ABSTRACT
The vancomycin loading dose (LD) of 25 to 30 mg/kg is a frequently practiced strategy to achieve effective concentrations from the first-treatment dose. However, considering only the body weight for dosing might be inadequate in critically ill patients due to pharmacokinetics changes. We sought to assess achieving optimal trough serum levels of vancomycin and AUC0–24/MIC in the first 24 h of treatment by using an LD based on population pharmacokinetic parameters of critically ill patients. We performed a concurrent cohort study over 22 months of patients with severe sepsis who received intravenous vancomycin. The patients were treated with three different strategies to initiate vancomycin: without an LD (group A), with an LD of 25 to 30 mg/kg (group B), and with an LD based on population pharmacokinetic parameters of the critically ill patient (group C). An optimal trough serum concentration was achieved in 5, 9, and 83% of patients in groups A, B, and C, respectively. The number of patients that reached optimal AUC0–24 was 2 of 18 (11%), 5 of 11 (46%), and 11 of 12 (92%) in groups A, B, and C, respectively. The statistical analysis for both parameters revealed significant differences in group C with respect to other groups. The administration of the LD calculated from population pharmacokinetic parameters from the beginning of therapy is a more efficient strategy to obtain adequate trough serum concentrations and AUC0–24/MIC in critical patients.
KEYWORDS: vancomycin, critical care, pharmacokinetics
INTRODUCTION
Infectious diseases are one of the main causes of mortality in intensive care units (ICUs) and a constant challenge faced by clinical teams (1). Some factors that influence this high mortality are severe hypoperfusion, multiple organ dysfunction syndrome, increased antimicrobial resistance, and a delayed start of proper antibiotic therapy and subtherapeutic concentrations of antibiotics (2–4), which can occur with medications that have complex pharmacokinetics, such as vancomycin (5).
Vancomycin is an antibiotic used in hospital-acquired infections caused by Gram-positive bacteria. Its pharmacokinetic profile can be characterized by either two or three compartments, renal elimination, variable tissue penetration, and pharmacokinetic/pharmacodynamic (PK/PD) characteristics, showing that its efficacy and safety depends on multiple factors (5).
At this time, it is well accepted that the best PK/PD index for predicting the activity of vancomycin is the ratio between the area under the 24-h concentration-time curve (AUC0–24) and the MIC (6). AUC0–24/MIC values of ≥400 have demonstrated clinical and microbiological efficacy against lower respiratory tract infections by methicillin-resistant Staphylococcus aureus (7), which have been later validated by international recommendations and guidelines (8–11). On the other hand, lower values have shown not only poor infection eradication but also require longer treatments and a higher mortality rate (12–14). In order to reach an appropriate PK/PD value, it has been proposed that trough levels between 15 and 20 μg/ml for complicated infections are required (8, 9, 15). Serum levels of <10 and >20 μg/ml have been linked to antibiotic resistance and nephrotoxicity, respectively (8, 16).
Septic critical patients, who receive high-volume resuscitation, also display an increase in vascular permeability, resulting in fluid shifts from the intravascular compartment to the interstitial space. This directly affects the pharmacokinetics of hydrophilic drugs, such as vancomycin (17). These situations increase its volume of distribution (V) and generate lower concentrations than those achieved with the same dose in noncritical patients (18). Furthermore, delay in its renal elimination causes an increase in its half-life, requiring extended administration intervals of several days before the desired serum levels are reached (17).
Use of the vancomycin loading dose (LD) has been reported as a strategy to achieve effective concentrations from the first-treatment dose, avoiding the appearance of resistance and treatment failure in critical patients (15, 19–23). Three studies have suggested a standard prescribing dose of 25 to 30 mg/kg based mainly on body weight (8–10). However, considering only body weight for dosing might be inadequate in critically ill patients due to the characteristics and pharmacokinetic changes mentioned earlier. Since it is crucial to obtain optimal serum levels rapidly in critical patients, with a proper AUC0–24/MIC, the use of an LD based on pharmacokinetic parameters is an appropriate alternative to consider (3).
