ABSTRACT
Studies with methicillin-resistant Staphylococcus aureus (MRSA) strain COL have shown that the optimal resistance phenotype requires not only mecA but also a large number of “auxiliary genes” identified by Tn551 mutagenesis. The majority of auxiliary mutants showed greatly increased levels of oxacillin resistance when grown in the presence of sub-MICs of mupirocin, suggesting that the mechanism of reduced resistance in the auxiliary mutants involved the interruption of a stringent stress response, causing reduced production of penicillin-binding protein 2A (PBP 2A).
KEYWORDS: stress response, auxiliary genes, oxacillin resistance expression
TEXT
All methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates carry the mecA determinant, most often accompanied by the regulatory genes mecI and mecR and/or blaI and blaR (1, 2), which control expression of the oxacillin resistance phenotype. Transposon inactivation studies identified a large number of additional genes that are not directly involved in the transcription or translation of mecA and yet had a profound effect on the oxacillin resistance phenotype (3, 4). The earlier studies (4, 5) identified five such genes in the so-called “fem” (factors essential for methicillin resistance) mutants. A subsequent and more extensive transposon (Tn551) mutagenesis study produced a surprisingly large library of additional so-called “auxiliary mutants,” in which the resistance level of the parental MRSA strain COL was reduced by ≥10-fold (3, 6). Strain COL expresses antibiotic resistance in a homogeneous fashion: all cells in a culture share a very high MIC value of 800 μg/ml oxacillin. In this strain, the transposon inactivation library identified as many as 35 loci each with major impacts on resistance, resulting in reduced and heterogeneous resistance to oxacillin (3, 6). This heterogeneous phenotype of auxiliary mutants is reminiscent of the antibiotic resistance phenotypes of most clinical MRSA strains (7–9).
While most auxiliary genes were identified in the laboratory by transposon mutagenesis of strain COL, the impact of mutations in numerous genetic determinants on the oxacillin resistance phenotype is not restricted to this library of Tn551 mutants but was also detected in other S. aureus genetic backgrounds (see Table S1). Several auxiliary genes identified by Tn551 mutagenesis exhibited reduced resistance level by orders of magnitude of 1 μg/ml or less (Table 1).
TABLE 1.
Induction of stringent stress response by mupirocin: impact on the resistance level and relative amounts of PBP 2A in auxiliary mutantsa
| Mutation no.b | Strain or mutant | Inactivated gene | MIC of oxacillin (μg/ml) |
Relative amt of PBP 2A (%)c |
||
|---|---|---|---|---|---|---|
| None | With mupirocind | None | With mupirocind | |||
| COL | None | >256 | >256 | 100e | 100 | |
| 1 | RUSA162 | SACOL0181 | 32 | >256 | 30 | 110 |
| 5 | RUSA262 | SACOL0830 | 1.5 | 128 | 10 | 90 |
| 6 | RUSA235 | murE (femF) | 1 | >256 | 20 | 95 |
| 8 | SABS1 | ptc1 | 0.38 | >256 | ND | ND |
| 11 | RUSA209 | glnR (femC) | 0.19 | >256 | 10 | 100 |
| 12 | RUSA164 | tkt | 16 | >256 | ND | ND |
| 15 | RUSA279 | trpG | 64 | >256 | ND | ND |
| 16 | BB308 | femA | 0.094 | 0.094 | ND | ND |
| 17 | RUSA10 | femB | 0.032 | 0.064 | 0.5 | 5 |
| 19 | RUSA239 | lysA | 0.25 | >256 | 25 | 120 |
| 21 | RUSA130 | pbp2 | 48 | >256 | 30 | 100 |
| 24 | RUSA188 | pepV | 64 | >256 | ND | ND |
| 31 | RUSA315 | glmM (femD) | 0.094 | >256 | 0.5 | 95 |
Determination of MIC and amount of PBP 2A was performed three times independently, with similar results.
