ABSTRACT
Candida auris is an emerging multidrug-resistant threat. The pharmacodynamics of three antifungal classes against nine C. auris strains was explored using a murine invasive candidiasis model. The total drug median pharmacodynamic (PD) target associated with net stasis was a fluconazole AUC/MIC (the area under the concentration-time curve over 24 h in the steady state divided by the MIC) of 26, an amphotericin B Cmax/MIC (maximum concentration of drug in serum divided by the MIC) of 0.9, and a micafungin AUC/MIC of 54. The micafungin PD targets for C. auris were ≥20-fold lower than those of other Candida species in this animal model. Clinically relevant micafungin exposures produced the most killing among the three classes.
KEYWORDS: C. auris, pharmacodynamics, antifungal therapy, antifungal resistance
TEXT
Candida auris is an emerging multidrug-resistant threat to human health across the globe (1, 2). The first documented clinical case of this species occurred in Japan in 2009 (3). Since then, infections due to C. auris have been published from numerous countries throughout the world (1, 2, 4–17). Unfortunately, antifungal therapeutic failure and mortality have been commonly reported. This is attributed in part to antifungal resistance. Many isolates exhibit high triazole and polyene MICs. This might be expected, as the species is phylogenetically related to Candida krusei, Candida lusitaniae, and Candida haemulonii, which are known to be less susceptible to these antifungal classes (18, 19). Variable in vitro susceptibility results have been noted for the echinocandins (1), rendering some isolates potentially clinically resistant to all three classes of commonly used antifungal agents. The optimal antifungal agent and dosing regimen for treatment of these infections have not been defined. As such, preliminary susceptibility breakpoints are based on limited in vitro data using breakpoints developed for other Candida species.
The goal of the present studies was to define the pharmacokinetic/pharmacodynamic (PK/PD) target for the three available antifungal drug classes against this emerging pathogen. Specifically, we designed an in vivo PK/PD study to compare the treatment effects of fluconazole, micafungin, and amphotericin B, using the neutropenic murine model of invasive candidiasis against nine clinical isolates of C. auris (Table 1). Strains were chosen to include those with variable in vitro susceptibility to available antifungal drug classes. Strains were screened for fitness in the animal model prior to treatment studies (Table 1). Fluconazole, micafungin, and amphotericin B deoxycholate were prepared as described in manufacturer instructions. Antifungal susceptibility testing was performed according to CLSI guidelines for fluconazole and micafungin or Etest for amphotericin B (20, 21). MICs varied by 128-fold for fluconazole (range, 2 to 256 mg/liter), 32-fold for micafungin (range, 0.125 to 4 mg/liter), and 10-fold for amphotericin B (range, 0.38 to4 mg/liter). The neutropenic disseminated candidiasis model was used for all experiments. Three mice per treatment or control group were included. Mice were inoculated with 6.34 ± 0.08 log10 CFU/ml via the lateral tail vein with each of the nine strains. Antifungal treatment began 2 h after inoculation and continued for 96 h, at which time mice were euthanized for CFU determination in the kidneys. Drug dosing consisted of 0.78, 3.125, 12.5, 50, and 200 mg/kg fluconazole every 12 h by subcutaneous (s.c.) administration, 0.3125, 1.25, 5, 20, and 80 mg/kg micafungin every 24 h by intraperitoneal (i.p.) administration, or 0.078, 0.3125, 1.25, 5, and 20 mg/kg amphotericin B deoxycholate every 24 h by i.p. administration. The treatment studies were designed to include clinically relevant exposures. Organism burden in mouse kidneys after 4 days (96 h) of therapy was compared to the Candida quantity at the start of therapy. The treatment results were analyzed using a sigmoidal maximum effect (Emax) model (22). Pharmacokinetic exposures were obtained from our lab in this mouse model (23–25). The PK exposures were plotted relative to MIC and the previously defined PK/PD driver. Specifically, AUC/MIC (area under the concentration-time curve over 24 h in the steady state divided by the MIC) was used for fluconazole and micafungin, and Cmax/MIC (the maximum concentration of drug in serum divided by the MIC) was used for amphotericin B (26, 27). The magnitude of the PK/PD index (AUC/MIC or Cmax/MIC) associated with net stasis and 1-log kill (when achieved) for each strain was calculated with the equation log10 D = log10 (E/[Emax − E])/(N + log10 ED50), where E is the control growth for the static dose (D), E + 1 is the control growth for the 1-log kill dose (D), and ED50 is the 50% effective dose.
TABLE 1.