Our objective was to assess achieving optimal trough serum levels of vancomycin and obtaining the AUC0–24/MIC in the first 24 h of treatment by comparing three different LD administration protocols in order to determine the most appropriate one. A second objective was to assess the renal safety of each protocol.
RESULTS
Demographics.
Forty-one patients were included and classified according to the strategy for the outset of the therapy of vancomycin (Table 1). No significant differences were detected between groups in their clinical characteristics, or their pharmacokinetic characteristics.
TABLE 1.
Demographic and clinical characteristics of patients in this study
| Parametera | Group |
Pb | ||
|---|---|---|---|---|
| A | B | C | ||
| No. of patients | 18 | 11 | 12 | |
| No. (%) male | 11 (61.1) | 9 (81.8) | 8 (66.7) | NS* |
| Mean age in yrs (SD) | 59.4 (15.8) | 55.4 (19.4) | 53.2 (17.3) | 0.610† |
| Mean (SD)c | ||||
| Wt (kg) | 81.4 (21.0) | 81.8 (16.0) | 81.3 (19.1) | 0.997† |
| Ht (cm) | 167.4 (7.2) | 169.9 (4.6) | 169.8 (6.3) | 0.481† |
| BMI (kg/m2) | 28.8 (6.2) | 28.3 (5.1) | 27.9 (5.3) | 0.919† |
| Basal serum creatinine (mg/dl) | 1.7 (1.0) | 1.8 (1.3) | 1.2 (0.6) | 0.205† |
| Median APACHE II score (range) | 20 (10–38) | 20 (4–49) | 19 (14–28) | 0.494† |
| Vvanco (liters/kg) | 0.67 (0.14) | 0.71 (0.18) | 0.67 (0.09) | 0.778† |
| CLvanco (liters/h) | 3.0 (2.6) | 2.8 (2.7) | 2.9 (1.1) | 0.977† |
Vvanco, volume of distribution for vancomycin; CLvanco, clearance of vancomycin; BMI, body mass index.
NS, not significant. *, Fisher exact test; †, ANOVA.
Except as noted for the APACHE score.
Evaluation of pharmacokinetic modeling.
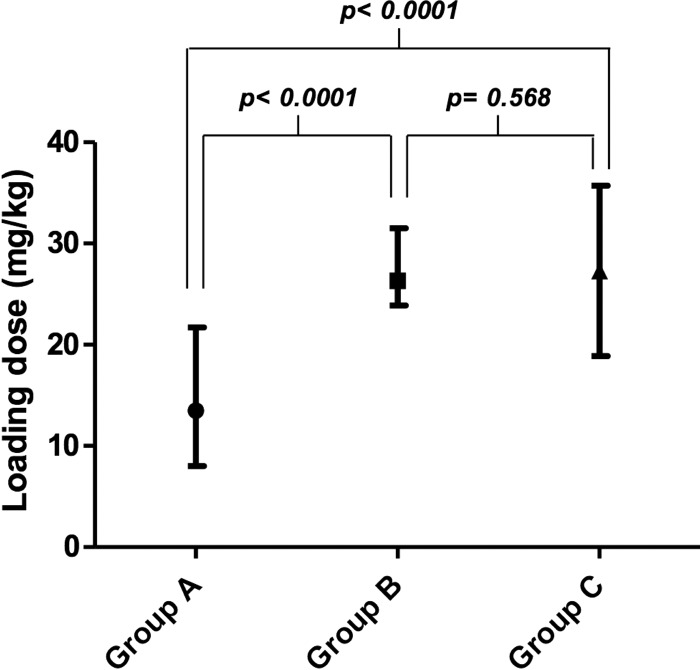
Figure 1 shows the mean and range for the vancomycin LD administered to each group. There were no statistically significant differences between an LD of 25 to 30 mg/kg (group B) and an LD based on population pharmacokinetic parameters (group C). However, there was a broader dose range in group C.
FIG 1.
Mean and range of administered loading dose for each group.
