin was less active against Klebsiella spp., as shown by a decrease of the PTA of the fosfomycin PD target index of an AUC/MIC of 23 in both serum and urine to below 90%.
The current study evaluated the MICs only of Enterobacteriaceae. Our findings for the fosfomycin MIC against E. coli and Klebsiella spp. were consistent with those reported in recent studies that evaluated in vitro susceptibility profiles (1, 19, 20). Fosfomycin presented high levels of activity against E. coli. However, this drug was less active against Klebsiella spp., which displayed a higher MIC distribution (21–23). Falagas et al. reported that the fosfomycin MIC distribution can be quite variable and can also be influenced by several factors, including bacterial species (24). A retrospective study from a hospital in Oxfordshire, UK, found the rate of fosfomycin resistance to be 1% for E. coli isolates but 19% for Klebsiella isolates when oral fosfomycin was used to treat UTIs (25). Their results corroborated the CFR estimated for the Maringá State University Hospital in Brazil. The current study shows that the standard single oral dose of fosfomycin is inadequate when Klebsiella spp. are the predominant bacteria, even in uncomplicated UTIs.
The success of antimicrobial therapy against UTIs in a population can be estimated by the PK/PD profiles inferred from the drug concentrations at the site of action (26). The fosfomycin dosing regimen tested showed sufficient antimicrobial coverage for bacteria with MICs of up to 64 μg/ml in urine. A study by Rhodes et al. found that a single oral dose of 3,000 mg of fosfomycin was suitable against pathogens with an MIC value of up 4 μg/ml in the prostate (27). On the basis of simulations, an oral dose of 3,000 mg fosfomycin was effective against E. coli isolates at both pH 6.0 and 7.0; however, the fosfomycin dosing regimen evaluated would not achieve a satisfactory PTA at an MIC of >64 μg/ml. Consequently, a PTA of ≥90% for the MIC50 against Klebsiella isolates was unattainable.
Albiero and colleagues evaluated treatment regimens consisting of fosfomycin alone and in combination with meropenem and also showed that the administration of fosfomycin as monotherapy against KPC-2-producing K. pneumoniae (MIC50, 64 μg/ml) was not able to achieve a PTA of ≥90% even at higher dosages and when given as 3-h infusions in patients with normal renal function or renal impairment (28). Combination of fosfomycin with a carbapenem is required to confer bacterial susceptibility to both fosfomycin and meropenem in KPC-producing K. pneumoniae (28).
CLSI recommends a breakpoint of ≤64 μg/ml to differentiate E. coli and Enterococcus faecalis isolates from patients with UTIs susceptible and resistant to oral fosfomycin (29). The EUCAST MIC breakpoint of 32 μg/ml for the susceptibility of Enterobacteriaceae and Staphylococcus spp. to intravenous fosfomycin, irrespective of the site of infection, is lower (30). Fosfomycin is excreted in the active form in the urine via the kidneys and might achieve in vivo concentrations above the usual MIC against common uropathogens (9, 10). The same studies demonstrated that serum susceptibility data overestimated the resistance of urinary isolates in the presence of high urine antibiotic levels (10, 31–33). Even though only the higher doses may be required to achieve the PTA for an MIC of 64 μg/ml, fosfomycin becomes highly concentrated in the urine. It remains to be evaluated in a clinical setting whether the current oral dosing regimen supplemented with urine acidification would be sufficient to treat UTIs caused by MDR bacteria or whether a dose adjustment is required.
