ABSTRACT
There are no data comparing outcomes of patients treated with ceftazidime-avibactam versus comparators for carbapenem-resistant Enterobacteriaceae infections. At our center, ceftazidime-avibactam treatment of carbapenem-resistant Klebsiella pneumoniae bacteremia was associated with higher rates of clinical success (P = 0.006) and survival (P = 0.01) than other regimens. Across treatment groups, there were no differences in underlying diseases, severity of illness, source of bacteremia, or strain characteristics (97% produced K. pneumoniae carbapenemase). Aminoglycoside- and colistin-containing regimens were associated with increased rates of nephrotoxicity (P = 0.002).
KEYWORDS: carbapenem-resistant Enterobacteriaceae, Klebsiella pneumoniae carbapenemase, ceftazidime-avibactam, Klebsiella pneumoniae, bacteremia, clinical success
TEXT
Optimal management of carbapenem-resistant Enterobacteriaceae (CRE) infections is limited by a paucity of effective treatment options. Before 2015, frontline regimens included combinations of agents with high toxicity rates (aminoglycosides, colistin), suboptimal pharmacokinetics (aminoglycosides, colistin, tigecycline), and/or known microbiological resistance (carbapenems). In 2015, the U.S. Food and Drug Administration (FDA) approved ceftazidime-avibactam (C-A), a novel β-lactam/β-lactamase inhibitor with in vitro activity against CRE expressing Klebsiella pneumoniae carbapenemases (KPCs) but not Ambler class B or some class D β-lactamases. Since the majority of CRE infections in the United States are caused by KPC-producing K. pneumoniae, C-A may offer a significant improvement over previous treatment regimens. At present, however, there are no data directly comparing the outcomes of CRE-infected patients treated with C-A versus other regimens.
Shortly after FDA approval, C-A was endorsed at the University of Pittsburgh Medical Center (UPMC) as the frontline agent against CRE infections. Our objective in this study was to compare the outcomes of patients with carbapenem-resistant K. pneumoniae (CR-Kp) bacteremia who received definitive treatment with a regimen containing C-A or alternative regimens (carbapenem plus aminoglycoside [CB+AG], carbapenem plus colistin [CB+COL], or others [including monotherapy with AG or COL]).
We conducted a retrospective study of UPMC patients with CR-Kp bacteremia between January 2009 and February 2017 who received ≥3 days of treatment. CR-Kp was defined by resistance to any carbapenem (1); only the first episode of CR-Kp bacteremia was included. Clinical success was defined at 30 days as survival, resolution of signs and symptoms of infection, sterilization of blood cultures within 7 days of treatment initiation, and absence of recurrent infections.
MICs were determined using Clinical and Laboratory Standards Institute (CLSI) broth microdilution methods. Based on our previously published in vitro studies and relevant pharmacokinetic-pharmacodynamic (PK-PD) data, carbapenems were considered inactive if MICs were >8 μg/ml (2–4). Strains were tested for KPC variants and the presence of other β-lactamases as described previously (5).
Comparisons between groups were made by Fisher's exact or chi-square tests for categorical variables and the Mann-Whitney U test for continuous variables. The Kruskal-Wallis test was used to compare two or more groups. Multivariate logistic regression was performed by backward selection procedures to identify factors associated with clinical success.
One hundred nine patients with CR-Kp bacteremia were included (Table 1). Median age of the patients was 61 years (range, 25 to 91 years); 56% (61/109) of the patients were men. At the onset of bacteremia, 50% (55/109) of the patients resided in an intensive care unit. Median APACHE II and Pitt bacteremia scores were 18 (4 to 38) and 4 (0 to 9), respectively. Primary bacteremia was diagnosed in 26% (28/109) of the patients; secondary bacteremia resulted from abdominal (46%, 50/109), respiratory tract (13%, 14/109), urinary tract (13%, 14/109), or soft tissue (3%, 3/109) sites.
TABLE 1.