Mutation numbers as shown in Table S1.
ND, not determined.
Mupirocin (0.03 μg/ml) was added to the growth medium as a reagent to induce the stringent stress response (11).
The amount of PBP 2A in COL was set to 100% as a control.
The parental strain COL carries the mecA determinant on a type I staphylococcal cassette chromosome mec element (SCCmec) (10); the strain does not have any mecA regulatory genes (mecI and mecR) or regulatory determinants of penicillin resistance (blaI and blaR), and appropriate experiments demonstrated that mecA was intact in each of the auxiliary mutants (6). Thus, the massive drop in β-lactam antibiotic resistance by mutations in auxiliary genes was surprising, since most of these genes were involved in diverse metabolic functions not associated with β-lactam resistance.
In a recent study, we used whole-genome sequencing to compare isogenic strains of MRSA that differed only in their degrees of oxacillin resistance. Heterogeneously resistant MRSA strains were converted to H*R mutants, which showed homogeneous and high-level antibiotic resistance associated with a variety of different mutations (11, 12). A major result of this study was identification of the relA gene and the stringent stress response as critical contributors to the resistance phenotypes of MRSA strains (11–14). In addition to the relA gene, rpoB, encoding the β-subunit of RNA polymerase, was another important determinant inducing a stringent stress response. An rpoB mutant showed a stringent response profile even in the absence of the inducing agent, guanosine tetraphosphate (ppGpp) (15).
Based on the comparison of 27 mutated genes in H*R mutants with those identified in strain COL, it was possible to show that COL carried mutations in four genes relevant for the resistance phenotype: in prsA (ribose-phosphate pyrophosphokinase), in gltX (glutamyl-tRNA synthetase), in rplK (ribosomal protein L11), and in rpoB (the β-subunit of DNA-directed RNA polymerase) (12). Strain COL also exhibits slow growth, which is a typical phenotype of bacteria under stress. These observations suggest that in the parental strain COL, the stringent stress response seems to be turned “on” without any external inducers. We used this information to reexamine the resistance phenotypes of several auxiliary mutants.
We first determined the basal level of guanosine pentaphosphate [(p)ppGpp] in the parental strain COL, in an auxiliary mutant, RUSA239, and in an archaic MRSA strain, UK13136, used as a control. COL maintained a 1.4-fold higher (p)ppGpp level than RUSA239 and UK13136 (Fig. 1). In addition to the mutation in rpoB, this subtle elevation of (p)ppGpp in COL rendered a substantial increase in oxacillin resistance. The auxiliary mutant RUSA239 exhibited significantly decreased MIC value of oxacillin (0.25 μg/ml), and the inactivated gene (SACOL1435) was identified as lysA (Table S1). The lysA gene encodes a lysine biosynthesis protein, diaminopimelate decarboxylase. It has been reported that inactivation of lysA reduced the activity of σB factor (16, 17). The knockout of sigB in COL drastically reduced the oxacillin MIC from 800 μg/ml to 100 to 200 μg/ml (18, 19). The σB factor is one of the cell wall stress response regulators linked to antimicrobial resistance (20). Inactivation of lysA in RUSA239 seems to make the stringent response profile of COL relaxed through the reduced activity of the σB factor, resulting in a decrease in (p)ppGpp. Moreover, induction of the stringent stress by mupirocin was able to increase the antibiotic resistance of each of the auxiliary mutants to the level of the parental strain COL (Table 1), suggesting that induction of the stringent stress response is one of the key mechanisms responsible for the antibiotic resistance level of MRSA isolates. These observations also indicate that most auxiliary mutants are able to “interrupt” or “relax” the stringent stress response of strain COL by somehow reducing the (p)ppGpp level. In two mutants, the femA and femB mutants, which are responsible for incorporation of five glycine residues into the pentapeptide of lipid II, the induction of stringent stress did not “correct” the low MIC value. These particular mutants are known to carry alterations in the primary structure of the cell wall peptidoglycan (21, 22).