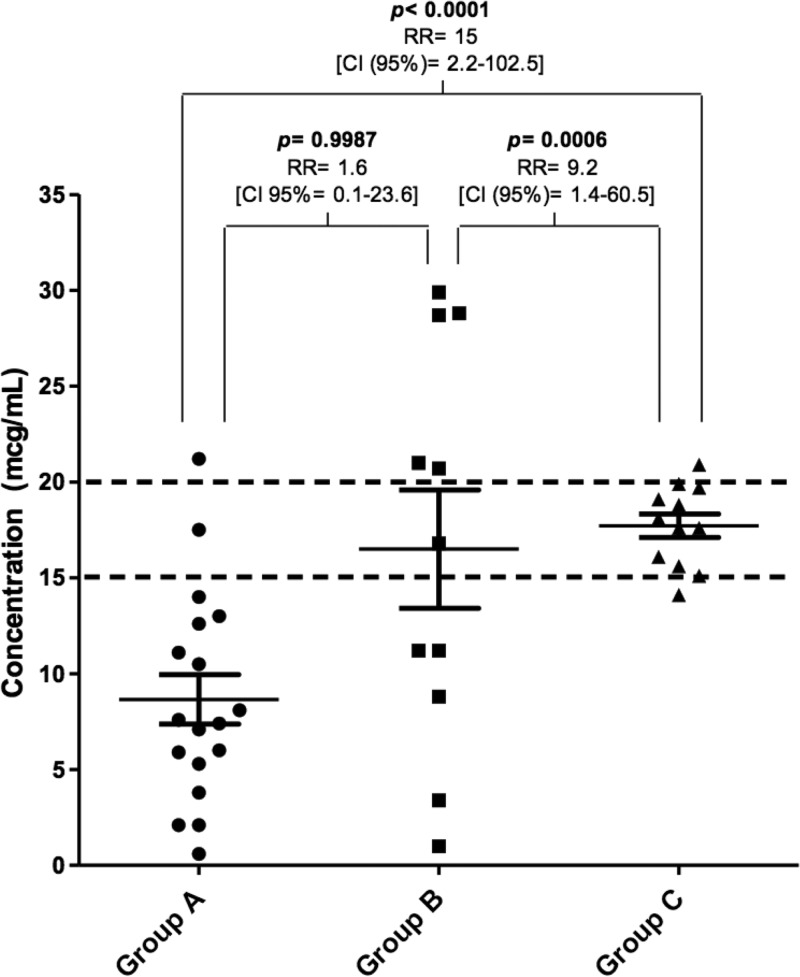
The LD obtained through the pharmacokinetic parameters of the critical patient generated an optimal average of trough serum concentrations of vancomycin (17.7 μg/ml, group C), with 83% of them (10/12 patients) in recommended concentrations of 15 to 20 mg/liter (Fig. 2). This was statistically different from patients that did not receive the LD (group A, P < 0.0001) and from patients that received an LD of 25 to 30 mg/kg (group B, P < 0.0006). In this last group there was a great variability, where only one patient (9%) achieved optimal therapeutic levels.
FIG 2.
Trough serum vancomycin concentrations achieved after the first dose, considering the three strategies for the therapy onset.