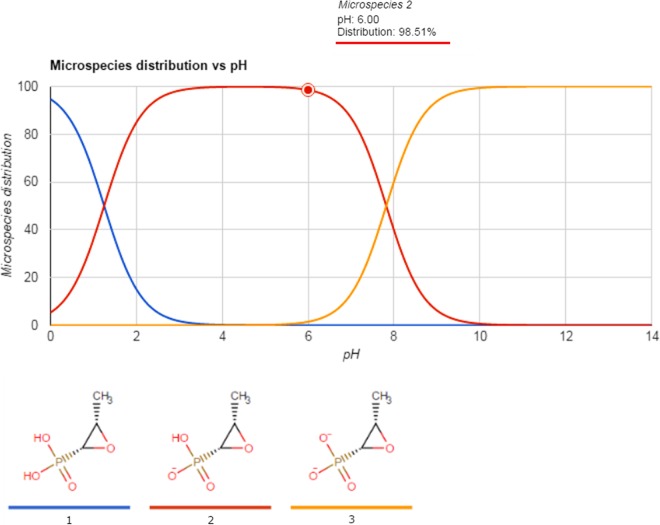
Acidification of the bacterial growth medium was an important factor affecting the efficacy of fosfomycin and, consequently, improved the antimicrobial coverage against the majority of the E. coli and Klebsiella isolates studied (71%). There was a significant difference in the MIC for these isolates between pH 6.0 and 7.0, which corroborates the findings of other studies that demonstrated a pH effect on the in vitro activity of antimicrobial agents and the therapeutic response (12–17, 34, 35). The enhanced activity of fosfomycin in an acidic environment can be explained by its physicochemical properties. The molecular structure of fosfomycin contains an epoxide ring linked to a phosphate group that is ionized, depending on the pH. It has two pKa values: pKa 1 is 1.25, and pKa 2 is 7.82 (Fig. 3). The fosfomycin molecule is less protonated at pH 6.0, where the predominant microspecies has an electric charge of −1, than at pH 7.0, where the predominant microspecies has an electric charge of −2 (Fig. 4). At an acidic pH where fosfomycin is in its least ionized and more lipophilic state, a major fraction of the available antibiotic molecules can enter the bacteria, resulting in greater antimicrobial activity in acidic urine.
FIG 3.
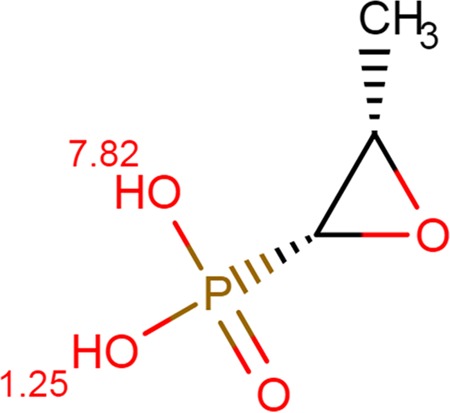
Molecular structure of fosfomycin and pKa values derived from the Chemicalize database (https://chemicalize.com/#/calculation).
FIG 4.
Relationship between the microspecies distribution percentage for fosfomycin and pH adapted from the Chemicalize database (https://chemicalize.com/#/calculation).
It is known that some urinary pathogens, such as Proteus mirabilis and Klebsiella species, are capable of producing ammonia from urea, resulting in an increased urine pH (36). The urine alkalization caused by these microorganisms can hinder antimicrobial treatment using fosfomycin. Alternative complementary strategies have been used for the treatment of UTIs, including the use of agents that acidify the urine (37). Ascorbic acidic (vitamin C) is regarded as safe and effective in altering the urinary pH (14, 38). It is often used as an agent to prevent UTI, although there is no evidence to support this indication (39). Some studies have shown the benefits of using vitamin C together with antimicrobials. Carlsson et al. investigated the inhibition of growth of different bacterial strains, including E. coli, by ascorbic acid at various pH levels in human urine and demonstrated that vitamin C may be used for the treatment and prophylaxis of UTIs (38). However, its use should not be excessive because excess ascorbic acid can induce tissue damage and salt precipitation, causing urinary stones and/or encrustation in humans (14).
The present study has some limitations. First, the isolates came from a public hospital that provides services for the population of 808,241 people residing in the Maringa metropolitan region, which covers 30 municipalities, but this population may not be representative of the overall Brazilian population. Second, the narrow range of pH values investigated with the E. coli and Klebsiella isolates (6.0 to 7.0) precludes the ability to determine the effect of the whole spectrum of the pH range on the behavior of fosfomycin according to pH. We verified in the survival analysis that the decrease in MIC values with a decrease in pH was statistically significant.
In conclusion, PK/PD analyses of fosfomycin showed that a lower physiological pH improved attainment of the target PD index in the majority of the E. coli isolates but not in Klebsiella species. Fosfomycin activity was improved at an acidic pH; urine acidification can easily be achieved with supplemental vitamin C during fosfomycin treatment of uncomplicated UTI in clinical practice.
MATERIALS AND METHODS
Bacterial isolates.