FIG 1.
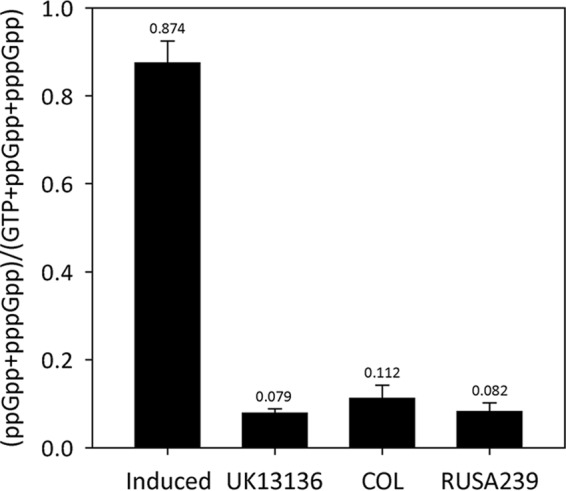
Basal levels of (p)ppGpp in UK13136, COL, and RUSA239. COL exhibited homogeneous oxacillin resistance, while a heterogeneous resistance phenotype was shown by UK13136, an archaic MRSA isolate, and RUSA239, an auxiliary mutant of COL. The amount of (p)ppGpp in COL was determined to be about 1.4-fold higher than in UK13136 and RUSA239. “Induced” (UK13136 plus mupirocin) in the first column is a positive control for (p)ppGpp induction by 60 μg/ml mupirocin. The experiment was carried out triplicate, and the averages and standard deviations of the data are shown in the graph.
As the stringent stress response induces PBP 2A production (23), we examined the transcription and translation of the mecA determinant in two auxiliary mutants, RUSA239 and RUSA262, in which the inactivated genes are SACOL1435 and SACOL0830, respectively (6). The mecA transcripts in these auxiliary mutants were maintained at the same level as in the parental strain COL (Fig. 2A). Surprisingly, the inactivation of auxiliary genes caused reduced PBP 2A expression by as much as 5- and 10-fold in RUSA239 and RUSA262, respectively, compared with that of strain COL (Fig. 2B), resulting in decreased oxacillin resistance. This suggests that relaxation of the stringent stress response in auxiliary mutants causes a reduction in PBP 2A production on the translational or posttranslational level but not on the level of transcription. Moreover, the mecA mRNA in the mutants was increased at most 2-fold in the presence of mupirocin, while the amount of PBP 2A was increased 6- to 9-fold in these mutants, compared to their level in the absence of mupirocin (Fig. 2A and B). This finding implies that the mupirocin-induced stringent stress response affected the translational or posttranslational level but not transcription of the mecA determinant in auxiliary mutants RUSA239 and RUSA262. The recovery of antibiotic resistance to parental levels in mutants RUSA239 and RUSA262 (and in most of the other auxiliary mutants as well) by mupirocin is most likely related to the increased production of PBP 2A (Table 1) through an unknown mechanism facilitating the translation or membrane integration of PBP 2A.
FIG 2.