Patient characteristics and clinical outcomes across treatment groups
| Characteristica | Treatment groupb |
P value | |||
|---|---|---|---|---|---|
| C-A (n = 13) | CB+AG (n = 25) | CB+COL (n = 30) | Otherc (n = 41) | ||
| Patient demographics | |||||
| Male (n [%]) | 7 (54) | 16 (64) | 18 (60) | 21 (51) | 0.75 |
| Age (median [range]) | 66 (32–91) | 57 (32–87) | 59 (26–84) | 62 (25–90) | 0.63 |
| Underlying disease | |||||
| Diabetes (n [%]) | 4 (31) | 8 (32) | 8 (27) | 15 (37) | 0.85 |
| Chronic liver disease (n [%]) | 0 (0) | 9 (36) | 9 (30) | 13 (32) | 0.11 |
| Chronic respiratory disease (n [%]) | 5 (38) | 5 (20) | 8 (27) | 8 (20) | 0.51 |
| Immunocompromised (n [%]) | 5 (38) | 13 (52) | 14 (47) | 22 (54) | 0.78 |
| Solid-organ transplant recipient (n [%]) | 3 (23) | 11 (44) | 9 (30) | 17 (41) | 0.46 |
| Severity of illness | |||||
| ICU at time of bacteremia (n [%]) | 6 (46) | 13 (52) | 12 (40) | 25 (61) | 0.36 |
| RRT (n [%]) | 2 (15) | 7 (28) | 7 (23) | 8 (20) | 0.79 |
| Pitt bacteremia score (median [range]) | 4 (1–6) | 4 (0–9) | 4 (0–9) | 4 (0–9) | 0.74 |
| APACHE II score (median [range]) | 20 (16–33) | 17 (8–38) | 16 (7–36) | 19 (4–34) | 0.46 |
| Strain characteristic | |||||
| Presence of KPC (n [%]) | 13 (100) | 24 (96) | 30 (100) | 39 (95) | 0.56 |
| KPC-2 | 9 | 19 | 24 | 29 | |
| KPC-3 | 4 | 5 | 6 | 10 | |
| Primary bacteremia (n [%]) | 3 (23) | 6 (24) | 5 (17) | 14 (34) | 0.41 |
| Secondary bacteremia (n [%]) | 10 (77) | 19 (76) | 25 (83) | 27 (66) | 0.41 |
| Abdominal | 2 | 12 | 16 | 20 | |
| Respiratory | 3 | 2 | 6 | 3 | |
| Urinary tract | 5 | 2 | 2 | 4 | |
| Soft tissue | 0 | 3 | 1 | 0 | |
| Treatment characteristic | |||||
| ≥2 active agentsd (n [%]) | 5 (38)e | 10 (40)f | 9 (30) | 8 (20) | 0.28 |
| Time to active treatment (median [IQR]) | 55.7 (25–67) | 52.5 (28–64) | 67.9 (30–133) | 65.0 (35–95) | 0.23 |
| Duration of treatment (median days [range]) | 13 (5–23) | 12 (3–28) | 14 (3–96) | 10 (3–47) | 0.31 |
| Patient outcome | |||||
| Clinical success (n [%]) | 11 (85) | 12 (48) | 12 (40) | 15 (37) | 0.02g |
| 30-Day survival (n [%]) | 12 (92) | 17 (68) | 21 (70) | 28 (68) | 0.37 |
| 90-Day survival (n [%]) | 12 (92) | 14 (56) | 19 (63) | 20 (49) | 0.04h |
| Persistent bacteremia (n [%]) | 0 (0) | 1 (4) | 5 (17) | 8 (20) | 0.13 |
| Recurrent bacteremia (n [%]) | 2 (15) | 5 (20) | 3 (10) | 9 (22) | 0.60 |
| Drug toxicityi | |||||
| Acute kidney injury at 48 h (n [%]) | 1 (9) | 0 (0) | 7 (30) | 4 (12) | 0.10 |
| Acute kidney injury at 7 days (n [%]) | 1 (9) | 3 (17) | 10 (43) | 4 (12) | 0.02 |
| Acute kidney injury at end of treatment (n [%]) | 2 (18) | 8 (44) | 13 (57) | 6 (18) | 0.01 |
| Initiation of renal replacement therapy (n [%]) | 0 (0) | 0 (0) | 5 (22) | 3 (9) | |
| Time to start of RRT (median days [range]) | 5 (2–8) | 8 (5–8) | |||
ICU, intensive care unit; IQR, interquartile range; RRT, renal replacement therapy.
