Abstract
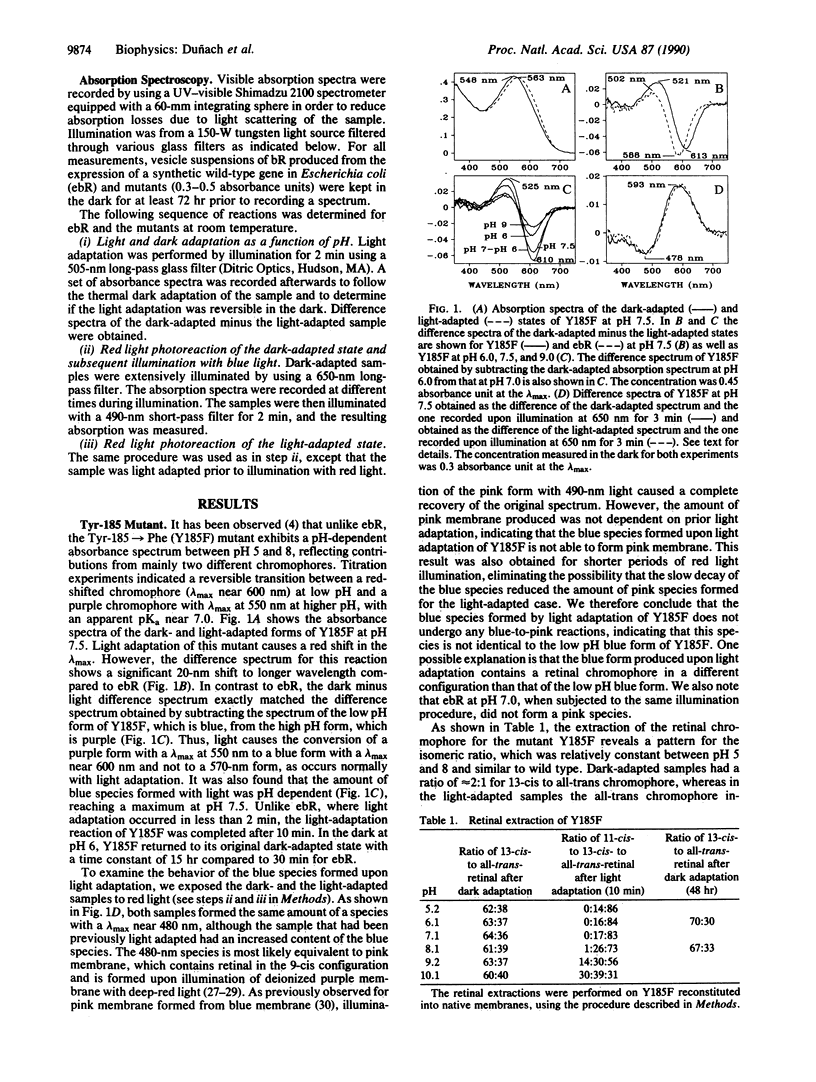
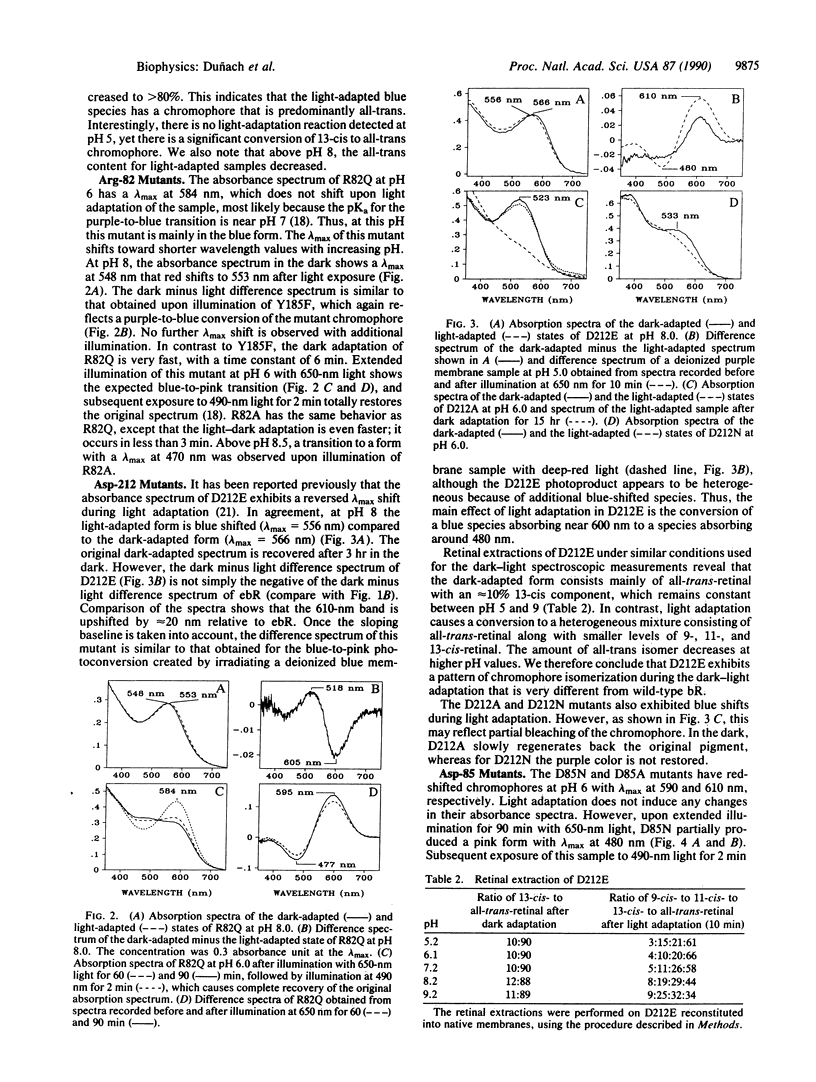
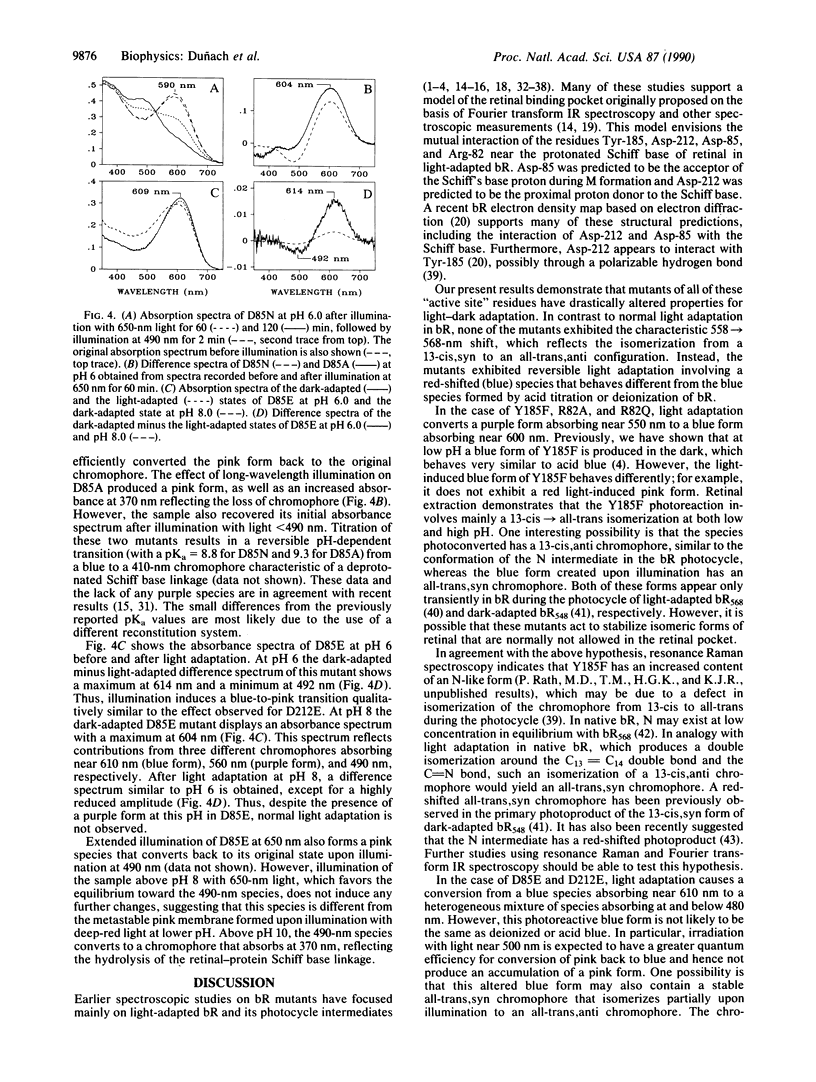
The light-dark adaptation reactions of a set of bacteriorhodopsin (bR) mutants that affect function and color of the chromophore were examined by using visible absorption spectroscopy. The absorbance spectra of the mutants Arg-82 in equilibrium Ala (Gln), Asp-85 in equilibrium Ala (Asn, Glu), Tyr-185 in equilibrium Phe, and Asp-212 in equilibrium Ala (Asn, Glu) were measured at different pH values during and after illumination. None of these mutants exhibited a normal dark-light adaptation, which in wild-type bR causes a red shift of the visible absorption maximum from 558 nm (dark-adapted bR) to 568 nm (light-adapted bR). Instead a reversible light reaction occurs in the Asp-85 and Asp-212 mutants from a blue form with lambda max near 600 nm to a pink form with lambda max near 480 nm. This light-induced shift explains the appearance of a reversed light adaptation previously observed for the Asp-212 mutants. In the case of the Tyr-185 and Arg-82 mutants, light causes a purple-to-blue transformation similar to the effect of lowering the pH. However, the blue forms observed in these mutants are not identical to those formed by acid titration or deionization of wild-type bR. It is suggested that in all of these mutants, the chromophore has lost the ability to undergo the normal 13-cis, 15-syn to all-trans, 15-anti light-driven isomerization, which occurs in native bR. Instead these mutants may have as stable forms all-trans,syn and 13-cis,anti chromophores, which are not allowed in native bR, except transiently.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahl P. L., Stern L. J., Düring D., Mogi T., Khorana H. G., Rothschild K. J. Effects of amino acid substitutions in the F helix of bacteriorhodopsin. Low temperature ultraviolet/visible difference spectroscopy. J Biol Chem. 1988 Sep 25;263(27):13594–13601. [PubMed] [Google Scholar]
- Ahl P. L., Stern L. J., Mogi T., Khorana H. G., Rothschild K. J. Substitution of amino acids in helix F of bacteriorhodopsin: effects on the photochemical cycle. Biochemistry. 1989 Dec 26;28(26):10028–10034. doi: 10.1021/bi00452a022. [DOI] [PubMed] [Google Scholar]