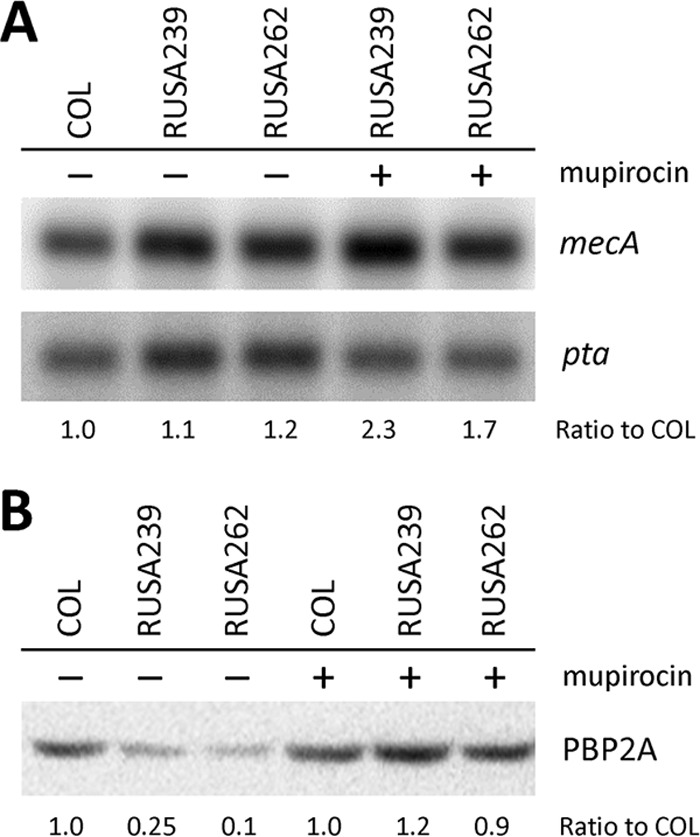
Relative amounts of mecA mRNA and PBP 2A in two auxiliary mutants. (A) The mecA transcript of parental strain COL (lane 1) is the same level at those of auxiliary mutants RUSA239 (lane 2) and RUSA262 (lane 3) in the absence of mupirocin. In mupirocin-treated auxiliary mutants (lanes 4 and 5), the mecA mRNA was increased at most 2-fold compared with mupirocin-untreated ones (lanes 2 and 3). (B) Relative amounts of PBP 2A on the cytoplasmic membrane of COL (lanes 1 and 4), RUSA239 (lanes 2 and 5), and RUSA262 (lanes 3 and 6) were determined by Western blotting. The PBP 2A intensity of COL without mupirocin was used as a standard to calculate the relative amounts of PBP 2A. In the absence of mupirocin, PBP 2A in RUSA239 (lane 2) and RUSA262 (lane 3) were lower by as much as 5- and 10-fold, respectively, compared to that in COL (lane 1). PBP 2A in auxiliary mutants (lanes 5 and 6) was elevated to the same level as COL by mupirocin. PBP 2A amount in COL was consistent regardless of mupirocin treatment. The ratios of mecA mRNA and PBP 2A were calculated through three independent experiments. The average of each ratio is shown in the figure.
The profound effect of induction of the stringent stress response by mupirocin on the antibiotic resistance phenotype of S. aureus was demonstrated in strains in which the phenotype was based on either the mecA or in the more recently identified mecC determinant (Fig. S1A) (24). Induction of the stringent stress response also increased β-lactam resistance in S. sciuri strain SS37 (Fig. S1B), which carries mecA1, an evolutionary precursor of mecA (25–27). In contrast, the oxacillin MIC value remained unaltered (MIC, 2 μg/ml) in the presence of mupirocin in strain M100 (a laboratory mutant of MSSA strain 27s), in which the mechanism of resistance is not associated with mecA but with a mutation(s) in pbpC encoding the native PBP 3 protein (28, 29). The introduction of three different auxiliary mutations into strain M100 caused only a minor reduction in MIC value (Table S2), in contrast to the massive change in resistance level demonstrated in the background of MRSA strain COL.
In conclusion, strain COL exhibits the stringent response profile regardless of external stress, such as nutrient starvation, cell wall-active agents (i.e., β-lactams), and high salt, because it carries mutations in rpoB as well as in prsA, gltX, and rplK. The mecA gene is a key determinant of antibiotic resistance in all MRSA strains, including COL. Inactivation of auxiliary genes in strain COL may cause shutting off of the rpoB-induced stringent response, resulting in a phenotype that is typical of most MRSA strains: low PBP 2A production and a heterogeneous resistance profile. Auxiliary mutants are able to revert their phenotypes to that of the parental strain COL when the ppGpp-dependent stringent stress response is turned on by mupirocin (Fig. 3).