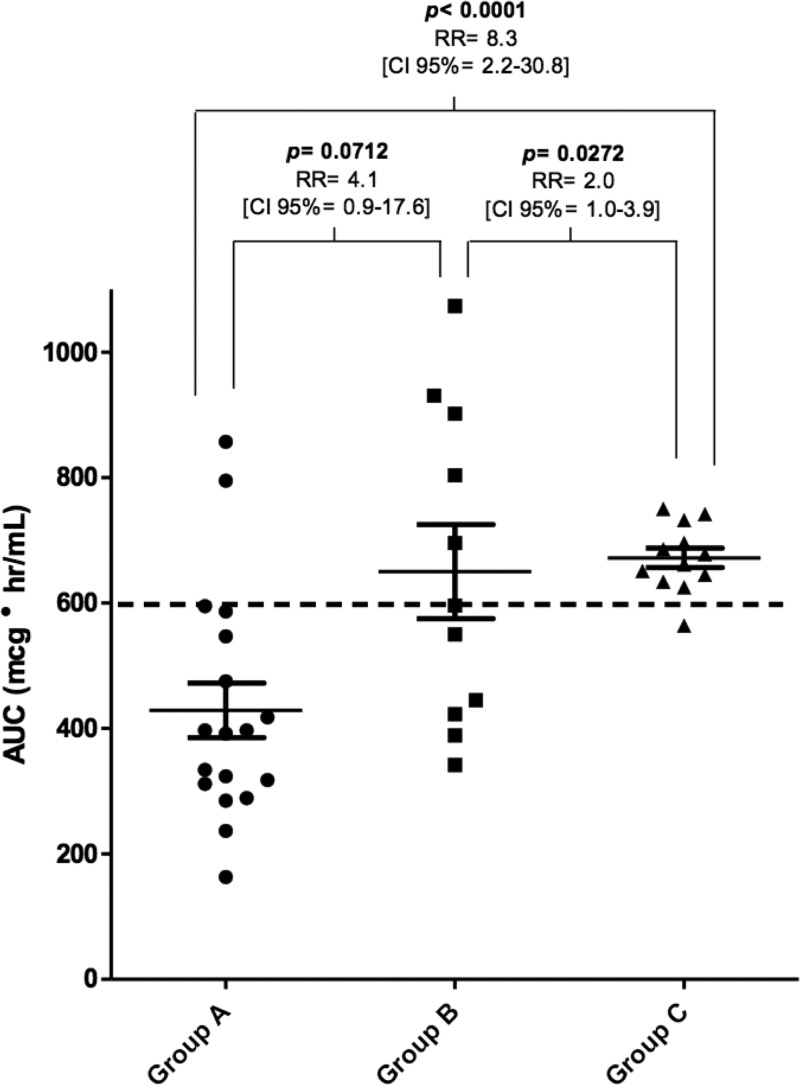
The number of patients that reached optimal AUC0–24, was 2 of 18 (11%), 5 of 11 (46%), and 11 of 12 (92%) in groups A, B, and C, respectively (Fig. 3). The probability of achieving an optimal AUC when using an LD based on population pharmacokinetic parameters (group C) was increased 8.3 times compared to patients without the LD (group A, P < 0.0001). This value was doubled compared to patients with an LD based on their weight (group B, P < 0.05).
FIG 3.
Vancomycin AUC curve for the first 24 h of treatment, according to the strategy of therapy onset.
Although there were six patients in group A (33.3%), four patients in group B (36.3%), and two patients in group C (16.6%) that presented an increase in serum creatinine levels during the first 5 days of treatment, a causality analysis showed that in all cases these elevations were related to the hypoperfusion state dependent on the acute septic condition. Therefore, no nephrotoxicity related with vancomycin was observed.
DISCUSSION
The use of an LD in critically ill patients is a widely recommended strategy and has been supported by several guides and studies (8–11, 20–23). Although there is no strong evidence to support the real clinical impact of this method, it has been suggested that its use is associated with better clinical cure rates in critically ill patients (24). In fact, the use of the LD allows optimal serum concentrations to be reached quickly, which improves the start of adequate antibiotic therapy. This reduces mortality and the length of hospitalization in patients with bacteremia by S. aureus (2, 25). Moreover, the use of an LD represents a safe strategy, which does not increase the incidence of nephrotoxicity (26).
In our study, the strategy of establishing an LD based on the actual body weight of the patient revealed a large variability. This is expected in critical patients that might present great changes in the volume of distribution and clearance of hydrophilic medications, such as vancomycin (17, 18). Some patients from groups A and B have very low serum concentrations (<2.5 μg/ml, Fig. 2), which was explained by their obesity and increased CLvanco (>5 liters/h). On the other hand, there were three group B patients that reached elevated serum concentrations (>28 μg/ml). All of these patients presented a decreased vancomycin clearance (CLvanco < 1 liters/h) from the beginning of the study, which could explain the higher concentrations reached. However, only one patient in group C had a concentration above the therapeutic range (20.9 μg/ml). Considering CLvanco in LD estimation would allow safer and more effective vancomycin concentration.