A total of 245 nonduplicated consecutive E. coli and Klebsiella isolates recovered from patients with suspected UTIs with a colony count of greater than 105 CFU per milliliter were selected from the medical microbiology laboratory organism bank of the Maringá State University Hospital. All isolates were identified by means of the BD Phoenix automated microbiology system and were stored at −20°C in Trypticase soy broth (Difco Laboratories, Detroit, MI) with 30% glycerol until they were tested. The isolates were recovered on MacConkey agar plates to verify the purity of the culture. These plates were incubated at 35 ± 2°C in ambient air for 24 h. The isolates, which were collected between January 2011 and June 2015, included 158 Escherichia coli isolates and 87 Klebsiella isolates. Only one isolate per patient was included in the study. The study was approved by the “Permanent Committee of Ethics in Research Involving Human Beings” of the Maringá State University (CAAE no. 318.0.093.000-11).
Antimicrobial agents.
Fosfomycin (Sigma-Aldrich, St. Louis, MO, USA) was purchased from LabCompany (Londrina, Paraná, Brazil). Fosfomycin was dissolved in water to form a 10-μg/ml stock solution, which was stored at −20°C (stock solution).
Antimicrobial susceptibility testing.
The susceptibilities of the isolates to fosfomycin were determined by the agar dilution method, as described in CLSI guidelines (29, 40), at pH 6.0 and 7.0 using Mueller-Hinton agar (MHA; Difco Laboratories, Detroit, MI), which was supplemented with an additional 25 μg/ml of glucose-6-phosphate (G6-phosphate). The pH of the MHA was adjusted by adding either 1 N HCl or NaOH and tested using a pH meter before autoclaving. During the experiment, G6-phosphate was diluted along with 2-fold serial dilutions of antimicrobial agent in water in glass tubes. After shaking, the serial dilution containing the antimicrobial agent plus G6-phosphate was incorporated into liquid MHA, which was kept in a constant 50°C water bath. The pH of the medium to which the antimicrobial solution plus G6-phosphate was added was then verified using Merck universal indicator strips. The solution was then poured onto plates.
The test isolates and ATCC reference strains were suspended in sterile Mueller-Hinton broth (Difco Laboratories, Detroit, MI) and adjusted to the equivalent of a 0.5 McFarland standard. Cell suspensions were further diluted and were delivered onto plates using a Steers replicator, which carried approximately 104 CFU of each isolate. The inoculated plates were incubated in ambient air at 35 ± 2°C for 16 to 20 h.
The tested fosfomycin concentrations ranged from 0.25 to 512 μg/ml. The MIC against a bacterial strain was defined as the lowest concentration that inhibited visible growth of the organism. MIC values were determined 2 to 4 times per isolate to identify the modal value, which is reported in this study. Control strains, including Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Enterococcus faecalis ATCC 29212, were included in each set of tests.
Interpretation of susceptibility results.
The susceptibility categories by MIC were determined using both the CLSI interpretive criteria for urinary tract isolates of E. coli and E. faecalis (29) and the EUCAST interpretive criteria for all isolates of the Enterobacteriaceae (30).
Pharmacokinetics and Monte Carlo simulation.
Monte Carlo simulation of 10,000 virtual exposure parameters was carried out in R software (v.3.1.1; R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria). Exposure data previously reported in the literature and the package insert for a single oral dose of 3,000 mg fosfomycin in both serum and urine were used to generate simulated distributions for the AUC for serum (AUCserum) of 296 ± 46.4 μg · h/ml for healthy volunteers (41) and an average urine concentration (Cavg,urine) of 537 ± 252 μg/ml within 6 to 8 h after a single dose in the fed state (42). The fosfomycin AUC for urine (AUCurine) was computed as Cavg,urine multiplied by 8 h (AUCurine, 4,296 ± 2,016 μg · h/ml). A log-normal distribution was assumed in order to avoid negative exposure values. In the simulation, the variability, characterized by the coefficient of variation or the AUCserum, was increased to 50% to mimic the variability in patients. The level of protein binding of fosfomycin in plasma is negligible (42). The area under the concentration-time curve-to-MIC (AUC/MIC) ratios in serum and urine for the single oral dose regimen were then determined and compared to the target PD index determined from the literature (43).
Pharmacodynamics.