AG, aminoglycoside; C-A, ceftazidime-avibactam; CB, carbapenem; COL, colistin.
Other regimens included patients treated with monotherapy (n = 29) or combination therapy (n = 12). Monotherapy consisted of an AG (n = 11), CB (n = 8), COL (n = 4), tigecycline (n = 4), and ciprofloxacin (n = 2). Combination regimens were COL+tigecycline (n = 3), AG+tigecycline (n = 2), and 1 each of AG+cefepime, AG+COL+tigecycline, COL+aztreonam, COL+cefepime, COL+ciprofloxacin, CB+doxycycline, and CB+tigecycline.
Active agents were defined by in vitro susceptibility according to CLSI interpretive criteria for all agents except the carbapenems, which were defined as active in vitro if the MIC was ≤8 μg/ml.
Five patients received gentamicin in combination with C-A.
Six patients received CB+AG+COL.
Patients treated with C-A had higher rates of clinical success than those treated with CB+AG (P = 0.04), CB+COL (P = 0.009), or other regimens (P = 0.004). Clinical success rates were higher among patients treated with C-A than in those treated with all other regimens (P = 0.006).
Patients treated with C-A had higher rates of 90-day survival than those treated with CB+AG (P = 0.03) or other regimens (P = 0.008); there was a trend toward higher 90-day survival for C-A compared with CB+COL (P = 0.07). The 90-day survival rates were higher among patients treated with C-A than in those treated with all other regimens (P = 0.01).
Among patients not requiring RRT at baseline, which included those receiving CA (n = 11), CB+AG (n = 18), CB+COL (n = 23), or other regimens (n = 33). Acute kidney injury was defined by KDIGO criteria as a ≥0.3-mg/dl increase in serum creatinine from baseline at 48 h and as a 1.5× increase in serum creatinine from baseline at 7 days or the end of treatment.
Ninety-seven percent (106/109) of the isolates harbored blaKPC, of which 76% (81/106) and 24% (25/106) encoded KPC-2 and KPC-3 subtypes, respectively. All isolates were meropenem resistant (median MIC, 32 μg/ml; range, 4 to 512 μg/ml). Eighty-four percent (92/109) and 76% (83/109) of isolates were susceptible to COL and gentamicin, respectively. Among baseline CR-Kp isolates from patients treated with C-A, the median C-A MIC was 1 μg/ml (range, 0.25 to 2 μg/ml); all isolates were susceptible according to the FDA breakpoint (MIC, ≤8 μg/ml).
Thirty- and 90-day mortality rates were 28% (31/109) and 40% (44/109), respectively. Clinical success at 30 days was achieved in 46% (50/109) of patients. Treatment failures were due to death (n = 31; 4 patients had persistent bacteremia before death), recurrent CR-Kp infection (n = 18), or persistent bacteremia (n = 10). Bacteremia recurred within 90 days in 17% (19/109) of the patients, at a median of 42 days (range, 13 to 90 days). Definitive treatment was initiated with monotherapy or combination therapy in 33% (36/109) and 67% (73/109), respectively. Treatment regimens included ≥2, 1, or 0 in vitro active agents in 29% (32/109), 60% (65/109), and 11% (12/109) of the patients, respectively. Seventeen percent (19/109) of patients received in vitro active empirical therapy. The median time to in vitro active treatment initiation was 58 h (interquartile range [IQR], 29.5 to 92.0) after the first positive blood culture.