Treatment effects for nine select Candida auris strains used in the studies, with country of origin, antimicrobial susceptibility results, and 24-h total drug PK/PD target exposures in the murine invasive candidiasis model
| Strain | Country of origin | 96 h growth in untreated controls (CFU/kidney) | Fluconazole |
Micafungin |
Amphotericin B |
||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) | Stasis (AUC/MIC)a | MIC (mg/liter) | Stasis (AUC/MIC)a | 1-log kill (AUC/MIC)a | MIC (mg/liter) | Stasis (Cmax/MIC)a | |||
| B11804 | Colombia | 2.17 | 2 | 51.2 | 0.5 | 48.1 | 120.3 | 0.5 | 0.87 |
| B11801 | Colombia | 2.86 | 16 | 26.3 | 1 | 32.9 | 49.9 | 2 | NAb |
| B11799 | Colombia | 2.08 | 16 | 36.3 | 2 | 18.5 | 92.1 | 0.5 | 1.29 |
| B11221 | South Africa | 1.85 | 128 | 6.3 | 1 | 47.6 | 140.6 | 0.38 | 0.52 |
| B11211 | India | 1.97 | 256 | NA | 4 | NA | NA | 1.5 | 0.69 |
| B11785 | Colombia | 2.32 | 8 | 34.1 | 0.5 | 59.4 | 119.2 | 1.5 | 1.50 |
| B11220 | Japan | 1.04 | 4 | 5.0 | 0.125 | 286.5 | 674.4 | 0.38 | NA |
| B11203 | India | 2.13 | 256 | NA | 0.25 | 117.0 | 376.4 | 4 | 0.51 |
| B11104 | Pakistan | 1.71 | 256 | 4.1 | 0.25 | 134.3 | 536.8 | 1 | 2.13 |
| Median | 26.3 | 53.7 | 130.5 | 0.87 | |||||
| Standard deviation | 18.5 | 87.9 | 235.3 | 0.60 | |||||
After 24 h.
NA, not applicable (endpoint not achieved).
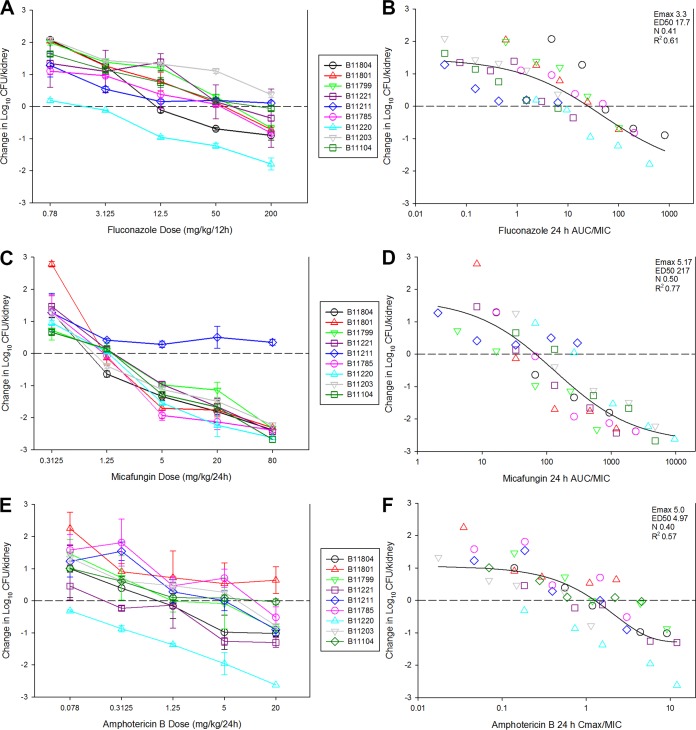
The results of the dose-ranging studies with the nine C. auris isolates for each drug are shown in Fig. 1A through C. Dose-dependent activity was observed with each strain. Net stasis was achieved against 7 of 9 strains for fluconazole. The two strains that did not achieve stasis over the dose range had an MIC of 256 mg/liter. Fluconazole therapy resulted in a 1-log kill for only one of the strains. In micafungin experiments, stasis and 1- and 2-log kill endpoints were achieved against 8 of 9 strains. The single strain in which these endpoints were not met (B11211) had the highest micafungin MIC of the group at 4 mg/liter. Finally, treatment of amphotericin B resulted in stasis with 8 of 9 strains. The single strain for which stasis was not observed with amphotericin B had an elevated MIC at 2 mg/liter. However, only 3 of 9 strains achieved 1-log kill endpoints for amphotericin B. Thus, for each drug, the dose-effect relationship against C. auris was proportional to the MIC.
FIG 1.