PD analyses were performed using Monte Carlo simulations based on the distribution of the AUC in urine and serum. This method accounts for the variability in the pharmacokinetics of the drug and the distribution of MIC data to determine the probability of reaching a target AUC/MIC ratio of 23 in serum and urine. This target value was selected on the basis of the report by Lepak et al. showing that the 24-h AUC/MIC ratio of 23 after fosfomycin injection in the neutropenic murine thigh infection model is associated with stasis in Enterobacteriaceae (43).
The PTA was determined from the distribution of the AUC/MIC in incremental MIC values. The PTA for a drug regimen was considered adequate when ≥90% of the simulated population achieved or exceeded the target PD index (44, 45). CFR for a single oral 3-g fosfomycin dose at an AUC/MIC of 23 was computed as the summation of the density or percentage of bacteria at each MIC across the distribution multiplied by the PTA value at the MIC for the regimen (46, 47).
Statistical analysis.
Survival analysis for interval-censored data was used to compare the effect of pH on the survival curve for all bacteria used in the study. For the comparison of the survival curves, the log-rank test was used to determine whether the curves were significantly different (48). A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
The funding agency, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), had no role in the study design, data collection and interpretation, or the decision to submit the work for publication, as these government funds are designed to encourage training in higher education in Brazil and cover only the cost of laboratory materials.
REFERENCES
- 1.Demir T, Buyukguclu T. 2013. Evaluation of the in vitro activity of fosfomycin tromethamine against Gram-negative bacterial strains recovered from community- and hospital-acquired urinary tract infections in Turkey. Int J Infect Dis 17:e966–e970. doi: 10.1016/j.ijid.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Neuner EA, Sekeres J, Hall GS, van Duin D. 2012. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 56:5744–5748. doi: 10.1128/AAC.00402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giancola SE, Mahoney MV, Hogan MD, Raux BR, McCoy C, Hirsch EB. 2017. Assessment of fosfomycin for complicated or multidrug-resistant urinary tract infections: patient characteristics and outcomes. Chemotherapy 62:100–104. doi: 10.1159/000449422. [DOI] [PubMed] [Google Scholar]
- 4.Los-Arcos I, Pigrau C, Rodriguez-Pardo D, Fernandez-Hidalgo N, Andreu A, Larrosa N, Almirante B. 2015. Long-term fosfomycin-tromethamine oral therapy for difficult-to-treat chronic bacterial prostatitis. Antimicrob Agents Chemother 60:1854–1858. doi: 10.1128/AAC.02611-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendlin D, Stapley EO, Jackson M, Wallick H, Miller AK, Wolf FJ, Miller TW, Chaiet L, Kahan FM, Foltz EL, Woodruff HB, Mata JM, Hernandez S, Mochales S. 1969. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science 166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 6.Skarzynski T, Mistry A, Wonacott A, Hutchinson SE, Kelly VA, Duncan K. 1996. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure 4:1465–1474. doi: 10.1016/S0969-2126(96)00153-0. [DOI] [PubMed] [Google Scholar]
- 7.Endimiani A, Patel G, Hujer KM, Swaminathan M, Perez F, Rice LB, Jacobs MR, Bonomo RA. 2010. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 54:526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis 46:1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 9.Frossard M, Joukhadar C, Erovic BM, Dittrich P, Mrass PE, Van Houte M, Burgmann H, Georgopoulos A, Muller M. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob Agents Chemother 44:2728–2732. doi: 10.1128/AAC.44.10.2728-2732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frimodt-Moller N. 2002. Correlation between pharmacokinetic/pharmacodynamic parameters and efficacy for antibiotics in the treatment of urinary tract infection. Int J Antimicrob Agents 19:546–553. doi: 10.1016/S0924-8579(02)00105-X. [DOI] [PubMed] [Google Scholar]
- 11.Milne MD, Scribner BH, Crawford MA. 1958. Non-ionic diffusion and the excretion of weak acids and bases. Am J Med 24:709–729. doi: 10.1016/0002-9343(58)90376-0. [DOI] [PubMed] [Google Scholar]