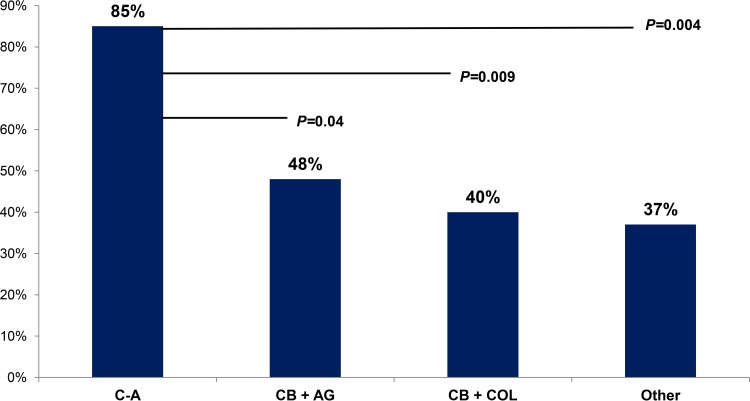
Across treatment groups, there were no differences in underlying diseases, severity of illness, source of bacteremia, or strain characteristics (Table 1). Treatment regimens included C-A (n = 13), CB+AG (n = 25), CB+COL (n = 30), and others (n = 41); the corresponding clinical success rates by regimen were 85% (11/13), 48% (12/25), 40% (12/30), and 37% (15/41), respectively (Fig. 1; P = 0.02). C-A was administered as monotherapy (n = 8) or in combination with gentamicin (n = 5; median duration of gentamicin, 4 days; range, 3 to 7 days); corresponding success rates were 75% (6/8) and 100% (5/5), respectively (Table 2).
FIG 1.
Rates of 30-day clinical success across treatment regimens. Among patients with carbapenem-resistant Klebsiella pneumoniae bacteremia, rates of clinical success were significantly higher among patients receiving ceftazidime-avibactam than among those who received a carbapenem plus aminoglycoside (P = 0.04) or colistin (P = 0.009) or other regimens (P = 0.004). Other regimens included aminoglycoside (n = 11), carbapenem (n = 8), colistin (n = 4), tigecycline (n = 4), and ciprofloxacin (n = 2) monotherapy, as well as combination regimens of colistin plus tigecycline (n = 3), aminoglycoside plus tigecycline (n = 2), and 1 each of aminoglycoside plus cefepime, aminoglycoside plus colistin plus tigecycline, colistin plus aztreonam, colistin plus cefepime, colistin plus ciprofloxacin, carbapenem plus doxycycline, and carbapenem plus tigecycline.
TABLE 2.
Clinical characteristics of patients with CR-Kp bacteremia treated with ceftazidime-avibactam
| Agea (sex) | Underlying diseasea | KPC variant | C-A MIC (μg/ml) | Creatinine clearance (ml/min)b | Source of bacteremia | Treatment regimen (days duration) | Clinical outcome at 30 days | Clinical outcome at 90 days |
|---|---|---|---|---|---|---|---|---|
| 47 (M) | Renal transplantation | KPC-2 | 1 | 64 | Urine | C-A (16) | Clinical success | Alive |
| 68 (F) | Stem cell transplantation | KPC-2 | 0.5 | 25 | Urine | C-A (19) plus gentamicin (5) | Clinical success | Alive |
| 75 (F) | Seizure disorder, chronic PEG/trach | KPC-2 | 0.25 | 47 | Urine | C-A (13) plus gentamicin (4) | Clinical success | Alive |
| 33 (M) | Paraplegia s/p hemorrhagic stroke | KPC-2 | 0.5 | >120 | Central venous catheter | C-A (8) plus gentamicin (3) | Clinical success | Alive |
| 79 (M) | Trauma leading to splenectomy | KPC-3 | 1 | 46 | Respiratory | C-A (13) | Recurrent pneumonia and death | Dead |
| 60 (F) | Double lung transplantation | KPC-3 | 1 | iHD | Central venous catheter | C-A (24) | Clinical success | Alive |
| 67 (M) | Short-gut syndrome | KPC-3 | 2 | 51 | Abdominal | C-A (4) plus gentamicin (7) | Clinical success | Alive |
| 91 (F) | Dementia | KPC-2 | 1 | 48 | Urine | C-A (7) followed by doripenem and gentamicin (7)c | Clinical success | Alive |
| 66 (M) | Charcot-Marie-Tooth disease | KPC-2 | 1 | >120 | Respiratory | C-A (14) | Recurrent bacteremia and survived | Alive |