A AUC0–24/MIC value of ≥400 was defined primarily for the clinical and microbiological efficacy of vancomycin in patients with hospital-acquired pneumonia with S. aureus (7). Later studies have corroborated this pattern with minimal variations with other types of infections and clinical outcomes (12–14). Obtaining adequate magnitudes would allow us to ensure clinical and microbiological efficacy. Although the strategy of using an LD based on actual body weight could be a reasonable alternative to reach the optimal AUC0–24, four patients had supratherapeutic concentrations (>20 μg/ml).
The highest proportion of patients in group A reached an AUC0–24 between 200 and 400 μg·h/ml (Fig. 3), which would be effective against pathogens with an MIC ≤ 0.5 μg/ml and an MIC ≤ 1.0 μg/ml, in some cases. In all of these situations, optimal trough serum concentrations were not achieved, affecting an appropriate drug penetration particularly in tissues poorly perfused and to prevent microbial resistance (8). It is important to note that despite the existence of patients in groups A and B with very high or low AUC0–24 values, we cannot infer a correlation with toxicity or the loss of clinical efficacy. Therefore, it seems more appropriate to use a strategy that does not generate variability, which was only obtained in group C.
Our results show that it is difficult to achieve the objective from the first dose without administering an LD. This was an expected result and has been reported in other studies (20–23). It is noteworthy that in group B the objectives proposed were not reached when doses based on actual body weight (25 to 30 mg/kg) were used, even though it is recommended by the guidelines and by consensus (8–10). In group C, several parameters were considered, such as V, which is increased in critical patients due to multiple factors and could explain the lower variability. To calculate the dose for group C in our study, we increased the V of the peripheral compartment by 13%, although some studies show that in critical patients these values may be up to two times the normal value (18). The V values estimated in this study by Bayesian methods are in concordance to the value of 0.7 liter/kg, which has been discussed and previously reported (5). Our results obtained in group C patients support the idea of considering V during vancomycin LD estimation in order to achieve adequate trough serum concentrations after the first dose.
Administered LDs in groups B and C were similar (2,045 ± 350 mg and 2,167 ± 444 mg, respectively), but the dose range used in group C was 19 to 36 mg/kg. This difference with group B (25 to 30 mg/kg) demonstrates a more flexible and personalized pharmacotherapy than the standard (Fig. 1).
No patient had nephrotoxicity associated with the use of LD, although some patients reached higher trough serum concentrations, the primary predictor of risk associated with this complication (16). It is important to note that a clinical pharmacist conducted a daily pharmacokinetic monitoring and a pharmacotherapeutic follow-up to adjust doses and to ensure that serum concentrations were within the therapeutic range.
One limitation of our study was the low patient numbers. However, our sample size allowed the evaluation of the objectives proposed in this research, providing an adequate rationale for further studies with more patients. A second limitation was the nonrandomized choice of the strategy to start vancomycin in each patient; it was related to the current protocol of the ICU at the admission of each patient, and therefore we cannot exclude a selection tendency. However, the clinical characteristics that were statistically evaluated allowed us to determine the comparable groups.
An adequate determination of trough serum concentration of vancomycin should be made just before the administration of the next dose and, in order to determine the AUC0–24, it is necessary to obtain multiple serum vancomycin ongoing determinations. However, it can be difficult to perform this protocol in clinical practice. For this reason, we calculated these parameters in our study with Bayesian models using pharmacokinetic software, a strategy that has been validated in several studies and is frequently used in current clinical practice (27–29). We believe it is necessary to complement our results with a multicentric study with a higher number of patients to evaluate the clinical outcome in response to vancomycin LD in critically ill patients.
In conclusion, the administration of the LD from the beginning of vancomycin therapy and calculated from population pharmacokinetic parameters of critical patients is an efficient strategy to obtain adequate trough serum concentrations and AUC0–24/MIC values compared to not administering the LDs or administering them based on actual body weight. Our results suggest that the administration of higher doses at baseline is not related to adverse events on renal function, when there is a permanent pharmacokinetic monitoring.