- 12.Burian A, Erdogan Z, Jandrisits C, Zeitlinger M. 2012. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology 90:281–287. doi: 10.1159/000342423. [DOI] [PubMed] [Google Scholar]
- 13.Erdogan-Yildirim Z, Burian A, Manafi M, Zeitlinger M. 2011. Impact of pH on bacterial growth and activity of recent fluoroquinolones in pooled urine. Res Microbiol 162:249–252. doi: 10.1016/j.resmic.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Wang K, Li H, Denstedt JD, Cadieux PA. 2014. The influence of urinary pH on antibiotic efficacy against bacterial uropathogens. Urology 84:731.e1–7. doi: 10.1016/j.urology.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Kamberi M, Tsutsumi K, Kotegawa T, Kawano K, Nakamura K, Niki Y, Nakano S. 1999. Influences of urinary pH on ciprofloxacin pharmacokinetics in humans and antimicrobial activity in vitro versus those of sparfloxacin. Antimicrob Agents Chemother 43:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorian V, Sabath LD. 1970. Effect of pH on the activity of erythromycin against 500 isolates of gram-negative bacilli. Appl Microbiol 20:754–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalhoff A, Schubert S, Ullmann U. 2005. Effect of pH on the in vitro activity of and propensity for emergence of resistance to fluoroquinolones, macrolides, and a ketolide. Infection 33(Suppl 2):S36–S43. [DOI] [PubMed] [Google Scholar]
- 18.Seroy JT, Grim SA, Reid GE, Wellington T, Clark NM. 2016. Treatment of MDR urinary tract infections with oral fosfomycin: a retrospective analysis. J Antimicrob Chemother 71:2563–2568. doi: 10.1093/jac/dkw178. [DOI] [PubMed] [Google Scholar]
- 19.Villar HE, Jugo MB, Macan A, Visser M, Hidalgo M, Maccallini GC. 2014. Frequency and antibiotic susceptibility patterns of urinary pathogens in male outpatients in Argentina. J Infect Dev Ctries 8:699–704. doi: 10.3855/jidc.3766. [DOI] [PubMed] [Google Scholar]
- 20.Sultan A, Rizvi M, Khan F, Sami H, Shukla I, Khan HM. 2015. Increasing antimicrobial resistance among uropathogens: is fosfomycin the answer? Urol Ann 7:26–30. doi: 10.4103/0974-7796.148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho YH, Jung SI, Chung HS, Yu HS, Hwang EC, Kim SO, Kang TW, Kwon DD, Park K. 2015. Antimicrobial susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in health care-associated urinary tract infection: focus on susceptibility to fosfomycin. Int Urol Nephrol 47:1059–1066. doi: 10.1007/s11255-015-1018-9. [DOI] [PubMed] [Google Scholar]
- 22.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 23.Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. 2016. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents 47:269–285. doi: 10.1016/j.ijantimicag.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews PC, Barrett LK, Warren S, Stoesser N, Snelling M, Scarborough M, Jones N. 2016. Oral fosfomycin for treatment of urinary tract infection: a retrospective cohort study. BMC Infect Dis 16:556. doi: 10.1186/s12879-016-1888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez D, Schmidt S, Derendorf H. 2013. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev 26:274–288. doi: 10.1128/CMR.00092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes NJ, Gardiner BJ, Neely MN, Grayson ML, Ellis AG, Lawrentschuk N, Frauman AG, Maxwell KM, Zembower TR, Scheetz MH. 2015. Optimal timing of oral fosfomycin administration for pre-prostate biopsy prophylaxis. J Antimicrob Chemother 70:2068–2073. doi: 10.1093/jac/dkv067. [DOI] [PubMed] [Google Scholar]
- 28.Albiero J, Sy SK, Mazucheli J, Caparroz-Assef SM, Costa BB, Alves JL, Gales AC, Tognim MC. 2016. Pharmacodynamic evaluation of the potential clinical utility of fosfomycin and meropenem in combination therapy against KPC-2-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 60:4128–4139. doi: 10.1128/AAC.03099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; 26th informational supplement, 26th ed M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/clinical_breakpoints/. [Google Scholar]
- 31.Stamey TA, Fair WR, Timothy MM, Millar MA, Mihara G, Lowery YC. 1974. Serum versus urinary antimicrobial concentrations in cure of urinary-tract infections. N Engl J Med 291:1159–1163. doi: 10.1056/NEJM197411282912204. [DOI] [PubMed] [Google Scholar]