FIG 3.
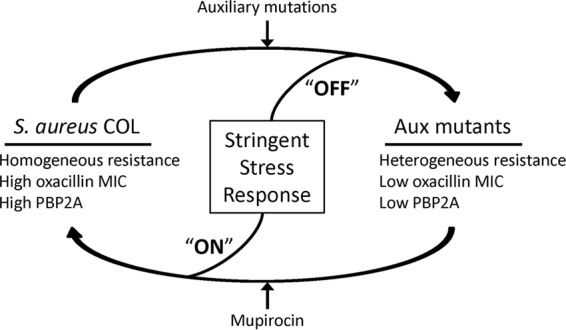
Proposed model for the mechanism of lower resistance in auxiliary (Aux) mutants.
Auxiliary genes are critical factors for resistance of MRSA strains to β-lactam antibiotics by ensuring an optimal level of resistance. Many auxiliary genes required for high-level β-lactam resistance are involved in important cellular events, such as cell wall biosynthesis and cell division. Investigators have tried to develop compounds that target auxiliary factors and show synergistic antimicrobial activities with well-known β-lactam drugs (30). An important and novel line of investigation is focusing on the discovery of inhibitors that shut off the stringent stress response and target GTP pyrophosphokinase encoded by relA and on synthesizing (p)ppGpp (31). A combination of these inhibitors with β-lactam antibiotics may provide novel ways to overcome the potential toxicity of the drugs by rendering better efficacy at a lower dosage. The discovery of inhibitors against auxiliary factors should also broaden the spectrum of targets for the treatment of drug-resistant staphylococcal infections.
MATERIALS AND METHODS
Susceptibility of auxiliary mutants to oxacillin.
Tests for the susceptibility of S. aureus COL and the auxiliary mutants to oxacillin were done by the Etest (bioMérieux, Inc.) that involved spreading small aliquots of overnight cultures (diluted to an optical density at 620 nm [OD620] of 0.08) on tryptic soy agar (TSA) plates (occasionally also containing 0.03 μg/ml mupirocin to induce the stringent stress response), followed by placing oxacillin Etest strips on the plates (11). The MIC values of oxacillin were evaluated after incubation at 37°C for 24 h, according to the Etest reading guide distributed by bioMérieux, Inc. In more-detailed tests of susceptibility, cultures were plated for population analysis profiles (PAPs) (8) on TSA plates containing a range of concentrations of oxacillin with or without mupirocin in the agar medium.
Determination of the basal level of (p)ppGpp.
The basal level of (p)ppGpp (GDP, 3′-di-phosphate) in an archaic MRSA UK13136 strain, COL, and an auxiliary mutant (RUSA239) was determined as previously described, with some modification (14). A 5-ml culture of cells grown in low-phosphate medium containing 100 μCi/ml [32P]orthophosphate was harvested at an OD of 0.5 and extracted with 50 μl of 5 M formic acid by repeating freeze/thaw cycles four times, followed by incubation for 30 min on ice. Cells were removed by centrifugation at 20,000 × g for 5 min at 4°C. The extracted samples (15 μl each) were spotted on polyethyleneimine-cellulose F thin-layer chromatography (TLC) plates, which were developed with 1.5 M monopotassium phosphate (pH 3.5) to separate the phosphorylated nucleotides. 32Pi-labeled nucleotides were visualized with a Typhoon9400 image scanner, and nucleotide spots were quantified by the ImageQuant software. The amounts of (p)ppGpp were described as fractions of total guanine nucleotides, including GTP and (p)ppGpp.
The sample extracted from UK13136 treated with 60 μg/ml mupirocin at 15 min before harvest was used as a positive control for the identification of (p)ppGpp spots on TLC plates.
Transduction of three auxiliary mutations into strain M100, a methicillin-resistant laboratory construct.
Auxiliary mutations identified in mutant strains RUSA130, RUSA235, and RUSA262 were transduced with phage 80α, as previously described (32), into the recipient strain M100 (28, 29). Transductants were selected using erythromycin.