MATERIALS AND METHODS
Patients and data.
A concurrent cohort study was conducted in the ICU at Hospital del Salvador, an adult tertiary hospital, between January 2012 and October 2013. This research was approved by the Scientific Ethics Committee of the Hospital. The Pharmacy Department of the Faculty of Chemistry of Pontificia Universidad Católica de Chile (Pontifical Catholic University of Chile) collaborated on the methodology and analysis of results.
In this study, we included both the critically ill patients with severe sepsis that received intravenous vancomycin and patients that were correctly monitored for vancomycin serum levels in order to guide and monitor their therapy. Patients that received hemodialysis after the first dose were excluded. During the study period, three different strategies to initiate empirical vancomycin therapy were considered. Patients were classified on the following groups: (i) group A, patients that did not receive the LD; (ii) group B, patients that received an LD of 25 to 30 mg/kg (based on actual body weight); and (iii) group C, patients that received an LD based on the population pharmacokinetic parameters of the critically ill patient, and on vancomycin's infusion rate, according the following equation:
where tinf is the infusion time of the LD (10 mg/kg/h), Vc is the volume of distribution of the central compartment (0.17·kg; if CLCR ≤ 10 ml/min, then 0.45·kg) (30, 31), Cp is the serum concentration 1 h after of the end of infusion (35 μg/ml) (32), k21 is the transfer rate constant between the peripheral and central compartments (0.46 h−1) (33), t is the time to serum concentration 1 h after the end of vancomycin infusion (5), α is the rate constant of the distribution phase [(k21·CLvanco)/(Vc·β)] (34), CLvanco is [(0.79·CLCR) + (0.5·kg)]·0.06 (34), β is the rate constant of the elimination phase (CLvanco/Vp) (34), and Vp is the volume of distribution of the peripheral compartment, which was increased 13% (0.7·kg·1.13) (18).
The assignment of patients to each group depended on the existing institutional protocol at the time of admission to the ICU. The sample size of patients was determined by Kelsey method and using the software OpenEpi (v3.01), with a 95% confidence and 80% powered, respectively. In addition, the percentages of patients that would reach the optimal trough serum concentrations and optimal AUC0–24/MIC in each group (15% for group A, 30% for group B, and 90% group C) were considered in the sample size, as previously described (20, 21).
Each maintenance dose scheme was established with the Therapeutic Drug Monitoring System program (TDMS 2000; v12.04.26 for Windows from Healthware, Inc.). The dosage interval was adjusted according to the renal function of each patient every 8, 12, 24, 48, or 72 h. The clinical pharmacist requested serum level measurements, based on local protocols and international recommendations (8, 9, 35). The occurrence of nephrotoxicity was determined as an increase in serum creatinine levels of 0.5 mg/dl or 50% from the baseline during the first 5 days after the first dose and excluding any other reason, using the causality analysis of the Naranjo algorithm with the treating team (36).
Vancomycin blood concentration assay.
Blood samples were drawn just prior of the next maintenance dose through an arterial line or an alternate venous line to vancomycin administration. The samples were processed on heparin-free tubes and then analyzed by homogeneous immunoassay technique using Roche COBAS C311 equipment.
Trough serum concentrations of vancomycin, after the first dose, were analyzed using Bayesian methods, with the TDMS 2000 software, which has a database to estimate population pharmacokinetic parameters based on clinical and demographic data for the patients. Values between 15 and 20 μg/ml were considered optimal serum concentrations (8, 9, 11). In addition, the CLvanco and Vvanco were determined using Bayesian methods for each patient.
The AUC0–24 of vancomycin was determined using the trapezoid method, based on the methodology of DeRyke and Alexander (15) but considering a bicompartmental kinetic and the stages of administration, distribution, and elimination. An infusion rate of 10 mg/kg/h and a distribution phase of 1 h were applied. For a calculation basis, simulated concentrations with Bayesian methods were used, with the TDMS 2000 program. Considering that most therapies were initiated empirically, an AUC0–24 of ≥600 μg·h/ml was considered optimal in order to ensure effectiveness against pathogens with an MIC of ≤1.5 μg/ml (600/1.5 = 400 AUC0–24/MIC).