| 68 (F) | Intracranial hemorrhage | KPC-2 | 1 | 56 | Urine | C-A (21) | Clinical success | Alive |
| 65 (F) | Schizophrenia | KPC-2 | 1 | 14 | Abdominal | C-A (15) | Clinical success | Recurrent bacteremia and survived |
| 52 (M) | Renal and pancreas transplantation | KPC-3 | 2 | iHD | Central venous catheter | C-A (13) plus gentamicin (3) | Clinical success | Alive |
| 32 (M) | Intravenous drug user | KPC-2 | 0.5 | 63 | Respiratory | C-A (13) | Clinical success | Alive |
F, female; iHD, intermittent hemodialysis; M, male; PEG, percutaneous endoscopic gastrostomy; s/p, status post.
All ceftazidime-avibactam doses were adjusted according to manufacturer recommendations.
Patient was treated successfully with C-A for 7 days but switched to doripenem plus gentamicin on discharge due to an ongoing national shortage of C-A.
Clinical success was achieved more frequently among patients treated with a regimen including C-A than with other regimens (P = 0.006), including those comprised of ≥2 in vitro active agents (44% [12/27]; P = 0.02). By multivariable logistic regression, primary bacteremia (odds ratio [OR], 4.50; 95% confidence interval [CI], 1.53 to 13.21; P = 0.006) and receipt of C-A (OR, 8.64; 95% CI, 1.61 to 43.39; P = 0.01) were independent predictors of clinical success (Table 3).
TABLE 3.
Multivariable analysis of factors associated with clinical success at 30 days
| Factora | Treatment outcome |
P value | Multivariate P value (OR, 95% CI)a | |
|---|---|---|---|---|
| Success (n = 50) | Failure (n = 59) | |||
| Male (n [%]) | 26 (52) | 36 (61) | 0.34 | |
| Age (median [range]) | 59 (26–91) | 61 (25–85) | 0.49 | |
| Diabetes (n [%]) | 13 (26) | 22 (37) | 0.21 | |
| Chronic liver disease (n [%]) | 11 (22) | 20 (34) | 0.17 | |
| Chronic respiratory disease (n [%]) | 14 (28) | 12 (20) | 0.35 | |
| Immunocompromised (n [%]) | 25 (50) | 29 (49) | 0.93 | |
| Any malignancy (n [%]) | 7 (14) | 17 (29) | 0.06 | 0.10 |
| Solid-organ transplant recipient (n [%]) | 18 (36) | 22 (37) | 0.89 | |
| Primary bacteremia (n [%]) | 19 (38) | 9 (15) | 0.007 | 0.006 (4.50, 1.53–13.21) |
| Renal replacement therapy (n [%]) | 6 (12) | 18 (31) | 0.02 | 0.20 |
| Pitt bacteremia score (median [range]) | 3 (0–9) | 5 (0–9) | 0.003 | 0.15 |
| APACHE II score (median [range]) | 17 (7–38) | 21 (4–36) | 0.02 | Excluded for confounding |
| ICU (n [%]) | 21 (42) | 35 (59) | 0.07 | 0.24 |
| Treatment with ≥2 active agents (n [%]) | 21 (42) | 11 (19) | 0.008 | Excluded for confounding |
| Time to active treatment (median [IQR]) | 55.6 (23.5–74.6) | 66 (34.3–105) | 0.08 | 0.28 |
| Treatment with C-A (n [%]) | 11 (22) | 2 (3) | 0.003 | 0.01 (8.64, 1.61–46.39) |
CI, confidence interval; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
Survival rates at 30 and 90 days were 92% (12/13) among patients receiving C-A regimens versus 69% (66/96) and 55% (53/96), respectively, among patients receiving any other regimen (P = 0.10 and 0.01, respectively). Survival rates were 87.5% (7/8) and 100% (5/5) among patients receiving C-A alone or in combination with gentamicin, respectively.