- Birge R. R. Photophysics of light transduction in rhodopsin and bacteriorhodopsin. Annu Rev Biophys Bioeng. 1981;10:315–354. doi: 10.1146/annurev.bb.10.060181.001531. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Stern L. J., Hackett N. R., Chao B. H., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: I. Tyrosine-185 protonates and deprotonates during the photocycle. Proteins. 1988;3(4):219–229. doi: 10.1002/prot.340030403. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Liu S. Y., Jonas R., Govindjee R. The pink membrane: the stable photoproduct of deionized purple membrane. Biophys J. 1987 Oct;52(4):617–623. doi: 10.1016/S0006-3495(87)83252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duñach M., Berkowitz S., Marti T., He Y. W., Subramaniam S., Khorana H. G., Rothschild K. J. Ultraviolet-visible transient spectroscopy of bacteriorhodopsin mutants. Evidence for two forms of tyrosine-185----phenylalanine. J Biol Chem. 1990 Oct 5;265(28):16978–16984. [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Lozier R. H. Photocycles of bacteriorhodopsin in light- and dark-adapted purple membrane studied by time-resolved absorption spectroscopy. Biophys J. 1989 Oct;56(4):693–706. doi: 10.1016/S0006-3495(89)82716-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S. S., Nassal M., Doi T., Jay E., Sgaramella V., Khorana H. G. Structure-function studies on bacteriorhodopsin. II. Improved expression of the bacterio-opsin gene in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9255–9263. [PubMed] [Google Scholar]
- Katre N. V., Wolber P. K., Stoeckenius W., Stroud R. M. Attachment site(s) of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4068–4072. doi: 10.1073/pnas.78.7.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Kouyama T., Nasuda-Kouyama A., Ikegami A., Mathew M. K., Stoeckenius W. Bacteriorhodopsin photoreaction: identification of a long-lived intermediate N (P,R350) at high pH and its M-like photoproduct. Biochemistry. 