- 32.Pea F, Pavan F, Di Qual E, Brollo L, Nascimben E, Baldassarre M, Furlanut M. 2003. Urinary pharmacokinetics and theoretical pharmacodynamics of intravenous levofloxacin in intensive care unit patients treated with 500 mg b.i.d. for ventilator-associated pneumonia. J Chemother 15:563–567. doi: 10.1179/joc.2003.15.6.563. [DOI] [PubMed] [Google Scholar]
- 33.Cunha BA. 2012. Predicting in vivo effectiveness from in vitro susceptibility: a step closer to performing testing of uropathogens in human urine. Scand J Infect Dis 44:714–715. doi: 10.3109/00365548.2012.673731. [DOI] [PubMed] [Google Scholar]
- 34.Danby CS, Boikov D, Rautemaa-Richardson R, Sobel JD. 2012. Effect of pH on in vitro susceptibility of Candida glabrata and Candida albicans to 11 antifungal agents and implications for clinical use. Antimicrob Agents Chemother 56:1403–1406. doi: 10.1128/AAC.05025-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha BA. 2016. An infectious disease and pharmacokinetic perspective on oral antibiotic treatment of uncomplicated urinary tract infections due to multidrug-resistant Gram-negative uropathogens: the importance of urinary antibiotic concentrations and urinary pH. Eur J Clin Microbiol Infect Dis 35:521–526. doi: 10.1007/s10096-016-2577-0. [DOI] [PubMed] [Google Scholar]
- 36.Vince A, Dawson AM, Park N, O'Grady F. 1973. Ammonia production by intestinal bacteria. Gut 14:171–177. doi: 10.1136/gut.14.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid G. 1999. Potential preventive strategies and therapies in urinary tract infection. World J Urol 17:359–363. doi: 10.1007/s003450050161. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson S, Wiklund NP, Engstrand L, Weitzberg E, Lundberg JO. 2001. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide 5:580–586. doi: 10.1006/niox.2001.0371. [DOI] [PubMed] [Google Scholar]
- 39.Hickling DR, Nitti VW. 2013. Management of recurrent urinary tract infections in healthy adult women. Rev Urol 15:41–48. [PMC free article] [PubMed] [Google Scholar]
- 40.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 41.Bergan T. 1990. Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection 18(Suppl 2):S65–S69. doi: 10.1007/BF01643430. [DOI] [PubMed] [Google Scholar]
- 42.Food and Drug Administration. 2011. Monurol (fosfomycin tromethamine) package insert. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050717s007lbl.pdf. [Google Scholar]
- 43.Lepak AJ, Zhao M, VanScoy B, Taylor DS, Ellis-Grosse E, Ambrose PG, Andes DR. 2017. In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (fosfomycin for injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e00476-17. doi: 10.1128/AAC.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sy SK, de Kock L, Diacon AH, Werely CJ, Xia H, Rosenkranz B, van der Merwe L, Donald PR. 2015. N-Acetyltransferase genotypes and the pharmacokinetics and tolerability of para-aminosalicylic acid in patients with drug-resistant pulmonary tuberculosis. Antimicrob Agents Chemother 59:4129–4138. doi: 10.1128/AAC.04049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Kock L, Sy SK, Rosenkranz B, Diacon AH, Prescott K, Hernandez KR, Yu M, Derendorf H, Donald PR. 2014. Pharmacokinetics of para-aminosalicylic acid in HIV-uninfected and HIV-coinfected tuberculosis patients receiving antiretroviral therapy, managed for multidrug-resistant and extensively drug-resistant tuberculosis. Antimicrob Agents Chemother 58:6242–6250. doi: 10.1128/AAC.03073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sy SK, Derendorf H. 2014. Pharmacometrics in bacterial infections, p 229–258. In Schmidt S, Derendorf H (ed), Applied pharmacometrics, 1st ed Springer, New York, NY. [Google Scholar]
- 47.Sy SK, Zhuang L, Derendorf H. 2016. Pharmacokinetics and pharmacodynamics in antibiotic dose optimization. Expert Opin Drug Metab Toxicol 12:93–114. doi: 10.1517/17425255.2016.1123250. [DOI] [PubMed] [Google Scholar]
- 48.van de Kassteele J, van Santen-Verheuvel MG, Koedijk FD, van Dam AP, van der Sande MA, de Neeling AJ. 2012. New statistical technique for analyzing MIC-based susceptibility data. Antimicrob Agents Chemother 56:1557–1563. doi: 10.1128/AAC.05777-11. [DOI] [PMC free article] [PubMed] [Google Scholar]