In vivo dose-response curves for 9 C. auris strains against fluconazole (A), micafungin (B), and amphotericin B (C). Each symbol represents the mean and standard deviation (error bars) of burden in the kidneys of three mice. The horizontal dashed line represents the burden at the start of therapy. The relationship between PK/PD index (AUC/MIC or Cmax/MIC) and efficacy for fluconazole (D), micafungin (E), and amphotericin B (F) are also shown. Each symbol represents the mean burden from three mice, and the horizontal dashed line is the burden at the start of therapy. A best-fit line based on the Hill equation is shown, as are the PD parameters maximum effect (Emax), ED50, slope (N), and the coefficient of determination (R2).
The degree to which MIC influences outcome in relation to pharmacokinetic exposures is the basis of PK/PD analyses. The results of these analyses are shown in Fig. 1D through F. There was a strong relationship between the PK/PD parameter (AUC/MIC or Cmax/MIC) and treatment outcome for each drug (R2, 0.61 for fluconazole, 0.77 for micafungin, and 0.57 for amphotericin). The stasis and 1-log kill target exposures (for micafungin only) are shown in Table 1. In the case of fluconazole, the stasis and ED50 targets (data not shown) were similar at 26 and 19, respectively. These values are consistent with prior fluconazole studies against Candida species demonstrating that AUC/MIC values of approximately 25 are associated with success in the animal model and in clinical studies in patients with candidemia (27). For amphotericin B, the data for C. auris were also remarkably congruent with prior studies, which have shown stasis to occur at Cmax/MIC exposures of 1 to 2 (27). The stasis Cmax/MIC for the groups of C. auris strains in this study was near 1. In contrast, micafungin efficacy differed in comparison to prior data in the invasive candidiasis model with other Candida species. Despite elevated MICs (range 0.125 to 4 mg/liter), micafungin drug exposures resulted in killing activity at relatively low drug exposures. The median total drug AUC/MIC associated with net stasis was only 53.7. Based on protein binding levels of 99.8% (28), this would translate into a free drug AUC/MIC target of 0.18. Previous micafungin studies demonstrated free drug AUC/MIC targets of 12, 4, and 5 for C. albicans, C. glabrata, and C. parapsilosis, respectively (29). Thus, the PD targets observed for micafungin against C. auris were ≥20-fold lower than those for other Candida species. 1-log kill exposures were also relatively low at a total drug AUC/MIC of 131 (free drug AUC/MIC of 0.26). The reasons for the enhanced efficacy observed for micafungin against C. auris compared to those of fluconazole and amphotericin B are unknown and an important area for future investigation.
Importantly, targets identified in this model with triazoles and echinocandins have correlated well with clinical outcomes in patients with invasive candidiasis (27). Thus, the present findings in this PK/PD study should be useful for forecasting effective treatment regimens for patients and in the development of preliminary susceptibility breakpoints. For example, a common daily dose of fluconazole in humans (400 mg) results in an AUC of nearly 400 mg · h/liter (30, 31). Therefore, using an AUC/MIC target exposure of 26, the MIC ceiling for which success would be predicted is approximately 16 mg/liter. Using the same approach, the MIC ceiling for amphotericin B would be 1 to 1.5 mg/liter. These are similar to PK/PD based breakpoints for other Candida species and these antifungals. Finally, a micafungin dosing regimen of 100 mg daily results in free drug exposures of approximately 0.3 to 0.4 mg · h/liter (32). Using the stasis PK/PD target data for micafungin, the PK/PD breakpoint could be as high as 2 to 4 mg/liter, with standard dosing of 100 mg/day.