Northern blotting of the mecA gene.
In order to determine the effect of mupirocin on mecA gene transcription, Northern blotting was carried out as previously described, with slight modifications (11). The parental strain COL and auxiliary mutants RUSA239 and RUSA262 (3, 6) were grown overnight and then diluted 1:200 in fresh tryptic soy broth (TSB) with and without 0.03 μg/ml mupirocin. The bacterial cultures were collected for total RNA extraction when the culture density (OD620) reached 0.75. Total RNAs were isolated using the RNeasy kit (Qiagen), according to the manufacturer's instructions. RNA (2.5 μg) from each strain was loaded for electrophoresis on a 1.2% agarose-0.7 M formaldehyde gel in 0.5× morpholinepropanesulfonic acid (MOPS) running buffer. Separated RNAs were transferred to a nylon membrane using TurboBlotter (Whatman) and fixed by UV cross-linking, and then the ethidium bromide-stained RNAs were visualized by UV illumination of the transfer membrane in order to quantify the transferred RNAs. The probe used for detecting the mecA transcripts was a 500-bp PCR product of chromosomal DNA from S. aureus strain COL. The probe was labeled using Amersham Ready-To-Go DNA labeling beads (GE Healthcare) with [α-32P]-dCTP. The probe was hybridized with RNA at 65°C in an SDS hybridization solution containing 5× SSPE (1× SSPE is 0.18 NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]; Invitrogen), 5× Denhardt's reagent (Invitrogen), 0.5% SDS, and 100 μg/ml salmon sperm DNA (Invitrogen). The washed membrane was exposed to a phosphor screen for 5 h and scanned on a Typhoon9400 image scanner.
Titration of PBP 2A on the plasma membrane.
Membrane fractions were prepared from S. aureus COL and its auxiliary mutant derivatives, according to the method described previously (12). The strains were grown in 200 ml of TSB with and without 0.03 μg/ml mupirocin at 37°C. The relative amounts of PBP 2A on the plasma membrane were determined in each of the isolates using Western blotting. All cultures were harvested at an OD620 of 0.5, washed, and resuspended in 3 ml of 20 mM Tris-HCl (pH 7.6) containing 1× Halt protease inhibitor cocktail (Thermo Fisher Scientific, Inc.), 10 mM MgCl2, 100 μg/ml lysostaphin, 100 μg/ml lysozyme, 50 μg/ml DNase I, and 50 μg/ml RNase A. The cells were incubated for 1 h on ice and disrupted by sonication with a pulse of 40% output for 5 min. The supernatants were transferred to ultracentrifuge tubes after centrifugation at 7,000 × g for 20 min. Membrane fractions were collected by centrifugation at 100,000 × g for 1 h. The collected membranes were resuspended in 20 mM Tris-HCl (pH 7.6) and stored at −70°C. The concentration of total membrane proteins was determined using a bicinchoninic acid (BCA) assay.
Membrane proteins (80 μg) were loaded on the polyacrylamide gel (8% resolving gel, 4% stacking gel) for SDS-PAGE. The primary anti-PBP 2A antibody and the secondary horseradish peroxidase (HRP)-conjugated antibody (0.5 mg/ml; PerkinElmer) were diluted to 1:15,000 and 1:10,000, respectively.
ChromPure human IgG Fc fragment (Millipore) was added to the blocking solution at a final concentration of 3 μg/ml in order to prevent the antibodies from nonspecific binding. Pierce ECL 2 substrate (Thermo Fisher Scientific, Inc.) was used for visualization of PBP 2A bands after the X-ray film exposure.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the U.S. Public Health Service, 2 RO1 AI457838-15, by grant UL1 TR000043-07S1 from the National Center for Advancing Translational Sciences (NCATS; National Institutes of Health [NIH] Clinical and Translational Science Award [CTSA]) program awarded to A. Tomasz, by project LISBOA-01-0145-FEDER-007660 (Microbiologia Molecular, Estrutural e Celular) funded by FEDER funds through COMPETE2020-Programa Operacional Competitividade e Internacionalização (POCI), and by national funds through the Fundação para a Ciência e a Tecnologia (FCT). C. Milheiriço was supported by grants SFRH/BPD/63992/2009 and SFRH/BPD/111697/2015 from the FCT, Portugal.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00313-17.