Even if the sensibility breakpoint was ≤2 μg/ml for S. aureus, the most common Gram-positive pathogen in the ICU setting, we based our criteria according to a meta-analysis that suggested a greater likelihood of adverse outcomes (higher mortality and treatment failure), when vancomycin was administered to infected patients for pathogens with higher MIC values (>1.5 μg/ml) (37).
Data extraction and evaluation.
Data were analyzed by one-way analysis of variance (ANOVA), followed by a Bonferroni multiple-comparison post hoc test, in order to evaluate the clinical and pharmacokinetic characteristics of each group. A Fisher exact test was performed to determine differences in the achieved through serum levels, as well as the optimal AUC0–24 results and the occurrence of nephrotoxicity. A confidence interval of 95% was used, and a P value of ≤0.05 was considered statistically significant. All analyses were performed using GraphPad Prism version 5.01.
REFERENCES
- 1.Hotchkiss RS, Karl IE. 2003. The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Labelle A, Juang P, Reichley R, Micek S, Hoffmann J, Hoban A, Hampton N, Kollef M. 2012. The determinants of hospital mortality among patients with septic shock receiving appropriate initial antibiotic treatment. Crit Care Med 40:2016–2021. doi: 10.1097/CCM.0b013e318250aa72. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. 2014. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 4.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 5.Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42(Suppl 1):35S–39S. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 6.Ebert S, Leggett J, Vogelman B. 1987. In vivo cidal activity and pharmacokinetics parameters (PKPs) for vancomycin against methicillin-susceptible (MSSA) and -resistant (MRSA) S. aureus, abstr 439. In Program and Abstracts of the 27th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, DC. [Google Scholar]
- 7.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 8.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K, Takesue Y, Ohmagari N, Mochizuki T, Mikamo H, Seki M, Takakura S, Tokimatsu I, Takahashi Y, Kasahara K, Okada K, Igarashi M, Kobayashi M, Hamada Y, Kimura M, Nishi Y, Tanigawara Y, Kimura T. 2013. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother 19:365–380. doi: 10.1007/s10156-013-0599-4. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. 2011. Clinical Practice Guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 52:975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 13.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Warren SJ, Gao W, Howden BP, Johnson PD. 2013. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gawronski KM, Goff DA, Brown J, Khadem TM, Bauer KA. 2013. A stewardship program's retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin Ther 35:772–779. doi: 10.1016/j.clinthera.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 15.DeRyke CA, Alexander DP. 2009. Optimizing vancomycin dosing through pharmacodynamic assessment targeting area under the concentration-time curve/minimum inhibitory concentration. Hosp Pharm 44:751–765. doi: 10.1310/hpj4409-751. [DOI] [Google Scholar]
- 16.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37:840–851. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 18.del Mar Fernández de Gatta Garcia M, Revilla N, Calvo MV, Domínguez-Gil A, Sánchez-Navarro A. 2006. Pharmacokinetic/pharmacodynamic analysis of vancomycin in ICU patients. Intensive Care Med 33:279–285. [DOI] [PubMed] [Google Scholar]
- 19.Reardon J, Lau TT, Ensom MH. 2015. Vancomycin loading doses: a systematic review. Ann Pharmacother 49:557–565. doi: 10.1177/1060028015571163. [DOI] [PubMed] [Google Scholar]