Twenty-two percent (24/109) of the patients required renal replacement therapy at baseline. Among the remaining patients, 21% (18/85) and 34% (29/85) developed acute kidney injury (AKI) by 7 days and end of treatment (EOT), respectively (Table 1). At EOT, AKI rates were 18% (2/11), 44% (8/18), 57% (13/23), and 18% (6/33) for C-A, CB+AG, CB+COL, and other regimens, respectively. EOT AKI rates were 25% (1/4) and 14% (1/7) for patients receiving C-A with and without an AG, respectively. Across groups, EOT AKI rates were significantly higher among patients receiving an AG or COL (42% [28/66]) than among patients not receiving these agents (5% [1/19]; P = 0.002). AKI rates were 26% (8/31), 55% (16/29), and 80% (4/5) for patients receiving an AG, COL, or both, respectively. AKI was significantly more common with COL-containing than with AG-containing regimens at 7 days (38% [11/29] versus 10% [3/31]; P = 0.01) and EOT (55% [16/29] versus 26% [8/31]; P = 0.03).
To our knowledge, this is the first study to compare the clinical outcomes of CRE-infected patients treated with C-A with those of patients treated with other regimens. Our most noteworthy finding is that clinical success and survival were significantly improved for patients with CR-Kp bacteremia who received C-A. These data build on findings from early observational reports, in which overall clinical response rates with C-A ranged from 53% to 68% across CRE infection types (6–8). Among previously reported patients with CRE bacteremia, the composite success rate was 69% (48/70) (6–9). This figure and our experience compare favorably with those reported for patients with bacteremia treated with ≥2 in vitro active agents, where survival rates ranged from 60% to 82% (3, 10). Compared to AG and COL, C-A demonstrates more reliable in vitro activity against KPC-producing CRE, exhibits more favorable PK characteristics, and is well tolerated. Indeed, rates of AKI were significantly higher among our patients who received AG- or COL-containing regimens (Table 1). Taken together, the data from this study and others establish that C-A is an important advance in the treatment of CRE infections.
The improved outcomes we observed following the introduction of C-A in 2015 stand in contrast to data from a recent multicenter study at hospitals in regions of New York/New Jersey where CRE is endemic. At these centers, mortality rates for CRE bacteremia in 2013 (before availability of C-A) were unchanged from those a decade earlier (11). Notably, time to receipt of active treatment in our study was not an independent predictor of clinical success or survival. Indeed, the rather lengthy times to receipt of an in vitro active agent (median, 58 h [IQR, 29.5 to 92 h]) or C-A specifically (median, 55.7 h [IQR, 25 to 67 h]) suggest an area for future improvement. In this regard, it will be important to determine whether rapid CRE detection assays can shorten times to appropriate treatment and improve outcomes.
To date, clinical response rates have been similar for CRE-infected patients treated with C-A monotherapy or combination therapy (6–8). However, as in our experience here, sample sizes in earlier reports were small, and indication biases cannot be excluded. We used combination therapy with short-course gentamicin followed by deescalation to C-A alone in five patients, which may represent a strategy for maximizing treatment effectiveness while limiting toxicity. In fact, AKI occurred at 7 days in only 10% of patients receiving an AG. Clearly, the roles of combination regimens in treating various types of CRE infections merit further investigation. In particular, it will be important to determine if combination therapy (and shorter time to active treatment) can improve outcomes among patients with CRE pneumonia, in whom success rates with C-A have been consistently lower than for patients with bacteremia (6, 7), and suppress the emergence of C-A resistance, which has been noted after short courses of treatment (12).