In sum, the current animal model PK/PD study suggests that echinocandins are likely to be the most efficacious drug class for most C. auris isolates. The results suggest that traditional MIC breakpoints are likely to be relevant for fluconazole and amphotericin B, as the drug exposures associated with optimal outcome for C. auris were similar to those of previous Candida species studies. However, micafungin demonstrated a potent cidal effect against almost all strains with an MIC of <4 mg/liter, and the drug exposure targets (AUC/MIC) were significantly lower than those for other Candida species. Based on these data, echinocandins should be considered first-line therapy for patients with C. auris infection with regimen tailoring based on susceptibility results.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Msd Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. MMWR Morb Mortal Wkly Rep 65:1234–1237. doi: 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- 3.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, Maor Y, Tarabia J, Schechner V, Adler A, Finn T. 2017. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg Infect Dis 23. doi: 10.3201/eid2302.161486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borman AM, Szekely A, Johnson EM. 2017. Isolates of the emerging pathogen Candida auris present in the UK have several geographic origins. Med Mycol. doi: 10.1093/mmy/myw147. [DOI] [PubMed] [Google Scholar]
- 6.Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. 2016. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect 73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, Dinesh KR, Karim S, Singh SK, Hagen F, Meis JF. 2014. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis 33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, Jain S, Kathuria S, Randhawa HS, Hagen F, Meis JF. 2013. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis 19:1670–1673. doi: 10.3201/eid1910.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emara M, Ahmad S, Khan Z, Joseph L, Al-Obaid I, Purohit P, Bafna R. 2015. Candida auris candidemia in Kuwait, 2014. Emerg Infect Dis 21:1091–1092. doi: 10.3201/eid2106.150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang HC. 2011. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magobo RE, Corcoran C, Seetharam S, Govender NP. 2014. Candida auris-associated candidemia, South Africa. Emerg Infect Dis 20:1250–1251. doi: 10.3201/eid2007.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy M. 2016. Hospital transmitted Candida auris infections confirmed in the US. BMJ 355:i5978. doi: 10.1136/bmj.i5978. [DOI] [PubMed] [Google Scholar]
- 13.Morales-Lopez SE, Parra-Giraldo CM, Ceballos-Garzon A, Martinez HP, Rodriguez GJ, Alvarez-Moreno CA, Rodriguez JY. 2017. Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg Infect Dis 23:162–164. doi: 10.3201/eid2301.161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakash A, Sharma C, Singh A, Kumar Singh P, Kumar A, Hagen F, Govender NP, Colombo AL, Meis JF, Chowdhary A. 2016. Evidence of genotypic diversity among Candida auris isolates by multilocus sequence typing, matrix-assisted laser desorption ionization time-of-flight mass spectrometry and amplified fragment length polymorphism. Clin Microbiol Infect 22:277.e1–277.e9. doi: 10.1016/j.cmi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz Gaitan AC, Moret A, Lopez Hontangas JL, Molina JM, Aleixandre Lopez AI, Cabezas AH, Mollar Maseres J, Arcas RC, Gomez Ruiz MD, Chiveli MA, Canton E, Peman J. 2017. Nosocomial fungemia by Candida auris: first four reported cases in continental Europe. Rev Iberoam Micol 34:23–27. doi: 10.1016/j.riam.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. 2016. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R. 2017. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis 23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Prakash A, Singh A, Kumar H, Hagen F, Meis JF, Chowdhary A. 2016. Candida haemulonii species complex: an emerging species in India and its genetic diversity assessed with multilocus sequence and amplified fragment-length polymorphism analyses. Emerg Microbes Infect 5:e49. doi: 10.1038/emi.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma C, Kumar N, Pandey R, Meis JF, Chowdhary A. 2016. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect 13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Alexander BD, Byrne TC, Smith KL, Hanson KE, Anstrom KJ, Perfect JR, Reller LB. 2007. Comparative evaluation of Etest and sensititre yeastone panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J Clin Microbiol 45:698–706. doi: 10.1128/JCM.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 23.Andes D, Stamsted T, Conklin R. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob Agents Chemother 45:922–926. doi: 10.1128/AAC.45.3.922-926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andes D, van Ogtrop M. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother 43:2116–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:3497–3503. doi: 10.1128/AAC.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepak A, Andes D. 2011. Fungal sepsis: optimizing antifungal therapy in the critical care setting. Crit Care Clin 27:123–147. doi: 10.1016/j.ccc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Lepak AJ., Andes DR. 2014. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 5:a019653. doi: 10.1101/cshperspect.a019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamato Y, Kaneko H, Hashimoto T, Katashima M, Ishibashi K, Kawamura A, Terakawa M, Kagayama A. 2002. Pharmacokinetics of the antifungal drug micafungin in mice, rats and dogs, and its in vitro protein binding and distribution to blood cells. Jpn J Chemother 50:74–79. [Google Scholar]
- 29.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother 54:2497–2506. doi: 10.1128/AAC.01584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant SM, Clissold SP. 1990. Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs 39:877–916. [DOI] [PubMed] [Google Scholar]
- 31.Louie A, Liu QF, Drusano GL, Liu W, Mayers M, Anaissie E, Miller MH. 1998. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob Agents Chemother 42:1512–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepak A, Castanheira M, Diekema D, Pfaller M, Andes D. 2012. Optimizing echinocandin dosing and susceptibility breakpoint determination via in vivo pharmacodynamic evaluation against Candida glabrata with and without fks mutations. Antimicrob Agents Chemother 56:5875–5882. doi: 10.1128/AAC.01102-12. [DOI] [PMC free article] [PubMed] [Google Scholar]