1988 Aug 9;27(16):5855–5863. doi: 10.1021/bi00416a006. [DOI] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande C., Callender R., Henderson R., Pande A. Purple membrane: color, crystallinity, and the effect of dimethyl sulfoxide. Biochemistry. 1989 Jul 11;28(14):5971–5978. doi: 10.1021/bi00440a038. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Gerchman S. E., Engelman D. M. Refolding of bacteriorhodopsin in lipid bilayers. A thermodynamically controlled two-stage process. J Mol Biol. 1987 Dec 20;198(4):655–676. doi: 10.1016/0022-2836(87)90208-7. [DOI] [PubMed] [Google Scholar]
- Roepe P. D., Ahl P. L., Herzfeld J., Lugtenburg J., Rothschild K. J. Tyrosine protonation changes in bacteriorhodopsin. A Fourier transform infrared study of BR548 and its primary photoproduct. J Biol Chem. 1988 Apr 15;263(11):5110–5117. [PubMed] [Google Scholar]
- Rothschild K. J., Braiman M. S., He Y. W., Marti T., Khorana H. G. Vibrational spectroscopy of bacteriorhodopsin mutants. Evidence for the interaction of aspartic acid 212 with tyrosine 185 and possible role in the proton pump mechanism. J Biol Chem. 1990 Oct 5;265(28):16985–16991. [PubMed] [Google Scholar]
- Rothschild K. J., Braiman M. S., Mogi T., Stern L. J., Khorana H. G. Conserved amino acids in F-helix of bacteriorhodopsin form part of a retinal binding pocket. FEBS Lett. 1989 Jul 3;250(2):448–452. doi: 10.1016/0014-5793(89)80774-4. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Gray D., Mogi T., Marti T., Braiman M. S., Stern L. J., Khorana H. G. Vibrational spectroscopy of bacteriorhodopsin mutants: chromophore isomerization perturbs tryptophan-86. Biochemistry. 1989 Aug 22;28(17):7052–7059. doi: 10.1021/bi00443a041. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., He Y. W., Mogi T., Marti T., Stern L. J., Khorana H. G. Vibrational spectroscopy of bacteriorhodopsin mutants: evidence for the interaction of proline-186 with the retinylidene chromophore. Biochemistry. 1990 Jun 26;29(25):5954–5960. doi: 10.1021/bi00477a011. [DOI] [PubMed] [Google Scholar]