Because our study was not a randomized controlled trial (RCT), bias in selection of therapy existed. Furthermore, treatment regimens were not blinded to investigators at the time of review. Nevertheless, we provide evidence to support C-A as the frontline agent for treatment of CR-Kp bacteremia. C-A treatment failures, particularly for infections such as pneumonia, and reports of emerging resistance among CRE isolates attest to the ongoing need for new drugs. Along these lines, recently completed RCTs of two antibiotics in development, meropenem-vaborbactam and plazomicin, may provide further clarity on the efficacy of newer agents compared with traditional CRE treatment regimens.
ACKNOWLEDGMENTS
We are indebted to Lloyd G. Clarke for assistance in data collection.
This work was funded, in part, by grants from the National Institutes of Health (K08AI114883 to R.K.S., R21AI117338 to L.C., R01AI090155 to B.N.K., R21AI128338 to M.H.N., and R21AI111037 to C.J.C.).
We declare no conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2016. Healthcare-associated infections (HAIs): FAQs about choosing and implementing a CRE definition. https://www.cdc.gov/hai/organisms/cre/definition.html Accessed 6 April 6.
- 2.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 4.Clancy CJ, Chen L, Hong JH, Cheng S, Hao B, Shields RK, Farrell AN, Doi Y, Zhao Y, Perlin DS, Kreiswirth BN, Nguyen MH. 2013. Mutations of the ompK36 porin gene and promoter impact responses of sequence type 258, KPC-2-producing Klebsiella pneumoniae strains to doripenem and doripenem-colistin. Antimicrob Agents Chemother 57:5258–5265. doi: 10.1128/AAC.01069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy CJ, Chen L, Shields RK, Zhao Y, Cheng S, Chavda KD, Hao B, Hong JH, Doi Y, Kwak EJ, Silveira FP, Abdel-Massih R, Bogdanovich T, Humar A, Perlin DS, Kreiswirth BN, Hong Nguyen M. 2013. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant 13:2619–2633. doi: 10.1111/ajt.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, Gilarranz R, Jeremiah C, Loeches B, Machuca I, Jimenez-Martin MJ, Martinez JA, Mora-Rillo M, Navas E, Osthoff M, Pozo JC, Ramos Ramos JC, Rodriguez M, Sanchez-Garcia M, Viale P, Wolff M, Carmeli Y. 2017. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 61:e01964-16. doi: 10.1128/AAC.01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King M, Heil E, Kuriakose S, Bias T, Huang V, El-Beyrouty C, McCoy D, Hiles J, Richards L, Gardner J, Harrington N, Biason K, Gallagher JC. 2016. Outcomes of ceftazidime/avibactam in patients with carbapenem-resistant Enterobacteriaceae (CRE) infections: a multi-center study, abstr. 2034. Abstr ID Week 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu G, Abraham T, Lee S. 2016. ceftazidime-avibactam for treatment of carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 63:1147–1148. doi: 10.1093/cid/ciw491. [DOI] [PubMed] [Google Scholar]
- 10.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, Isgri S. 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 11.Satlin MJ, Chen L, Patel G, Gomez-Simmonds A, Weston G, Kim AC, Seo SK, Rosenthal ME, Sperber SJ, Jenkins SG, Hamula CL, Uhlemann AC, Levi MH, Fries BC, Tang YW, Juretschko S, Rojtman AD, Hong T, Mathema B, Jacobs MR, Walsh TJ, Bonomo RA, Kreiswirth BN. 2017. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother 61:e02349-16. doi: 10.1128/AAC.02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]