REFERENCES
- 1.Hackbarth CJ, Chambers HF. 1993. blaI and blaR1 regulate beta-lactamase and PBP 2a production in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 37:1144–1149. doi: 10.1128/AAC.37.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiramatsu K, Asada K, Suzuki E, Okonogi K, Yokota T. 1992. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS Lett 298:133–136. doi: 10.1016/0014-5793(92)80039-J. [DOI] [PubMed] [Google Scholar]
- 3.de Lencastre H, Tomasz A. 1994. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 38:2590–2598. doi: 10.1128/AAC.38.11.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger-Bächi B. 1983. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol 154:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornblum J, Hartman BJ, Novick RP, Tomasz A. 1986. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol 5:714–718. doi: 10.1007/BF02013311. [DOI] [PubMed] [Google Scholar]
- 6.De Lencastre H, Wu SW, Pinho MG, Ludovice AM, Filipe S, Gardete S, Sobral R, Gill S, Chung M, Tomasz A. 1999. Antibiotic resistance as a stress response: complete sequencing of a large number of chromosomal loci in Staphylococcus aureus strain COL that impact on the expression of resistance to methicillin. Microb Drug Resist 5:163–175. doi: 10.1089/mdr.1999.5.163. [DOI] [PubMed] [Google Scholar]
- 7.Chung M, Antignac A, Kim C, Tomasz A. 2008. Comparative study of the susceptibilities of major epidemic clones of methicillin-resistant Staphylococcus aureus to oxacillin and to the new broad-spectrum cephalosporin ceftobiprole. Antimicrob Agents Chemother 52:2709–2717. doi: 10.1128/AAC.00266-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasz A, Nachman S, Leaf H. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother 35:124–129. doi: 10.1128/AAC.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lencastre H, Figueiredo AM, Tomasz A. 1993. Genetic control of population structure in heterogeneous strains of methicillin resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 12(Suppl 1):S13–S18. doi: 10.1007/BF02389872. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira DC, Tomasz A, de Lencastre H. 2001. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb Drug Resist 7:349–361. doi: 10.1089/10766290152773365. [DOI] [PubMed] [Google Scholar]
- 11.Kim C, Mwangi M, Chung M, Milheirico C, de Lencastre H, Tomasz A. 2013. The mechanism of heterogeneous beta-lactam resistance in MRSA: key role of the stringent stress response. PLoS One 8:e82814. doi: 10.1371/journal.pone.0082814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dordel J, Kim C, Chung M, Pardos de la Gandara M, Holden MT, Parkhill J, de Lencastre H, Bentley SD, Tomasz A. 2014. Novel determinants of antibiotic resistance: identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. mBio 5(2):e01000-13. doi: 10.1128/mBio.01000-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aedo S, Tomasz A. 2016. Role of the stringent stress response in the antibiotic resistance phenotype of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 60:2311–2317. doi: 10.1128/AAC.02697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mwangi MM, Kim C, Chung M, Tsai J, Vijayadamodar G, Benitez M, Jarvie TP, Du L, Tomasz A. 2013. Whole-genome sequencing reveals a link between beta-lactam resistance and synthetases of the alarmone (p)ppGpp in Staphylococcus aureus. Microb Drug Resist 19:153–159. doi: 10.1089/mdr.2013.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalebroux ZD, Swanson MS. 2012. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol 10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- 16.Shaw LN, Aish J, Davenport JE, Brown MC, Lithgow JK, Simmonite K, Crossley H, Travis J, Potempa J, Foster SJ. 2006. Investigations into sigmaB-modulated regulatory pathways governing extracellular virulence determinant production in Staphylococcus aureus. J Bacteriol 188:6070–6080. doi: 10.1128/JB.