- 20.Truong J, Levkovich BJ, Padiglione AA. 2012. Simple approach to improving vancomycin dosing in intensive care: a standardised loading dose results in earlier therapeutic levels. Intern Med J 42:23–29. doi: 10.1111/j.1445-5994.2011.02459.x. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Udy AA, Kirkpatrick CM, Lipman J, Roberts JA. 2012. Improving vancomycin prescription in critical illness through a drug use evaluation process: a weight-based dosing intervention study. Int J Antimicrob Agents 39:69–72. doi: 10.1016/j.ijantimicag.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Golenia BS, Levine AR, Moawad IM, Yeh DD, Arpino PA. 2013. Evaluation of a vancomycin dosing nomogram based on the Modification of Diet in Renal Disease equation in intensive care unit patients. J Crit Care 28:710–716. doi: 10.1016/j.jcrc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Denetclaw TH, Dowling TC, Steinke D. 2013. Performance of a divided-load intravenous vancomycin dosing strategy for critically ill patients. Ann Pharmacother 47:1611–1617. doi: 10.1177/1060028013510395. [DOI] [PubMed] [Google Scholar]
- 24.Mohammedi I, Descloux E, Argaud L, Le Scanff J, Robert D. 2006. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents 27:259–262. doi: 10.1016/j.ijantimicag.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 36:1418–1423. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 26.Rosini JM, Davis JJ, Muenzer J, Levine BJ, Papas MA, Comer D, Arnold R. 2016. High single-dose vancomycin loading is not associated with increased nephrotoxicity in emergency department sepsis patients. Acad Emerg Med 23:744–746. doi: 10.1111/acem.12934. [DOI] [PubMed] [Google Scholar]
- 27.Nunn MO, Corallo CE, Aubron C, Poole S, Dooley MJ, Cheng AC. 2011. Vancomycin dosing: assessment of time to therapeutic concentration and predictive accuracy of pharmacokinetic modeling software. Ann Pharmacother 45:757–763. doi: 10.1345/aph.1P634. [DOI] [PubMed] [Google Scholar]
- 28.Avent ML, Vaska VL, Rogers BA, Cheng AC, van Hal SJ, Holmes NE, Howden BP, Paterson DL. 2013. Vancomycin therapeutics and monitoring: a contemporary approach. Intern Med J 43:110–119. doi: 10.1111/imj.12036. [DOI] [PubMed] [Google Scholar]
- 29.Aubron C, Corallo CE, Nunn MO, Dooley MJ, Cheng AC. 2011. Evaluation of the accuracy of a pharmacokinetic dosing program in predicting serum vancomycin concentrations in critically ill patients. Ann Pharmacother 45:1193–1198. doi: 10.1345/aph.1Q195. [DOI] [PubMed] [Google Scholar]
- 30.Matzke GR, Zhanel GG, Guay DR. 1986. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet 11:257–282. doi: 10.2165/00003088-198611040-00001. [DOI] [PubMed] [Google Scholar]
- 31.Tan CC, Lee HS, Ti TY, Lee EJ. 1990. Pharmacokinetics of intravenous vancomycin in patients with end-stage renal failure. Ther Drug Monit 12:29–34. doi: 10.1097/00007691-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Levine DP, Fromm BS, Reddy BR. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 33.Rotschafer JC, Crossley K, Zaske DE, Mead K, Sawchuk RJ, Solem LD. 1982. Pharmacokinetics of vancomycin: observations in 28 patients and dosage recommendations. Antimicrob Agents Chemother 22:391–394. doi: 10.1128/AAC.22.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum S. 2017. Pharmacokinetics of an intravenous bolus injection in a two-compartment model, p 178–197. In Rosenbaum S. (ed), Basic pharmacokinetics and pharmacodynamics: an integrated textbook and computer simulations, 2nd ed, vol 1 John Wiley & Sons, Princeton, NJ. [Google Scholar]
- 35.Alvarez-Lerma F, Olaechea P, Grau S, Marín M, Domínguez A, Martínez-Lanao J, Soy D, Alos M, Calvo MV, Sádaba B, Mediavilla A, Fatela D. 2008. Recommendations for antibiotic monitoring in ICU patients. Enferm Infecc Microbiol Clin 26:230–239. doi: 10.1016/S0213-005X(08)72695-8. [DOI] [PubMed] [Google Scholar]
- 36.Naranjo C, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. 1981. A method for estimating the probability of adverse drug reactions. Clin Pharm Ther 30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 37.Van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 54:755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]