- Scherrer P., Mathew M. K., Sperling W., Stoeckenius W. Retinal isomer ratio in dark-adapted purple membrane and bacteriorhodopsin monomers. Biochemistry. 1989 Jan 24;28(2):829–834. doi: 10.1021/bi00428a063. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Lugtenburg J., Mathies R. A. Determination of retinal chromophore structure in bacteriorhodopsin with resonance Raman spectroscopy. J Membr Biol. 1985;85(2):95–109. doi: 10.1007/BF01871263. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Pardoen J. A., Winkel C., Mulder P. P., Lugtenburg J., Mathies R. Determination of retinal Schiff base configuration in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2055–2059. doi: 10.1073/pnas.81.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W., Carl P., Rafferty Ch, Dencher N. A. Photochemistry and dark equilibrium of retinal isomers and bacteriorhodopsin isomers. Biophys Struct Mech. 1977 Jun 29;3(2):79–94. doi: 10.1007/BF00535798. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Ahl P. L., Marti T., Mogi T., Duñach M., Berkowitz S., Rothschild K. J., Khorana H. G. Substitution of membrane-embedded aspartic acids in bacteriorhodopsin causes specific changes in different steps of the photochemical cycle. Biochemistry. 1989 Dec 26;28(26):10035–10042. doi: 10.1021/bi00452a023. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Khorana H. G. Structure-function studies on bacteriorhodopsin. X. Individual substitutions of arginine residues by glutamine affect chromophore formation, photocycle, and proton translocation. J Biol Chem. 1989 Aug 25;264(24):14202–14208. [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Khorana H. G. Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82----Ala and Asp-85----Glu: the blue form is inactive in proton translocation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1013–1017. doi: 10.1073/pnas.87.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Pathways of the rise and decay of the M photointermediate(s) of bacteriorhodopsin. Biochemistry. 1990 Mar 6;29(9):2241–2250. doi: 10.1021/bi00461a006. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Keszthelyi L., Lanyi J. K. Transient spectroscopy of bacterial rhodopsins with an optical multichannel analyzer. 1. Comparison of the photocycles of bacteriorhodopsin and halorhodopsin. Biochemistry. 1989 Jun 13;28(12):5165–5172. doi: 10.1021/bi00438a038. [DOI] [PubMed] [Google Scholar]