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stapleton MR, Horsburgh MJ, Hayhurst EJ, Wright L, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J Bacteriol 189:7316–7325. doi: 10.1128/JB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh VK, Schmidt JL, Jayaswal RK, Wilkinson BJ. 2003. Impact of sigB mutation on Staphylococcus aureus oxacillin and vancomycin resistance varies with parental background and method of assessment. Int J Antimicrob Agents 21:256–261. doi: 10.1016/S0924-8579(02)00359-X. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, de Lencastre H, Tomasz A. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol 178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole K. 2012. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother 67:2069–2089. doi: 10.1093/jac/dks196. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge BL, Chang YS, Gage D, Tomasz A. 1992. Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Biol Chem 267:11255–11259. [PubMed] [Google Scholar]
- 22.de Jonge BL, Sidow T, Chang YS, Labischinski H, Berger-Bachi B, Gage DA, Tomasz A. 1993. Altered muropeptide composition in Staphylococcus aureus strains with an inactivated femA locus. J Bacteriol 175:2779–2782. doi: 10.1128/jb.175.9.2779-2782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C, Mwangi M, Chung M, Milheirco C, de Lencastre H, Tomasz A. 2013. The mechanism of heterogeneous beta-lactam resistance in MRSA: key role of the stringent stress response. PLoS One 8:e82814. doi: 10.1371/journal.pone.0082814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Álvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RL, Larsen AR, Skov RL, Peacock SJ, Maskell DJ, Holmes MA. 2011. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis 11:595–603. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couto I, Wu SW, Tomasz A, de Lencastre H. 2003. Development of methicillin resistance in clinical isolates of Staphylococcus sciuri by transcriptional activation of the mecA homologue native to S. sciuri. J Bacteriol 185:645–653. doi: 10.1128/JB.185.2.645-653.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Antignac A, Wu SW, Tomasz A. 2008. Penicillin-binding proteins and cell wall composition in beta-lactam-sensitive and -resistant strains of Staphylococcus sciuri. J Bacteriol 190:508–514. doi: 10.1128/JB.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolo J, Worning P, Boye Nielsen J, Sobral R, Bowden R, Bouchami O, Damborg P, Guardabassi L, Perreten V, Westh H, Tomasz A, de Lencastre H, Miragaia M. 2017. Evidence for the evolutionary steps leading to mecA-mediated beta-lactam resistance in staphylococci. PLoS Genet 13:e1006674. doi: 10.1371/journal.pgen.1006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinho MG, de Lencastre H, Tomasz A. 2000. Cloning, characterization, and inactivation of the gene pbpC, encoding penicillin-binding protein 3 of Staphylococcus aureus. J Bacteriol 182:1074–1079. doi: 10.1128/JB.182.4.1074-1079.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonin E, Tomasz A. 1986. Beta-lactam-specific resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother 30:577–583. doi: 10.1128/AAC.30.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roemer T, Schneider T, Pinho MG. 2013. Auxiliary factors: a chink in the armor of MRSA resistance to beta-lactam antibiotics. Curr Opin Microbiol 16:538–548. doi: 10.1016/j.mib.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Sivakumari N, Chiranjeevi P, RPradhan D, Umamaheswari A. 2015. Discovery of potent inhibitors against GTP pyrophosphokinase of Neisseria meningitidis serogroup B. Int J Sci Eng Res 6:273–278. [Google Scholar]
- 32.Oshida T, Tomasz A. 1992. Isolation and characterization of a Tn551-autolysis mutant of Staphylococcus aureus. J Bacteriol 174:4952–4959. doi: 10.1128/jb.174.15.4952-4959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


